2022 年 5 巻 5 号 p. 99-104
2022 年 5 巻 5 号 p. 99-104
Lysosome-associated membrane protein-1 (LAMP-1) is a type I membrane glycoprotein consisting of a large luminal domain, a membrane-anchoring domain, and a short cytoplasmic tail (CT). The tyrosine-based motif (G378Y379QTI382) in its CT exclusively binds to adapter protein complex-3 (AP-3), which may facilitate the incorporation of LAMP-1 into transport vesicles to late endosomes and lysosomes. Of this sequence, Y379 is critical, and hydrophobic I382 is optimal for the AP-3 binding and the efficient delivery to lysosomes. However, it is not clear how important G378 is in the AP-3 binding and lysosome transport. To clarify its importance, four mutants in which G378 was replaced with alanine, aspartic acid, glutamic acid, and asparagine (designated as G378A, G378D, G378E, and G378N, respectively) were prepared and their interaction strengths with AP-3 and lysosomal abundance were compared with those of wild-type (WT)-LAMP-1. A yeast two-hybrid system was applied to measure the interaction strengths of WT-, G378A-, G378D-, G378E-, and G378N-CTs with the medium subunit of AP-3 (μ3A). The G378A-, G378D-, and G378E-CTs as strongly interacted with μ3A as WT-CT, but the G378N-CT exhibited a very weak interaction with it. In the cell fractionation analyses, the lysosomal levels of G378A, G378D, and G378E were almost the same as that of WT whereas a lesser amount of G378N existed in the lysosomes. Taken together, it is considered that Y379 and I382 are essential for AP-3-mediated vesicular transport of LAMP-1 while the Y379-preceding amino acid is not restricted to glycine but asparagine at this position is less suitable for that.
Lysosomes are single membrane-enclosed organelles that play a prominent role in the intracellular degradation of various biomacromolecules.1,2) The degradative materials are delivered to lysosomes by way of biosynthetic transport, endocytosis, phagocytosis, and autophagy. More than 60 kinds of acid hydrolases degrade the biomacromolecule sequestered into lysosomal lumen. The limiting membrane of lysosomes is composed of over 200 integral proteins, of which two highly glycosylated lysosome-associated membrane proteins (LAMP-1 and LAMP-2) are principal components.3) LAMP-1 and LAMP-2 are structurally similar: an NH2-terminal major portion resides within the lysosomal lumen followed by a transmembrane domain and a short cytoplasmic tail (CT). The CTs have a tyrosine-based motif, GYXXΦ, at the COOH-terminus (Φ is a bulky hydrophobic amino acid), and the tyrosine-containing sequences serve as a lysosome-targeting signal.4–7) The lysosome-targeting signal of LAMP has been employed for the development of effective DNA vaccines.8,9) Precise research on the regulation of efficiency for lysosome trafficking of antigens by varying lysosome-targeted signals could lead to the development of more effective vaccines.
The tyrosine-based sequence interacts with tetrameric adaptor protein (AP) complexes and thereby mediates the incorporation of LAMPs into transport vesicles to late endocytic organelles (reviewed in Refs10,11). Four well-known AP complexes (AP-1, AP-2, AP-3, and AP-4) have been extensively studied for their interactions with various tyrosine-based sequences 12–16) and their roles in lysosomal delivery.11) LAMP-1’s tyrosine-based sequence, GY379QTI382, significantly interacts with a medium subunit of AP-3 (μ3A) but not the other three medium subunits, μ1, μ2, and μ4 of AP-1, AP-2, and AP-4, respectively.14–16) Furthermore, Y379 is crucial, and I382 is optimal for the interaction with μ3A. The role of amino acid preceding YXXΦ for binding to μ3A has been studied using combinatorial peptides with various amino acids at this position (Y-1).13) Glycine and acidic amino acids at Y-1 show significant interactions with μ3A in the yeast two-hybrid (Y2H) assays. The amino acids at Y-1 in lysosomal membrane proteins’ tyrosine-based motifs hitherto identified are glycine, alanine, glutamic acid, and asparagine among which glycine is the most common (Table 1).11,16,17) The importance of amino acids at Y-1 in the AP-3 binding and transport efficiency of LAMP-1 to lysosomes have not been well studied systematically.
| Lysosomal membrane protein | Amino acid sequence |
|---|---|
| LAMP-1 | GYQTI |
| LAMP-2A | GYEQF |
| LAMP-2B | GYQTL |
| LAMP-2C | GYQSV |
| DC-LAMP | GYQRI |
| CD63 | GYEVM |
| Acid phosphatase | GYRHV |
| Cystinosin | GYDQL |
| CD68 | AYQAL |
| NCU-G1 | EYQSI |
| Endolyn | NYHTL |
| TGN38* | DYQRL |
*TGN 38 is localized in the trans-Golgi network.
