2022 年 5 巻 6 号 p. 125-132
2022 年 5 巻 6 号 p. 125-132
Background: A rubric-based intervention program was designed to reduce the risk of developing coronary artery disease (CAD) in patients with dyslipidemia, and the effectiveness of community pharmacist-led intervention using the program was evaluated. Methods: We conducted an open-label, multicenter, randomized controlled trial. Participants included patients with dyslipidemia on statin medication recruited from August 2020 to July 2021. The intervention group received instructions from community pharmacists using a rubric-based intervention program in addition to usual pharmacist care. Conversely, the control group only received the usual care. The primary outcome was the change in the Suita score at month 6, which was compared between the intervention and control groups. The secondary outcome was the change in the rubric score, which was compared between the post-intervention and baseline. Results: We analyzed 15 participants (mean age, 61.9 years), whose mean Suita score was 42.6. No significant difference was observed in the change in the Suita score between the two groups. However, in the intervention group, the post-intervention Suita score was significantly lower than the baseline (43.9 vs. 38.7, p = 0.01), and the CAD risk classification based on Suita score decreased from medium to low risk. The post-intervention rubric score increased significantly compared with the baseline (2.0 vs. 3.1, p < 0.01). Conclusion: The rubric was a useful tool in promoting behavior changes in patients. The community pharmacist-led intervention for patients with dyslipidemia using a rubric-based intervention program showed the possibility of reducing the risk of developing CAD.
The number of patients with dyslipidemia in Japan exceeded 2.2 million in 2017 and has been increasing annually.1) Dyslipidemia is a risk factor for cardiovascular events. Strict control of blood pressure (BP), blood glucose, and blood lipids can reduce cardiovascular events.2–4) A reduction of 10 mmHg in the systolic blood pressure (SBP) or 5 mmHg in the diastolic blood pressure (DBP) is associated with a 20% reduction in the incidence of coronary artery disease (CAD).5) However, a 1% increase in HbA1c is associated with an 18% increase in cardiovascular events.6) Additionally, compared with a low-density lipoprotein cholesterol (LDL-C) level less than 80 mg/dL, LDL-C level above 140 mg/dL is associated with a 2.8-fold increase in the incidence of CAD.7) Despite the demonstrated importance of risk factor management, mortality remains high, and risk factor management remains inadequate.8)
Lifestyle improvement through diet and exercise therapy is important in treating dyslipidemia.9,10) Drug therapy is indicated when lipid management through lifestyle improvement is insufficient. However, the drug therapy continuation rate for dyslipidemia is lower than that for hypertension and diabetes.11) This is due to the absence of symptoms in dyslipidemia and the low level of knowledge and awareness about the disease. Therefore, improved lifestyle habits and continued drug therapy are important. Active intervention by community pharmacists for patients with dyslipidemia improved treatment continuation rate and medication adherence.12,13) Furthermore, community pharmacist-led intervention using the Framingham risk score reduced cardiovascular risk in Canada.14) Therefore, a pharmacist-led intervention is useful for managing the risk of cardiovascular events.
To validate the effectiveness of interventions by pharmacists, it is important to standardize the quality of pharmacists and introduce standardized intervention programs. The effectiveness of community pharmacist-led intervention programs has been reported for patients with dyslipidemia in Spain and for patients with hypertension in Japan.13,15) Additionally, it is necessary to promote behavioral changes in patients. In the education field, rubrics have been used to assess and enhance learning efficacy. Similarly, the use of rubrics in the healthcare field may enhance behavior changes in patients. However, only a few reports have evaluated the effect of community pharmacist-led interventions using a rubric-based program on patients with dyslipidemia. If the intervention effect by community pharmacists using a rubric-based program is obtained for patients with dyslipidemia, their risk for CAD will be reduced.
Therefore, a rubric-based intervention program was designed to reduce the risk of developing CAD in patients with dyslipidemia, and the effectiveness of community pharmacist-led intervention using the program were evaluated.
This study was an open-label, multicenter, and randomized controlled trial conducted in 10 community pharmacies in Japan. Participants were randomly assigned (2:2) using block randomization with a block size of four to the intervention or control groups. Fig. 1 is a flowchart of participant selection. The pharmacists obtained informed consent from the participants (Visit 0) and implemented the intervention four times (Visits 1–4). Data were collected within 3 months after the fourth intervention (post-intervention).

