2022 年 5 巻 6 号 p. 147-153
2022 年 5 巻 6 号 p. 147-153
Rosacea is an intractable skin disease and no effective treatment has been established, but there are cases of improvement with Kampo medicine. In this study, the efficacy of Keishi-Bukukryo-gan-ka-Yokuinin (KBY) in ameliorating the symptoms of rosacea was investigated in a steroid-induced rosacea mouse model. The mice were fed a KBY-containing diet for 28 d, after application of clobetasol propionate for 10 d; thereafter, the velocity of capillary blood flow, area of the skin purpura, histopathologic features, and gut microbiota were analyzed. The KBY-fed mice showed remarkable recovery in the capillary blood flow velocity and purpura area, as well as restoration of the epidermis features. Furthermore, gut microbiome analysis showed the enhancement of Lactobacillus population in the KBY-fed mice. The KBY-induced improvement of circulatory disturbances in skin might contribute to the alleviation of telangiectasia and erythema. Lactobacillus may be involved in alleviating the rosacea-like symptoms in association with KBY; however, this association should be investigated further by using other analysis methods. Our results provide evidence of the effectiveness of KBY in improving the pathology of rosacea.
Rosacea encompasses a constellation of clinical pathologies, including persistent erythema, inflammatory papulopustular rash, and facial telangiectasias. Additionally, rosacea is considered to be an important risk factor for various diseases, and it has been suggested to be related with inflammatory bowel disease, cardiovascular disease, hyperlipidemia, Parkinson’s disease, rheumatoid arthritis, malignant tumor, autoimmune diseases and so on.1,2,3) The major pathomechanisms of rosacea are environmental triggers (e.g., heat and alcohol) and genetic predisposition, which play a major role in neurovascular dysregulation. Other pathogenic factors of rosacea include ultraviolet radiation, skin barrier dysfunction, demodicosis and infection of Staphylococcus. Moreover, the improper and excessive use of topical corticosteroid creams on the face causes rosacea-like symptoms (steroid-induced rosacea).4) Recently, some studies have reported the importance of oxidative stress in the pathophysiology of rosacea about vascular lesions, inflammation, and oxidative tissue damage.5,6,7)
The Rosacea Medical Management Guidelines of the American Acne and Rosacea Society8) recommend certain topical and oral therapies for papules and pustules. Currently, standard medical and surgical therapies for rosacea include topical agents such as azelaic acid, metronidazole, and brimonidine tartrate; systemic doxycycline and isotretinoin; and vascular laser therapy;9,10) however, the therapeutic effect of the current treatment is rather limited, only palliative for a short span, and cannot prevent recurrence in many cases. In particular, telangiectasia and flushing, the clinical symptoms of rosacea, are only treated with the use of light devices, with weak trial evidence. Similarly, according to the guidelines of the Japanese Dermatological Association, many options for the treatment of acne vulgaris are ranked A (“highly recommended”), while most of the options for treating rosacea are ranked as only either C1 (“conditionally recommended”) or C2 (“not recommended”).11) Therefore, as mentioned above, the treatment recourse for the clinical symptoms of rosacea has not yet been sufficiently established.
However, some pharmaceutical-grade traditional Japanese medicines (Kampo) show antioxidative and antibacterial effects, especially the improvement of blood flow, and it has been reported that treatment with a traditional Kampo medicine has alleviated rosacea symptoms in many cases.12) In Kampo medicine, pathological changes in the capillaries of rosacea patients are considered the “Oketsu” state.13) Oketsu is similar in concept to blood stasis, but it is the characteristic pathophysiology in traditional Japanese medicine that describes a circulatory disturbance with vascular resistance and blood fluidity.