We here created the mouse LAMP-1 mutants in which G378 is substituted by amino acids found at the Y-1 position of other lysosomal membrane proteins and TGN38 (Table 1 and Fig. 1) and investigated the interaction strengths of their CTs with μ3A by the Y2H method. Moreover, these mutants transported to lysosomes were quantified by cell fractionation. The amino acid preceding Y379 is not restricted to glycine for the interaction with μ3A and efficient lysosomal targeting but asparagine at Y-1 is less suitable for them.

Schematic Representation of Wild-type (WT)-LAMP-1 and its Substitution Mutants
Mouse LAMP-1 is a 382-amino-acid protein composed of a luminal domain (LD), a transmembrane domain (TM), and a cytoplasmic tail (CT).The amino acid sequence of the WT-CT is shown in the top row. The underlined sequence (G378YQTI382) is the tyrosine-based motif for lysosome targeting. G378A, G378D, G378E, and G378N are mutant molecules in which G378 is replaced with alanine, aspartic acid, glutamic acid, and asparagine, respectively. Y379A is a mutant with Y379 being substituted for alanine.
MATCHMAKER two-hybrid kit was purchased from Clontech Laboratories Inc. (Palo Alto, CA). pGAD10-μ1, pGAD10-μ2, pGAD10-μ3A, pGAD10-μ4, and pcDNA3.1/mouse LAMP-1 cDNA were described previously.7,15)
Anti-mouse LAMP-1 rat monoclonal antibody (1D4B) was kindly provided by Dr. Thomas August (Johns Hopkins University, Baltimore, MD, USA). Horseradish peroxidase (HRP)-conjugated anti-rat IgG rabbit antibody and HRP-conjugated anti-mouse IgG goat antibody (Jackson ImmunoResearch Laboratories, West Grove, PA).
Plasmid ConstructionG378-substitution mutant cDNAs were produced by polymerase chain reaction (PCR) with the linear mouse LAMP-1 cDNA as a template. We used a forward primer, 5’-AGCAAAGAGATCTACACCATGGAT-3’, corresponding to a nucleotide sequence located upstream of the EcoRV site unique to mouse LAMP-1 cDNA as described. Reverse primers utilized for the generation of substitution mutants at G378 and Y379 (Fig. 1) were shown as follows.
Y379A, CCCCATCTAGACTAGATGGTCTGAGCGCCGGCGTCTG;
G378D, CCCCATCTAGACTAGATGGTCTGATAGTCGGCGTCTG:
G378E, CCCCATCTAGACTAGATGGTCTGATACTCGGCGTCTG;
G378A, CCCCATCTAGACTAGATGGTCTGATAAGCGGCGTCTG;
G378N, CCCCATCTAGACTAGATGGTCTGATAGTTGGCGTCTG.
A bold codon for an exchanged amino acid is written and an XbaI site introduced into the primer is underlined. The PCR products were purified and digested with EcoRV and XbaI. The pcDNA3.1/mouse LAMP-1 cDNA was cleaved with the same restriction enzymes and the insert released by this cleavage was removed. The enzyme-treated PCR cDNA fragment was subcloned into the EcoRV and XbaI sites of the pcDNA3.1/ mouse LAMP-1 cDNA.
For yeast two-hybrid assays, cDNA fragments corresponding to the cytoplasmic tails (CTs) (amino acids 372-382) of WT- and mutant LAMP-1 were amplified by PCR using the WT and mutant LAMP-1 pcDNA3.1 aliquots as templates and cloned into the EcoRI and BamHI sites of a pGBKT7 vector to produce pGBKT7-WT and mutants.