Flowchart of Participant Selection
The pharmacists obtained written informed consent at Visit 0. The participants were randomly assigned to intervention and control groups. The pharmacists intervened on Visit 1, 2, 3, and 4. The pharmacists collected clinical data at post-intervention.
The pharmacist recruited participants using a brochure describing the research content, from August 2020 to July 2021.
The inclusion criteria were as follows: i) participants with dyslipidemia who were on statins; ii) participants aged 35–69 years at enrolment; iii) participants whose components and/or dose of dyslipidemia therapeutic agent remain unchanged for 6 months or more; iv) participants with at least one of the following: LDL-C ≥ 140 mg/dL, high-density lipoprotein cholesterol (HDL-C) ≤ 40 mg/dL, SBP ≥ 140 mmHg, or DBP ≥ 90 mmHg or higher; and v) participants who obtained written informed consent.
The exclusion criteria were as follows: i) participants with familial hypercholesterolemia; ii) participants with a history of CAD; iii) participants with diabetes, chronic kidney disease, non-cardiogenic cerebral infarction, or peripheral arterial disease; iv) participants receiving treatment for dementia or mental illness; v) participants with serious complications including dialysis; vi) participants who could not obtain laboratory test results; and vii) participants who were considered unsuitable for this study.
To the best of our knowledge, no previous studies have evaluated changes in the Suita score in relation to dyslipidemia or CAD. Therefore, we set the sample size to 20 because the difference for changes in the Suita score between the intervention and control groups would improve by approximately eight points, a significance level of 5% and statistical power of 80%.
Pharmacist TrainingThirty-four pharmacists with more than 3 years of experience in pharmacist practice agreed to participate in this study. They underwent a 1-h video training on clinical research and dyslipidemia and a subsequent confirmation examination. The exam contained 30 questions, and it was passed when all the questions were answered correctly. The training videos included the following topics: clinical research, research ethics, research outline, pathology of dyslipidemia, treatment strategies for dyslipidemia, drug therapy for dyslipidemia, exercise therapy for dyslipidemia, diet for dyslipidemia, and how to use the instructional tools. Additionally, the pharmacists completed a 2-h online training on the intervention program, which included a lecture on the intervention program and role-playing in presenting the intervention method. The content of the lecture included the following topics: i) how to use the instructional tools, ii) how to obtain informed consent, and iii) the flow of intervention.
Intervention Group ToolsThe rubric consisted of assessment items, instructional items, and instructional tools (Supplementary table 1). The assessment items were classified into nine categories (pathology, pharmacotherapeutics, smoking cessation, alcohol consumption, weight control, exercise, dietary fiber, salt intake, and lipid intake). The level of assessment items were set at four levels, against which the pharmacists assessed the participants. Additionally, based on the rubric-based assessment, the pharmacist selected the instructional items appropriate for the participants and used the tools corresponding to the instructional items.
Healthcare notes are self-management tools for patients with dyslipidemia. The notes are designed to allow participants to check the contents of the pharmacists’ instruction and their health status at home. The notes have columns for recording assessment items, instructional tools, BP, body weight, laboratory data, and other data.