The Keishi-Bukuryo-gan-ka-Yokuinin (KBY) is one of the pharmaceutical-grade traditional Kampo, generally prescribed for Oketsu and has been widely used for the treatment of menstrual irregularity, automatic imbalance syndrome peculiar to women that resembles climacteric disturbance, acne, spots, and roughness of the hands and feet of patients with a comparatively strong constitution who sometimes complain of lower abdominal pain, shoulder stiffness, dull headache, dizziness, cold feet, and hot flush. The KBY is constituted by Yokuinin (coix seed) and another Kampo-medicine, Keishi-Bukuryo-Gan, which contains the following five crude drugs: Cinnamon Bark, Paeony Root, Peach Kernel, Poria sclerotium and Moutain Bark. Keishi-Bukuryo-gan effectively alleviates the symptoms of rosacea, possibly because it improves the peripheral blood flow as an anti-Oketsu drug.13,14,15,16,17) In addition, Yokuinin contains azelaic acid, which is used as a therapeutic agent for rosacea.18) Moreover, the gut microbiota and traditional Kampo are considered to interact significantly, resulting in therapeutic efficacy.19) Recently, rosacea patients have shown a high prevalence rate of small intestinal bacterial overgrowth (SIBO).20) The correlation between rosacea skin symptoms and gut microbiota is a noteworthy research target. Therefore, we hypothesized that the oral Kampo medicine therapy, especially KBY, may ameliorate rosacea with multifaceted effects; however, there is no clear evidence of such an effect.
In this study, we observed changes in the skin blood flow and purpura upon oral administration of KBY in a mouse model with rosacea-like symptoms. Moreover, the histopathological features and intestinal flora of mice pre- and post-KBY administration were investigated.
Male hairless mice (Hos:HR-1, 4-week-old) were purchased from Hoshino Laboratory (Bandou, Ibaraki, Japan). They were housed under pathogen-free conditions at a temperature of 25 ± 2°C, relative humidity of 55% ± 20%, and a 12 h light: 12 h dark cycle, with lights on from 07:00 to 19:00 h daily. The mice had free access to food and water. All protocols in this study were approved by the Animal Care and Use Committee at Hyogo Medical University (permit number: 21-004) and were in compliance with the Animal Experiment Regulations of Hyogo Medical University.
Experimental DietIn this study, the powdered extract of KBY (Tsumura & Co., Tokyo, Japan) was manufactured in compliance with good manufacturing practice requirements. KBY (lot.2190125010, 2210125010) used in our study was made using the following 6 kinds of crude drugs that were mixed in the proportions indicated in parentheses: Cinnamon Bark (4.0 g, The bark of Cinnamomum cassia Blume), Peony Root (4.0 g, The roots of Paeonia lactiflora Pallas), Peach Kernel (4.0 g, The seeds of Prunus persica Batsch or Prunus persica Batsch var. davidiana Maximowicz), Poria sclerotium (4.0 g, sclerotium of Wolfiporia cocos Ryvarden et Gilbertson), Moutan Bark (3 g, The bark of Paeonia suffruticosa Andrews (Paeonia moutan Sims), and Coix Seed (10.0 g, The seeds of Coix lacryma-jobi Linné var. mayuen Stapf). The mixture was then extracted using hot water and dried using the spray drying method. The quality of KBY was guaranteed by measuring following ingredients (E)-cinnamic acid and paeoniflorin. The representative chemical profiling in the extract of KBY via LC/MS/MS and GC/MS systems was previously reported.18) The KBY extract was included in the standard diet (MF diet; Oriental Yeast Co., Tokyo, Japan) at a concentration of 2.5%.
Experimental DesignThe experimental timeline of the study is presented in Fig. 1. After seven days of pre-breeding, clobetasol propionate ointment (0.05% clobetasol propionate ester, Sato Pharmacological Co., Tokyo, Japan) was applied to the dorsal side of the skin of each mouse, daily at 2 g for 10 d (days 0–9). Mice were fed a KBY-containing diet for 28 d (days 9–37), and the blood flow and purpura were observed during day 0–37. No-KBY mice were treated with clobetasol propionate for 10 d, but fed with KBY-containing diet. The control group is fed standard MF diet without clobetasol propionate application.

Experimental Timeline
Experimental timeline of the study. Clobetasol propionate ointment was applied from day 0–9. Mice were fed a KBY-containing diet during days 9–37. The capillary blood flow and skin purpura were observed during days 0–37.