Yeast Two-Hybrid AssaypGAD-T7 vector, pGAD10-μ1, pGAD10-μ2, pGAD10-μ3A or pGAD10-μ4 was transfected into Y187 strain, and pGBKT7-vector, pGBKT7-WT or one of the pGBKT7 mutants was transferred into Y2H gold strain according to the lithium acetate procedure.18) The Y187 and Y2H gold strains were then mated by the manufacturer’s protocol. A quantitative growth assay for the mated yeast strains was performed as described previously.15)
Cell Culture and TransfectionCOS7 cells were grown in Dulbecco’s modified eagle medium (Gibco ERL, Grand Island, NY) supplemented with 10% fetal calf serum, 100 units/mL penicillin/streptomycin, and 2 mM L-glutamine. COS7 cells were transfected with the pcDNA3.1 plasmids by Polyfect according to the manufacturer’s instruction (Qiagen Co., Tokyo, Japan).
Cell FractionationThe transfected cells were homogenized by nitrogen cavitation using Parr Cell Disruption Bombs (Central Scientific Commerce Inc., Tokyo, Japan). Post-nuclear supernatant (PNS) was prepared by centrifugation at 650 x g for 10 min. The PNS was subjected to fractionation by Percoll (GE Healthcare UK Ltd, Buckinghamshire, England) density gradient centrifugation according to the same method as that reported previously.7) An equal volume of the supernatant in each fraction obtained was used for assays of alkaline phosphodiesterase I (a marker enzyme for the plasma membrane), β-glucuronidase (a marker enzyme for lysosomes), and Western immunoblotting.
Enzyme AssayAlkaline phosphodiesterase I activity was calculated from the amounts of nitrophenol liberated from 1 mM thymidine 5′-monophosphate p-nitrophenyl ester (Sigma-Aldrich Japan, Tokyo) in 0.1 M glycine-NaOH buffer (pH 9.4) after incubation at 37°C for 6 h as described by Brightwell and Tappel.19) β-Glucuronidase activity was fluorometrically assayed with 1 mM 4-methylumbelliferyl-β-D-glucuronide (Nacalai, Tokyo) as a substrate by the method of Robins et al.20) The activity was calculated from the amounts of 4-methylumbelliferone released after incubation in 0.1 M acetate buffer (pH 3.8) 37°C for 20 min.
Western Immunoblot AnalysisWestern immunoblot analysis was performed using nitrocellulose sheets (Advantec. Tokyo Japan) as described previously.7) Densities of positive bands were measured using Lumino Graph II (ATTO, Tokyo, Japan).
StatisticsStatistical comparisons between different data were made using the Student’s t-test. Differences were considered if P < 0.01.
It has been shown that LAMP-1’s CT containing the tyrosine motif exclusively interacts with μ3A.14–16) In this study, to investigate the importance of G378 in the interaction between the μ3 subunit and LAMP-1’s CT, we measured the growth of the yeast that co-express μ3A and WT or one of G378-mutated CTs (Fig. 1) in a liquid medium without His, Leu, and Trp. The yeast co-expressing the WT-CT and μ3A began to proliferate after being incubated for 2 days and the growth attained almost the maximum value after 4-day-incubation (Fig. 2A, circles). Significant growth was observed for the yeast clones co-expressing μ3A and G378A-CT, G378D-CT, or G378E-CT with similar curves to that of the WT-CT/μ3A yeast clone (Fig. 2B, C, and D). In contrast, the yeast co-expressing μ3A and G378N-CT did not grow so much as WT (Fig. 2A, squares). Comparing OD600 values after incubation for 4 days, G378D-CT, G378A-CT, and G378E-CT interact with μ3A as strongly as WT-CT (Fig. 2E). In contrast, the interaction strength between G378N-CT and μ3A is approximately one-seventh of that between WT-CT and μ3A.