Intervention MethodsThe pharmacists intervened using the rubric-based intervention program, which had four components (Fig. 2):
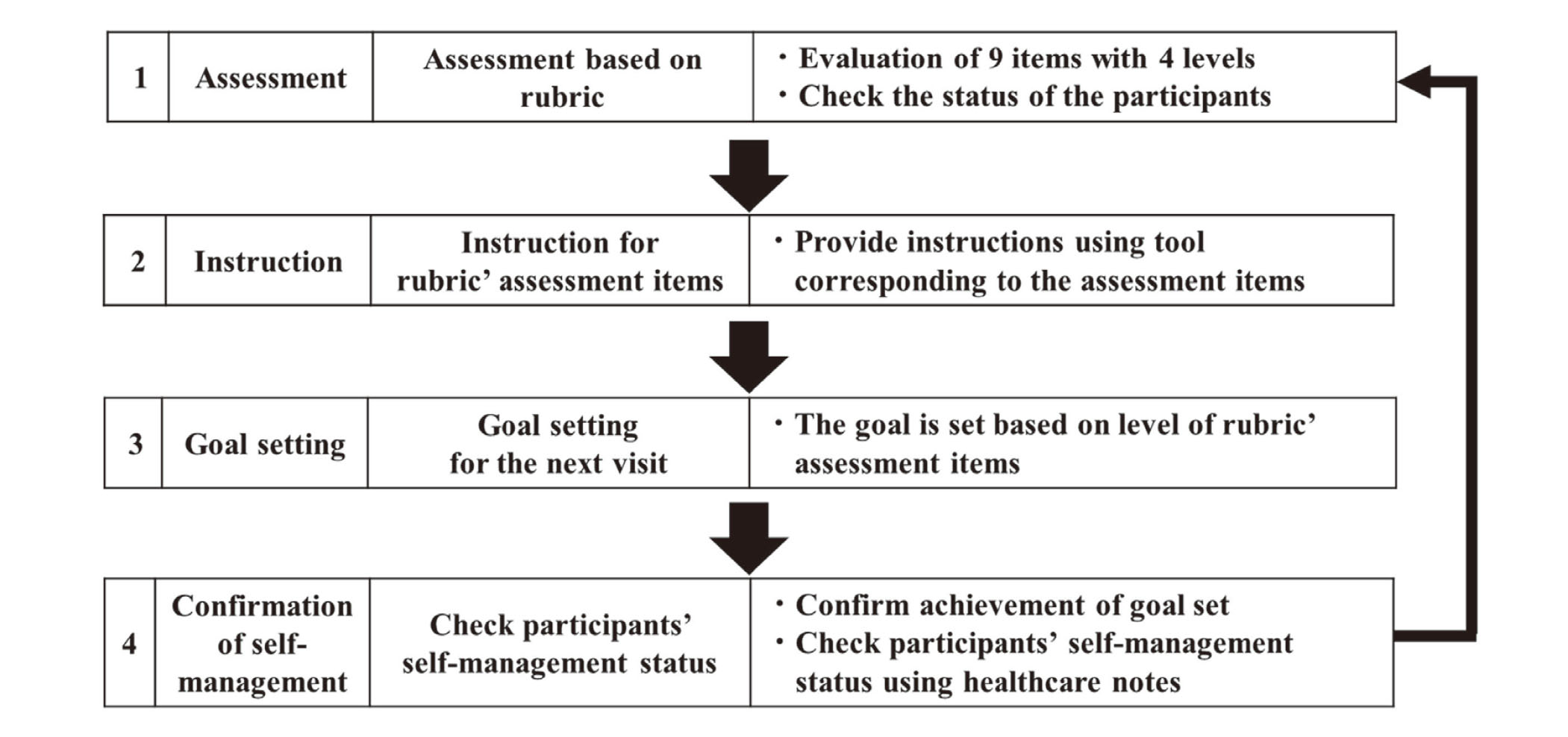
Rubric-Based Intervention Program
The pharmacists intervened in the following order: 1) Assessment, 2) Instruction, 3) Goal setting, and 4) Confirmation of self-management. This intervention was repeated four times.
Intervention Program 1 (Assessment): Pharmacists assessed participants’ awareness of the disease, medication knowledge, and lifestyle status using the rubric. Afterward, the participants selected three or more items out of the rubric’s nine assessments items.
Intervention Program 2 (Instruction): The pharmacists provided instructions using the tools corresponding to the assessment items selected by the participants.
Intervention Program 3 (Goal setting): The goal for the next visit was set based on the level of the assessment items instructed by the pharmacist.
Intervention Program 4 (Confirmation of self-management): Using healthcare notes, the pharmacist confirmed whether the participant achieved their goals.
Control GroupThe participants were checked for BP and body weight, in addition to the usual pharmacist care. They were provided with a healthcare note without the assessment items or instructional tools and only their BP, body weight, and laboratory data were recorded.