Video images of mouse skin were recorded with a capillary flow scope (TOKU Capillaro; Toku Co., Tokyo, Japan), and video analysis was performed using the capillary analysis software, Capimetrics (Toku Co.). The area of the purpura was measured using the Image J software. The images of the mouse’s skin were converted to grayscale (16-bit) and processed with Gaussian Blur. The purpuras were detected in the images by automatic selection of the threshold differentiating black and white, and the area of the black portion was automatically measured.
As of October 2022, only topical Metronidazole gel is officially approved for the treatment of rosacea under the Japanese health insurance system. Metronidazole is effective in treating the inflammatory papules of rosacea but not in telangiectasia. Therefore, there are no drugs which should be compared with KBY and we observed only the effective of Kampo medicine in this study.
Analysis of Gut MicrobiotaThe fecal samples were collected on day 0 and 37. Gut microbiota analysis was performed using the Nagashima method.21) DNA isolation from the fecal samples was performed using the MORA Extraction Kit (Kyokuto Pharmaceutical Industrial Co., Tokyo, Japan). PCR was performed with a model 2720 thermal cycler (Applied Biosystems, Waltham, USA) using the Tks Gflex™ DNA polymerase reaction mixture (Takara Bio, Tokyo, Japan), forward (516f) and reverse (1510r) primers, each at a concentration of 0.2 μM, and 20 ng of the fecal DNA. The primers 5’ FAM-labeled 516f (5’-TGCCAGCAGCCGCGGTA-3’) and non-labeled 1510r (5’-GGTTACCTTGTTACGACTT-3’) were purchased from Thermo Fisher Scientific (Massachusetts, USA). The amplification program was set as follows: preheating at 94°C for 60 s; 40 cycles of denaturation at 98°C for 10 s, annealing at 55°C for 15 s, and extension at 68°C for 30 s.
Histopathological Evaluation of the Dorsal SkinDorsal skin samples of the mice were excised on day 9 and day 37. The mice were anesthetized with isoflurane (2%–3%). Skin samples were immediately fixed in 4% formaldehyde at 4°C. For histological observation, the paraffin-embedded skin was cut into 2 μm cross-sections and stained with hematoxylin and eosin (HE).
Statistical AnalysisAll values are expressed as the mean ± standard error of the mean (SEM). Statistical analysis was performed using the t-test, analysis of variance (ANOVA) followed by Tukey’s post-hoc test, or Wilcoxon signed-rank test using Free JSTAT 22.0E. Differences were considered statistically significant at p < 0.05.