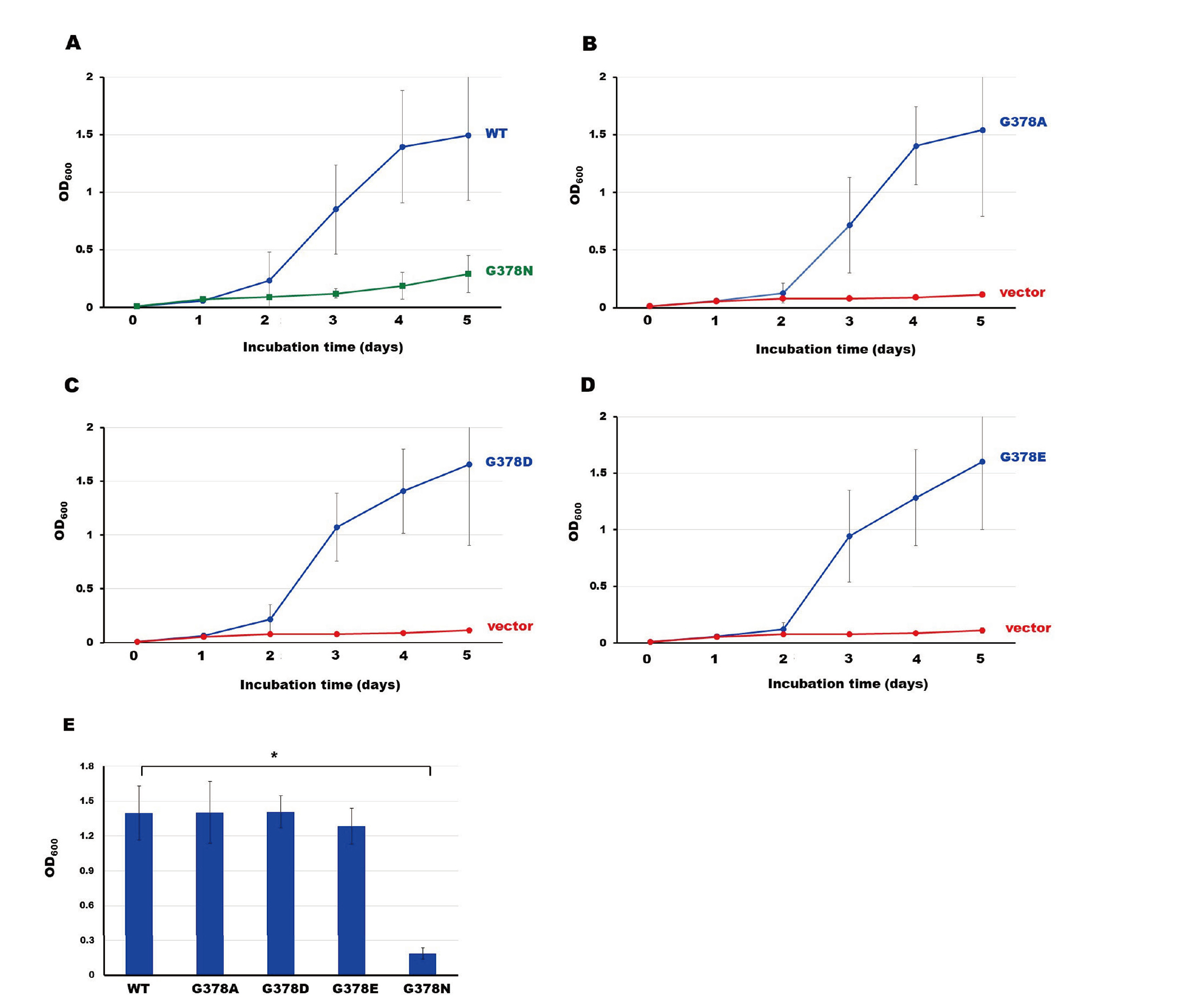
Yeast Two-Hybrid Interaction of WT and Mutated LAMP-1 Cytoplasmic Tails with μ3A-Subunit
The yeast cells co-expressing μ3A and WT or one of the mutant CTs were incubated in synthetically defined leucine, tryptophan, and histidine dropout medium (SD-LWH) for 5 days. The control culture was performed using the yeast cells co-transformed with pCAD-μ3A and pGBKT7 vector. OD600 values were measured at the times indicated. (A) WT (circles) and G378N (squares), (B) G378A, (C) G378D, and (D) G378E. Each value represents the mean ± SD (5 separate cultures). No significant growth was observed for yeast cells co-expressing μ1, μ2, or μ4 and any of the mutant CTs (data not shown). (E) Interaction strengths of WT and mutated CT peptides of LAMP-1 with μ3A. After the transformed yeasts were grown in SD-LWH for 4 days, OD600 was determined. Each value represents the mean ± SE (5 separate cultures). *P < 0.01.