OutcomesThe primary outcome was the change in the Suita score, which was calculated as the difference between the Suita scores at visit 0 (baseline) and post-intervention. The Suita score was calculated based on the risk factors for CAD: age, sex, smoking, BP, HDL-C, LDL-C, impaired glucose tolerance, and family history of premature CAD.16) The Suita score is proposed as an appropriate index for estimating the risk for CAD in the Japan Atherosclerosis Society Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases.7) The Suita Score was used to classify the risk for CAD as high, medium, and low risk. Therefore, the Suita score is the optimal index for assessing the effect of a pharmacist-led intervention in the Japanese population.
The secondary outcomes were the changes in the LDL-C, HDL-C, TG (triglyceride), non-HDL-C levels, BP, body weight, the proportion of days covered (PDC), and the rubric score of the intervention group.17) The PDC was calculated using the following formula: number of days covered by the target medication/number of days during the study period. The rubric score was assigned to 1 to 4 points based on the level of the rubric’s assessment.
MeasurementsThe BP was measured at the pharmacy, while body weight was measured at home on the day before the visit 1–4. The LDL-C, HDL-C, TG, and non-HDL-C levels were measured and recorded at the medical institution. The patients’ baseline laboratory data were measured within 3 months before enrollment in the study, whereas laboratory data for post-intervention was measured within 2 months before and after the visit.
Statistical AnalysisThe student’s t-test or Mann–Whitney U test was used to compare the scale variables while the Fisher’s exact test was used to compare categorical variables between the intervention and control groups. The paired t-test or Wilcoxon signed-rank test was used to compare the scale variables between the baseline and post-intervention. All p-values < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS software ver. 25 (IBM, Tokyo, Japan). To analyze cases with missing data during the post-intervention, we used the baseline data.
EthicsThis study was approved by the Ethics Committee of the Showa University School of Pharmacy, Japan (No. 354), and the study protocol complies with the principles of the Declaration of Helsinki. Informed consent was obtained from all participants. All patient data were analyzed anonymously. This study was registered with the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR; Identification No. UMIN000044945).
Pharmacists took the examination online and re-learned the material until they passed the exam. Among the 34 pharmacists who appeared for the exam, 20, 12, 1, and 1 cleared it on their first, second, third, and fifth attempts, respectively. The participating pharmacists learned intervention skills through role-playing.
ParticipantsTen community pharmacies participated in this study. A total of 20 participants were recruited, 17 of whom were eligible. We excluded one participant who met the exclusion criteria. A total of 16 participants were randomly assigned to the intervention group (9 participants) or the control group (7 participants). One participant withdrew in the control group. The final analysis included nine participants in the intervention group and six in the control group (Fig. 3).

CONSORT Flow Chart
Ten community pharmacies participated in this study. A total of 20 participants were recruited, 17 of whom were eligible. We excluded one participant who met the exclusion criteria. Sixteen participants were randomly assigned to the intervention group (9 participants) or the control group (7 participants).
No significant differences were observed between the baseline characteristics of the intervention and control groups (Table 1). The mean age of the participants was 61.9 years, and 13% were male. The mean Suita score of the participants was 42.6, and most participants were classified as at medium risk of CAD based on their Suita scores. The median duration of the study intervention was 126.5 d.
 Primary Outcome
Primary Outcome
The results of the primary outcome are shown in Table 2. The amount of change in Suita score was −5.2 ± 5.0 in the intervention group and −1.0 ± 4.9 in the control group (95% confidence interval [CI]: −9.9 to 1.4, p = 0.13). The percentage of decrease in the Suita score was 11.8% in the intervention group and 2.5% in the control group. The Suita score changed from 43.9 ± 5.9 to 38.7 ± 9.1 (95% CI: −9.0 to −1.4, p = 0.01) in the intervention group and from 40.7 ± 7.6 to 39.7 ± 7.3 (95% CI: −6.1 to 4.1, p = 0.64) in the control group. In the intervention group, the CAD risk classification based on the Suita score decreased from medium to low risk.