The daily food intake was 5.760 ± 0.160 g/mouse in the standard diet group and 6.041 ± 0.170 g/mouse in the KBY-diet group. There was no significant difference in the daily food intake (t = 1.777, p = 0.246, Fig. 2a). The average body weight on day 37 was 34.078 ± 0.520 g in the control group, 32.086 ± 0.577 g in the no-KBY group, and 32.059 ± 0.470 g in the KBY group, with no significant differences (Fig. 2b).
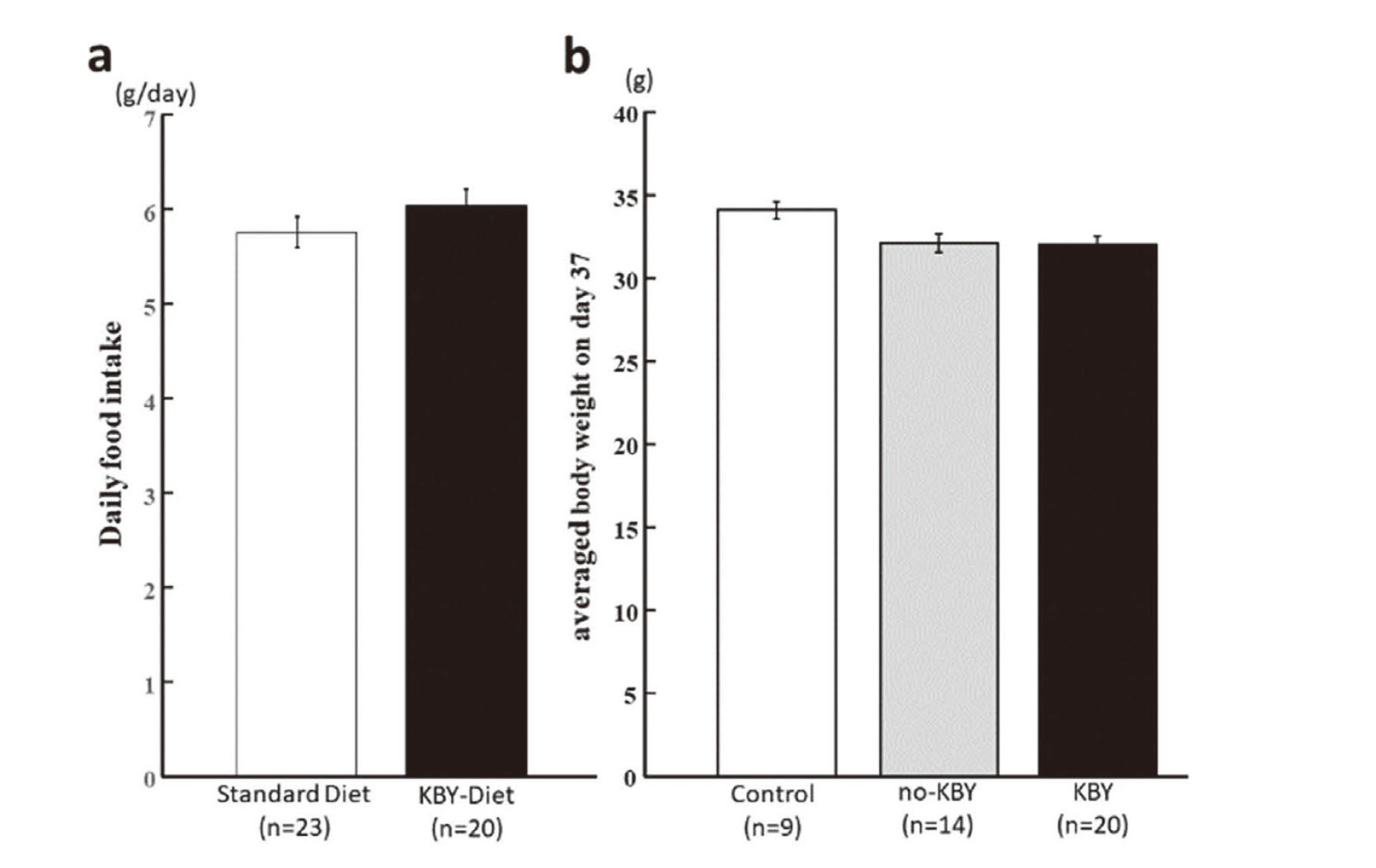
Daily Food Intake and Body Weight
Daily intake of standard and KBY-containing diet of mice (a) and average body weight on day 37 in each experimental group (b). Data represent the mean ± SEM.
The chronological changes in the average blood flow velocity within the skin capillaries in each group are shown in Fig. 3. Two-way ANOVA followed by Tukey’s post-hoc test detected significant differences between the no-KBY and other groups (F(2, 220) = 7.668, p < 0.001), while no significant difference was determined between the KBY and control group. At the individual measurement points, one-way ANOVA showed that the average blood velocity of the no-KBY group was significantly lower than that of the control group or both control and KBY groups on days 7, 23, 30, 35, and 37. The calculated average blood flow velocities during the experimental period (days 0–37) were 887.995 mm/s in the no-KBY, 1049.613 mm/s in the KBY, and 1113.847 mm/s in the control group.

Averaged Blood Flow Velocities in Skin
Chronological changes in the average blood flow velocity in skin capillaries in each group. The blood flow velocity of the no-KBY group (n = 9) was significantly lower than that observed in the KBY-fed (n = 9) and control groups (n = 5). At individual measurement points, the average blood flow velocity of the no-KBY group was significantly lower than that of the control group or both control and KBY groups on days 7, 23, 30, 35, and 37. Data represent the means ± SEM. **, ## p < 0.01, *, # p < 0.05.
The chronological changes in the average area of the skin purpura within the observation field in each group are shown in Fig. 4. Two-way ANOVA followed by Tukey’s post hoc test revealed significant differences among all experimental groups (F(2, 220) = 79.223, p < 0.0001), the skin purpura that occurred in the no-KBY group improved significantly in the KBY group, although not as much as in the control group.