In relation to the interactions of LAMP-1’s G378 mutants with μ3A, the G378 mutants were transiently expressed in COS7 cells (Fig. 3A) and the transport of these mutants to lysosomes was quantified by the cell fractionation method. Figure 3B shows the distribution of the plasma membrane marker enzyme, alkaline phosphodiesterase I, along with that of Percoll density. This enzyme was exclusively detected around fraction 3. As shown in Fig. 3C, a major portion of WT-LAMP-1 was localized in low-density fractions while a significant amount was present in the dense lysosomal fractions. WT-LAMP-1 in the low-density fractions is mainly derived from the plasma membrane and early endosomes (Fig. 3B and Ref. 7). Y379A was hardly detected in the dense lysosomal fraction (Fig. 3D). Notable amounts of G378A, G378D, and G378E were recovered in the dense lysosomal fractions (Fig. 3E, F, and G, respectively). Their distribution profiles are very similar to that of WT-LAMP-1. Meanwhile, G378N existed in the dense lysosomal fraction to a lesser extent (Fig. 3H). We summarized the lysosomal abundance of WT-LAMP-1 and the mutants in Fig. 3I. The lysosomal amounts of G378A, G378D, and G378E are comparable to that of WT-LAMP-1 whereas the lysosomal abundance of G378N is approximately half that of WT-LAMP-1.

Distribution of WT-LAMP-1 and its Mutants in the Percoll Density Gradient Fractions
(A) Western immunoblotting of WT-LAMP-1 and its mutants expressed in COS7 cells. Equal amounts (10 μg protein) of PNSs from the COS7 cells were analyzed by Western immunoblotting. Lane 1, parental; lane 2, WT; lane 3, G378A; lane 4, G378D, lane 5, G378E; lane 6, G378N, and lane 7, Y379A. Molecular mass (kDa) was indicated to the left. (B) The PNS from WT-LAMP-1-expressing COS7 cells was subjected to fractionation by Percoll density gradient centrifugation. Fractions of 0.5 mL were collected from the top of the gradient. Percoll densities (circles) and alkaline phosphodiesterase I activities (bar graphs) were measured for the fractions. (C–H) The fractions were subjected to Western immunoblotting with an anti-mouse LAMP-1 antibody and assayed for β-glucuronidase. The resultant blots with fraction numbers were shown in the upper panels. The distributions of WT-LAMP-1 and its mutants (bar graphs) and β-glucuronidase (line graphs) in the 18 fractions are represented as percentages of the total: (C) WT, (D) Y379A, (E) G378A, (F) G378D, (G) G378E, and (H) G378N. (I) Relative abundance of WT-LAMP-1 and the mutants in the lysosomal fraction. Lysosomal WT-LAMP-1 and mutants are sums of those in fractions 15–18. The data are shown as the means ± SE from four independent experiments. *P < 0.01.
The lysosomal amounts of these G378 mutants strongly correlate with the interaction strengths of their CTs with μ3A (Fig. 4), supporting the previous conclusion that AP-3 makes a major contribution to the vesicular transport of LAMP-1 to lysosomes.15)
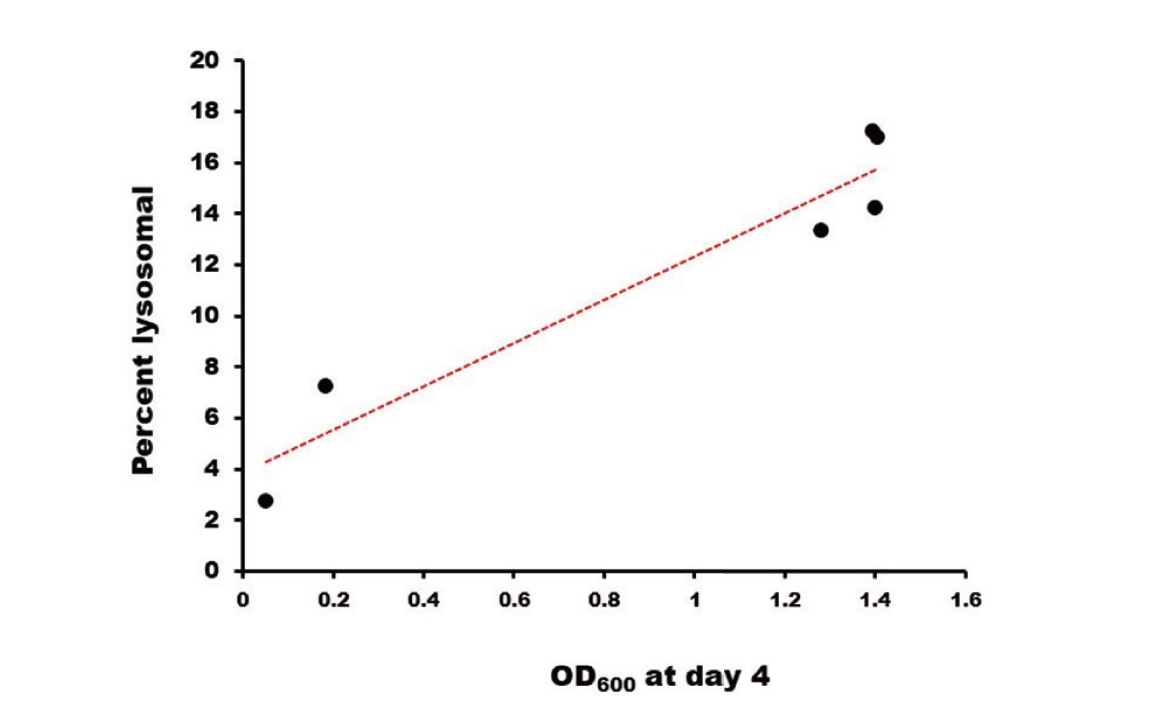
The Interaction Strengths of WT and Mutated Forms of LAMP-1 with μ3A Correlate with Their Localization in Lysosomes
The Y2H interaction strengths of WT and mutant LAMP-1 CTs (Fig. 2E) were plotted against their amounts in the lysosomal fraction (Fig. 3I). The correlation coefficient was calculated to be 0.95.