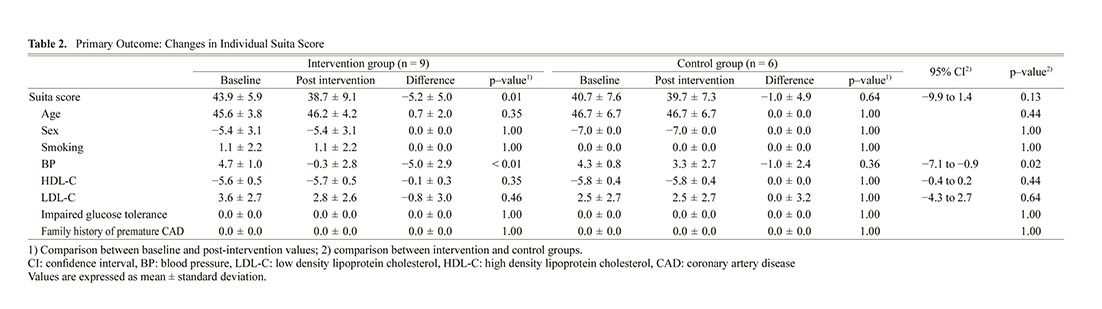
The LDL-C and HDL-C levels, which are components of the Suita score, did not change in the control group. However, in the intervention group, the LDL-C and HDL-C levels showed a trend toward improvement. BP level, which is also a component of the Suita score, was significantly lower in the intervention group than in the control group.
Secondary OutcomesThe LDL-C, HDL-C, TG, non-HDL-C, SBP, DBP, body weight, and PDC were not significantly different between the intervention and control groups (Table 3). However, in the intervention group, the post-intervention SBP level was significantly lower than the baseline (145.0 vs 127.7 mmHg, p < 0.01).

The post-intervention rubric score increased significantly from the baseline (2.0 vs 3.1, p < 0.01). The analysis of the rubric-based assessment items revealed that the post-intervention rubric scores were significantly higher than the baseline for pathology (2.0 vs 4.0, p = 0.04), pharmacotherapy (1.0 vs 4.0, p = 0.04), exercise (1.0 vs 3.0, p = 0.02), dietary fiber (2.0 vs 3.0, P < 0.01), salt intake (2.0 vs 3.0, p < 0.01), and lipid intake (1.0 vs 2.0, p = 0.03).
The change in the Suita score was not significantly different between the control and intervention groups. However, the CAD risk classification based on the Suita score decreased from medium to low risk in the intervention group. To our knowledge, this is the first study to evaluate the effectiveness of community pharmacists’ intervention for patients with dyslipidemia using a rubric-based intervention program. The rubric was a useful tool for promoting behavioral changes in patients with dyslipidemia. Additionally, we showed that community pharmacist-led intervention using a rubric-based program improved patients’ awareness of the disease, medication knowledge, and lifestyle habits, which may reduce the risk of developing CAD. This rubric-based intervention program could be used by health support pharmacies that support the health of local residents.
In the intervention group, the post-intervention Suita score decreased by 5.2 points from the baseline. The incidence of CAD is calculated for every 5 points of the Suita score.7) Therefore, a decrease in the Suita score in the intervention group means a 1%–8% reduced risk of developing CAD.7) Furthermore, in the intervention group, the CAD risk classification based on the Suita score decreased from medium (Suita score of 41–55 indicates < 2%–9% incidence of CAD within 10 years) to low risk (Suita score of ≤ 40 indicates < 2% incidence of CAD within 10 years).7) This finding suggests that community pharmacist-led intervention using the rubric-based program reduced the risk of developing CAD.
Among the components of the Suita score, HDL-C and LDL-C levels at baseline were controlled within the reference range. The HDL-C and LDL-C levels of most participants were considered to be well controlled through pharmacological treatment, because they were taking statins and had a high adherence rate. Thus, although detecting a significant difference between the intervention and control groups was difficult, the Suita score demonstrably decreased in the intervention groups.
We developed an original rubric-based intervention program in this study. This program was designed to promote behavioral changes in patients through pharmacist-led interventions. The assessment items of the rubric were selected based on the Japan Atherosclerosis Society Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases and guidelines for managing obesity.7,18) Particularly, the instructional items and their content were designed to promote behavioral changes in patients.19) The baseline and post-intervention rubric scores for each assessment item were compared. As a result, the rubric scores for the awareness of disease, medication knowledge, exercise, and diet significantly improved. The findings showed that pharmacist-led intervention using the rubric improved patients’ awareness of the disease, medication knowledge, and lifestyle habits, which led to lower lipid and BP levels.