Averaged Areas of Purpura in Skin
Chronological changes in the average area of the skin purpura in each experimental group. Average area of skin purpuras in the no-KBY group (n = 9) was significantly greater than that in the KBY (n = 9) and control groups (n = 5). After day 14, the mean purpura area in the no-KBY group was significantly greater than that in the other groups. Data represent the means ± SEM. **, ## p < 0.01. *, # p < 0.05.
The average area of skin purpura during the experimental period in the no-KBY group (0.225 mm2) was significantly greater than that in the KBY (0.199 mm2) and control groups (0.170 mm2). Moreover, the area of skin purpura in the KBY group was significantly greater than that in the control group.
At individual measurement points, one-way ANOVA showed that the purpura area was significantly larger in the no-KBY group than that in the control group after day 14. Likewise, the purpura area in the no-KBY group was significantly greater than that in the KBY group on days 23, 30, 35, and 37. Only on day 37, the purpura area in the KBY group was significantly larger than that of the control group. The typical purpuras of the skin in the KBY and no-KBY groups are shown in Fig. 5.

Images of Typical Skin Purpuras
The typical skin purpuras (arrows) in the KBY and no-KBY groups. The images were adjusted by the automatic selection of the threshold differentiating black and white, and the area of the purpura was automatically measured. Bar = 200 μm.
Typical histological images of the dorsal skin of mice are presented in Fig. 6. On day 9, both KBY-fed and non-KBY-fed mice showed horny layer peeling and thinning of the granular layer to almost the same degree (upper panels, arrows) following corticosteroid ointment application. On day 37, the histopathological characteristics of the dorsal skin of the non-KBY-fed mice still showed horny layer peeling, thinning of the granular layer, and insufficient recovery. However, the KBY-fed mice showed an almost normal epidermis and granular layer (lower panels, arrows) on day 37, and its histological images are similar to those of control mice on day 37.

Histological Images of the Dorsal Skin