The extensive Y2H analyses have shown that the μ3A subunit prefers aspartic acid, glutamic acid, and glycine to asparagine at Y-1,13) which agrees well with the interaction strengths of WT, G378D, G378E, and G378N with μ3A in Fig. 2E. Endolyn is a lysosomal type I membrane glycoprotein whose CT has a tyrosine-based motif of NYHTL (Table 1). Endolyn’s CT also interacts with μ3A, but not with other μ subunits.16) The Endolyn motif has asparagine at Y-1, and its CT has a considerably weaker interaction with μ3A than a mutant CT containing GYHTL, which is very similar to the weaker G378N’s interaction with μ3A. The molecular modeling illustrated that the tyrosine motif of LAMP-1 is sandwiched by two paralleled β-sheets (β1 and β16) occurring in the COOH-terminal domain of rat μ3A.15) The hydroxy group of Y379 makes hydrogen bonds with the side chains of Asp182 (β1) and Lys406 (β16). Our preliminary molecular simulations suggest that the replacement of G378 with N would change the orientation of the side chain of Lys406 and affect the hydrogen bond between Lys406 and Y379, resulting in a weakened interaction.
Harter and Mellman4) reported that the G to A-mutant of rat LAMP-1 (equivalent to G378A) is predominantly present in the intracellular organelles, probably late endosomes and lysosomes as much as WT in the steady-state distribution while the Y to A-substituted mutant (equivalent to Y379A) becomes largely distributed to the cell surface. Their data are consistent with the present results that the substitution of G378 to A hardly affects the lysosomal localization of LAMP-1 whereas the Y379 to A mutation almost abolished the lysosomal localization of LAMP-1 concomitant with its remarkable increase in the cell surface (Fig. 3 and Supplemental Figure).
Ihrke et al.16) examined AP-3-mediate subcellular trafficking of two chimeric proteins containing the tyrosine motifs of endolyn NYHTL and its mutant GYHTL, respectively. The high μ3A-affinity GYHTL chimera is preferentially sorted to the direct lysosomal pathway at the TGN while the low μ3A-affinity NYHTL chimera follows the indirect pathway; this fusion protein moves to the cell surface along the secretory pathway and then undergoes endocytosis to early endosomes where they enter the transport vesicles with the help of μ3A and are transported to late endosomes/lysosomes.
Since the CTs of WT-LAMP-1 and G378A have stronger interaction with μ3A than that of G378N, it is possible that the newly synthesized WT-LAMP-1 and G378A bind to AP-3 at the TGN and are directly sorted to late endosomes/lysosomes. Indeed, most newly synthesized WT-LAMP-1 and G378A are transported directly from the TGN to late endosomes and then to lysosomes.4,21) On the other hand, newly synthesized G378N possibly follows the indirect pathway. This possibility may be supported by the fact that the amount of G378N in the plasma membrane fraction is greater than that of WT (Fig. 3 and Supplemental Figure).
Because the indirect pathway involves more steps, mutating G378 of LAMP-1 to N may reduce its transport efficiency, causing G378N to be lower than WT and G378A in the lysosomal amount. In summary, the combination of G378, Y379, and I382 is optimal for the most efficient transport of LAMP-1 to lysosomes by the direct pathway.
Conflict of interestThe authors declare no conflict of interest.