In a meta-analysis examining the effect of pharmacist-led intervention, the difference in LDL-C level between the intervention and control groups was −7.9 mg/dL.20) The difference obtained in our study was very similar at −7.8 mg/dL (−1.8 vs 6.0). Conversely, the meta-analysis showed that the differences in HDL-C and TG levels between the intervention and control groups were 1.75 and −24.3 mg/dL, respectively, whereas those in our study improved demonstrably to 7.7 (2.2 vs −5.5) and −60.0 mg/dL (−29.7 vs 30.3), respectively. Additionally, LDL-C and HDL-C levels tended to improve in the intervention group, whereas they tended to worsen in the control group, thereby reflecting the effect of the pharmacist-led intervention.
No significant difference was observed in BP levels between the intervention and control groups. In a randomized controlled trial in which community pharmacists provided motivational interventions for patients with hypertension,15) the differences in SBP and DBP were −6.0 and −3.4 mmHg, respectively. Our results were similar: −6.9 (−17.3 vs −10.4) and −3.4 mmHg (−8.7 vs. −4.7), respectively. In the intervention group, the post-intervention SBP level was significantly lower than the baseline. Moreover, the rubric score of salt intake significantly improved post-intervention. It is reported that lifestyle modification is associated with reduced blood pressure.21) Furthermore, a 10-mmHg reduction in SBP or 5-mmHg reduction in DBP is associated with a 20% reduction in the incidence of CAD.5) Therefore, lifestyle modification was found to reduce BP, leading to a lower incidence of CAD.
PDC was not significantly different between the intervention and control groups. A PDC of 80% or higher is considered to indicate good adherence.22) Therefore, there was no significant difference in the PDC between the two groups due to good adherence at the baseline (PDC, 96.3%).
Our study focused on the frequency rather than the duration of intervention, since this was an intervention trial to promote behavioral changes through patient education. In previous studies, the frequency of pharmacist-led interventions has been set at three to six times.20,23,24) Similarly, in this study, the number of visits to the pharmacy was set at six times, including the visit 0 and post-intervention. Therefore, the frequency of intervention was considered appropriate for promoting behavioral changes in patients.
Studies on pharmacist-led intervention confirm the necessary of conducting training, such as those on knowledge and intervention methods for pharmacists, before the start of a study.12,15) Similarly, in this study, pharmacists went through the training program and since they all passed the confirmation examination, we may have standardized their quality.
LimitationsThis study has several limitations. First, no significant difference was noted in the amount of change in the Suita score. This may be attributed to the small sample size. Even in the control group, the amount of change in the Suita score improved. Therefore, a large sample size is required to demonstrate a significant difference in the amount of change in the Suita scores between the two groups. Second, the Suita score also decreased in the control group since the study was an open-label trial. The Hawthorne effect,25) defined as changes in the behavior of patients when involved in a trial because of increased knowledge or interest or else due to feeling observed in the trial, may have occurred in the control group, since the pharmacists monitored their BP and laboratory data. Third, the study population comprised primarily females. The number of female patients with dyslipidemia in Japan is 2.4 times that of male pateints.1) Therefore, the results obtained from this study reflective of the clinical practice.
ConclusionWe constructed rubric-based intervention program for patients with dyslipidemia. The rubric was a useful tool for promoting behavioral changes in patients. Additionally, we showed that pharmacist-led intervention using the rubric-based program improved patients’ awareness of the disease, medication knowledge, and lifestyle habits, which may reduce the risk of developing CAD.
AcknowledgmentsThis work was supported by JSPS KAKENHI Grant Number JP20K07138.
We thank Tanabe Pharmacy Inc., Ltd, Yoshitaka Aono, Maho Arita, Hiroyuki Okada, Shigeru Kurita, Shiho Nakatsu, Daisuke Matsushima, Akiko Murashige, Takahiro Yanagiya, and Takafumi Yoshida for their cooperation in conducting this study. We also thank Chisa Takeda, Miyu Watanabe, Kana Oono, Tomoko Eshita, and Miyu Suzuki for their cooperation in creating the tools and workshop materials.
Conflicts of InterestHisateru Ueki, Toshinori Yamamoto, and Tsuyoshi Inoue are employees of Tanabe Pharmacy Inc.