Images obtained following HE staining of the typical skin samples from the KBY and no-KBY groups. On day 9, both KBY- and no-KBY groups showed horny layer peeling and thinning of the granular layer (upper panels, arrows). On day 37, sufficient recovery of skin abnormalities was not observed in the no-KBY group, whereas the KBY group showed an almost normal epidermis and granular layer (lower panels, arrows). Bar = 100 μm.
Upon comparing the intestinal flora on days 0 and 37 in each experimental group, a significant difference was detected only in the KBY group, using the Wilcoxon signed-rank test (U = 113.0, p = 0.034, one-tail, data not shown). Therefore, we compared the relative peak areas of each Operational Taxonomic Unit (OTU) and found that the peak-area ratio of OTU657 (est. Lactobacillus)21) showed different tendencies between the experimental groups (Fig. 7).
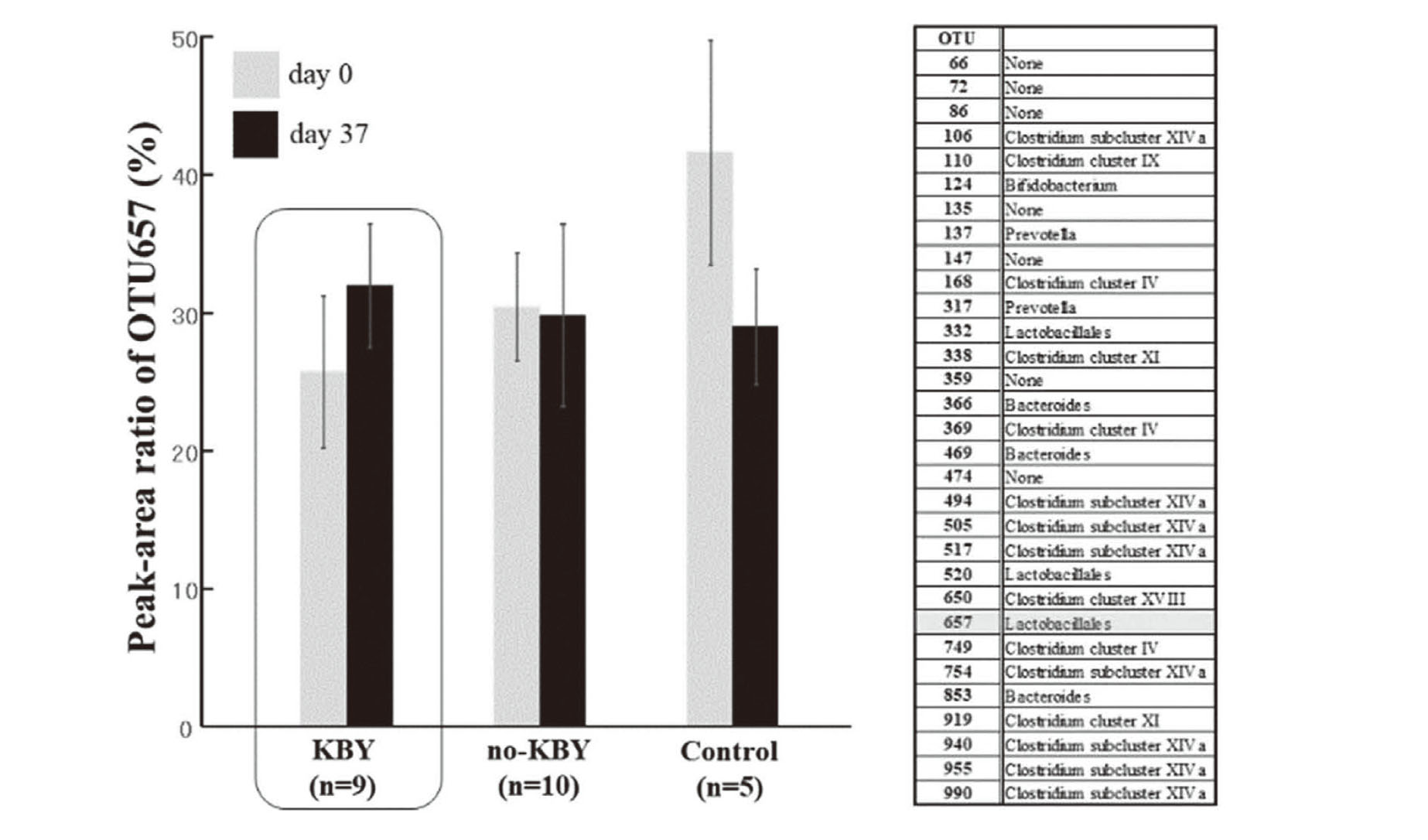
Averaged Peak-Area Ration of OTU657 (est. Lactobacillus)
Averaged peak-area ratio of OTU657 in each experimental group. Only in the KBY group, the peak area of OTU657 on day 37 was greater than that on day 0 (boxed area). OTU657 is estimated lactobacillus (Right Column).21)
In the KBY group, the area ratio of OTU657 on day 37 (31.935% ± 4.464%) was higher than that on day 0 (25.682% ± 5.491%). In the other group, the area ratio of OTU657 was comparable on days 0 and 37 (no-KBY group) or decreased on day 37 (control group). However, no significant differences were detected in the peak-area ratio of OTU657.
Our Rosacea model mouse is made by application of topical corticosteroid ointment on the dorsal skin the model of steroid induced Rosacea. Rosacea-like dermatitis manifests erythema, papules, pustules, and facial telangiectasias like rosacea. It is usually caused by long-term use of topical corticosteroid application on the face. In this study, we investigated the efficacy of KBY, a traditional Japanese medicine, in glucocorticoid-induced rosacea-like mouse model by assessing the capillary circulatory flow, purpura area, histopathological features of the dorsal skin, and gut microbiota.
Histopathologically, KBY had a significant recovery effect on the skin manifestations caused by the application of clobetasol propionate ointment.
In summary, the following therapeutic effects manifested upon oral KBY administration:
(a) Improvement of the capillary blood flow velocity
(b) Reduction of skin purpura
(c) Restoration of epidermal thinning
(d) Relative population of Lactobacillus was not decrease
Although the pathomechanism in the clobetasol propionate ester-induced rosacea-like mouse model has not been fully investigated in this study, the adverse effects of glucocorticoids may be involved in blood coagulation by activating the plasminogen activator inhibitor-1.22,23) Previous reports have supported a possible mechanism of alleviating the disturbances in microcirculation by KBY. Keishi-Bukuryo-gan, a component of KBY, exhibited platelet aggregation-inhibiting activity, mainly contributed by moutan bark, cinnamon bark, and peach kernel.24) The chemical constituents of KBY absorbed into the blood, such as paeonol, paeoniflorin, and (+)-catechin, improve the capillary blood flow by inhibiting platelet aggregation and/or blood coagulation.24,25) Therefore, improvement in the skin blood flow following KBY supplementation is extremely conclusive and is an associated therapeutic effect of KBY.
We also observed extensive purpura in the clobetasol propionate ester-induced rosacea-like mouse model, which demonstrated the vulnerability of skin capillaries to topical glucocorticoids. Interestingly, KBY effectively attenuated the purpura area in mice, as shown in Figs. 4 and 5. Although further research is needed to uncover the action mechanisms of KBY, previous reports have suggested possible amelioration of the skin capillary damage as an effect of KBY supplementation. Increased production of reactive oxygen species (ROS) by innate immune cells, such as neutrophils, in the skin of rosacea patients is involved in the disease pathogenesis. Thus, suppression of ROS production by topical drugs (azelaic acid, metronidazole) is considered effective in rosacea.26,27) A previous study reported that KBY significantly attenuated systemic oxidative stress in rats, and its chemical constituents, including gallic acid, 3-O-methyl gallic acid, (+)-catechin, and azelaic acid, are absorbed into the blood circulation.18) Furthermore, excessive activation of the stratum corneum tryptic enzyme kallikrein 5 in rosacea patients results in an increased production of LL-37 (cathelicidin, antimicrobial peptide), which exhibits not only antimicrobial activity at the epidermis but also induces neutrophil infiltration, leading to ROS generation. The enzymatic activity of kallikrein 5 was inhibited in a concentration-dependent manner by pachymic acid and tumulosic acid, which appeared in the rat plasma after oral administration of KBY.18)
Based on these previous studies, KBY may be more effective in treating the symptoms of rosacea by multiple mechanisms than the existing oral drugs. It may improve skin microcirculation, reduce oxidative stress, and inhibit kallikrein 5 activity through the action of its chemical constituents absorbed into the blood.
Skin atrophy is the most common adverse side effect of long-term use of steroids and can cause steroid-induced rosacea. In our present results, KBY-fed mice showed remarkable recovery of the epidermis and granular layer. These results provide considerable evidence of KBY’s efficacy against steroid-induced rosacea.
Analysis of the gut microbiota following KBY administration showed that the proportion of Lactobacillales (OTU 657) 21) was enhanced in the KBY-fed mice. A recent study has reported that the kampo “Daikenchuto” induces an increase in Lactobacillus abundance.28) However, the relationship between KBY and Lactobacillus is still unknown; therefore, the association of Lactobacillus with the therapeutic effects of KBY cannot be concluded. In contrast, several studies have suggested that lactic acid bacteria contribute to maintaining skin health29,30,31,32) and the intestinal microbiota is significantly involved in the medicinal efficacy of the Kampo medicines by metabolizing their constituents.19) Therefore, Lactobacillus may be involved in alleviating the rosacea-like symptoms in association with KBY; however, this association should be investigated further by using other analysis methods.
There are some limitations of this study. Firstly we made rosacea model mice, not human body model, so the accuracy of the model cannot be established. Secondly we did not obtain physiological measures, thus we cannot asses the activity of the medicine in the body. However, our present study provides evidence of the efficacy of KBY against skin diseases associated with blood circulatory disturbances including rosacea, and our findings suggest that the anti-oketsu drugs may contribute beneficially toward improving overall health.
We thank Mr. Yosuke Matsubara of Tsumura & Co. Ltd. for critically assessing the manuscript. This research was funded by Tsumura & Co. Ltd.
Conflict of interestThis research was supported by funding from Tsumura & Co. Ltd. Akinobu Gotoh received a research grant and a provision of drugs from Tsumura & Co, Ltd.