Abstract
Nanoparticles are used in many everyday products because of their innovative properties, but there are concerns that, being smaller than conventional materials, they have the potential to induce unexpected biological effects. Despite the need for the safety of nanoparticles to be assessed, action in this area is still lagging. Several studies, including those by us, have revealed that nanoparticles can reach the placenta and show placental toxicity, but it remains unclear how nanoparticles affect placental function. Here, we attempted to assess the effect of nanoparticles on placental hormone-producing function by using an in vitro forskolin-induced BeWo syncytialization model. BeWo cells were treated with amorphous silica nanoparticles with a diameter of 10 nm (nSP10) either during syncytialization or after being syncytialized by forskolin treatment. RT-PCR analysis showed that nSP10 inhibited forskolin-induced upregulation in the expression level of human chorionic gonadotropin β (hCGβ; gene name, CGB) during syncytialization, but not in syncytialized BeWo cells. Moreover, nSP10 downregulated forskolin-induced elevation in the expression of endogenous retrovirus group FRD member 1 (ERVFRD-1) and syndecan-1 (SDC1), which are involved in cell fusion during syncytialization. These results suggest that nSP10 could suppress trophoblast cell fusion, thus inhibiting the production of hCG in syncytialized BeWo cells.
INTRODUCTION
Because the placenta facilitates delivery of oxygen and nutrients and removal of waste products between mother and fetus, impairment of the placental structure and the resulting dysfunction leads to restricted fetal growth.1) Therefore, normal placental maintenance and development is essential for sustaining the growth of the fetus. The outermost layer of the villi that make up the placenta is covered with syncytiotrophoblast cells, which are formed by the differentiation/fusion (syncytialization) of cytotrophoblast cells localized inside the villi.2) This layer of syncytiotrophoblasts not only functions as a blood–placental barrier but also produces hormones, such as human chorionic gonadotropin (hCG), that perform multiple functions in the development of the placenta and fetus.3) Because hCG is a pregnancy-specific hormone produced by the syncytiotrophoblasts, its concentration is a marker for the subsequent clinical manifestation of preeclampsia.4) Therefore, the effects of environmental factors on syncytiotrophoblast cells and syncytiotrophoblast formation could impact hormone production in the placenta and subsequent growth of the fetus.
In recent years, the development and use of nanoparticles have been expanding.5) A characteristic function of nanoparticles is their greater specific surface area than that provided by conventional particles, and nanoparticles have come to be utilized in daily necessities and pharmaceuticals. For example, silica nanoparticles are frequently and repeatedly used as components of daily products such as cosmetics and foods.6) Thus, the risk of exposure to nanoparticles occurs regardless of age or gender.7) On the other hand, the collection of hazard information, which is essential for understanding the risk of nanoparticles, is inadequate, and particularly the lack of information worldwide on pregnant mothers has been noted.8) In this regard, we previously showed that the difference from conventional materials in the physical properties of silica nanoparticles induces collapse of the placental structure by damaging the placental trophoblast layer and retarding intrauterine growth.9),10) Therefore, we assessed the effect of silica nanoparticles on placental function in terms of hormone production in syncytiotrophoblast cells and during syncytiotrophoblast formation.
MATERIALS AND METHODS
Cell Line and Cell Culture
The human choriocarcinoma cell line BeWo was purchased from the Japanese Collection of Research Bioresources Cell Bank (JCRB9111; Osaka, Japan). BeWo cells were cultured in 10% inactivated fetal bovine serum (Biosera, Nuaille, France) and 1% (v/v) penicillin–streptomycin–amphotericin B suspension (Wako, Osaka, Japan) in Ham’s F-12 nutrient mixture (Wako). BeWo cells were maintained under standard cell-culture conditions at 37°C and > 95% humidity in a 5% CO2 atmosphere.
Reagents
An aqueous suspension of silica nanoparticles with a diameter of 10 nm (nSP10) was purchased from Micromod Partikeltechnologie (Rostock, Warnemünde, Germany). Before use in experiments, nSP10 was sonicated for 5 min at 400 W (Ultrasonic Cleaner, AS ONE, Osaka, Japan) and mixed for 1 min with a benchtop vortex mixer (Digital VORTEX-GENIE 2, Scientific Industries Inc., New York, USA). Forskolin and H-89 were purchased from Cayman Chemical (Ann Arbor, MI, USA). Forskolin was diluted with Ham’s F-12 to a final concentration of 50 µM, and H-89 was diluted with Ham’s F-12 to a final concentration of 10 µM.
Cell Viability Assay
BeWo cells were seeded at 1.0 × 104 cells/200 µL per well into a 96-well, flat, clear plate. To assess the effect of nSP10 on syncytialized trophoblasts, BeWo cells were pre-treated with 50 µM forskolin for 24 h. After removing forskolin, BeWo were treated with nSP10 (12.5, 25, 50, 100, or 200 μg/mL) for 72 h. Also, to assess the effect of nSP10 on trophoblasts during the syncytialization process, BeWo cells were treated with 50 µM forskolin and nSP10 (3.12, 12.5, 50, 100, 200, or 400 µg/mL) for 48 h. After treatment, cell viability was measured with the colorimetric dye MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] (Tokyo Chemical Industry, Tokyo, Japan) in accordance with the manufacturer’s instructions. Viability was determined with respect to the control (i.e. cells untreated with nSP10).
RT-PCR Analysis
BeWo cells were seeded at 1.0 × 105 cells/1 mL per well in 12-well, flat plates, pre-treated with 50 µM forskolin for 24 h, and then treated with nSP10 (12.5, 25, or 50 μg/mL) for 72 h. To assess the effect of co-treatment of nSP10 on the syncytialization process, BeWo cells were seeded at 1.5 × 105 cells/2 mL per well in 6-well, flat plates and treated with 50 µM forskolin and nSP10 (3.12, 12.5, or 50 µg/mL) for 48 h. H-89 was used as a positive control to suppress syncytialization. Total RNA was extracted by using a FastGene RNA Kit (Nippon Genetics, Tokyo, Japan) and reverse-transcribed into cDNA by using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA). A PCR mixture was prepared containing the above cDNA as a template and primers for the genes, CGB (forward, 5'-GCTCACCCCAGCATCCTATC-3' and reverse, 5'-CCTGGAACATCTCCATCCTTG-3'), endogenous retrovirus group FRD member 1 (ERVFRD-1) (forward, 5'-GCAGCACAGGGAGGAATTTG-3' and reverse, 5'-TGGCTCGTTCCCGTAAACTG-3'), syndecan-1 (SDC1) (forward, 5'-AGCACTTACTGGTAGGACCAAGC-3' and reverse, 5'-TTCTTAACCCTGATGCTGTCTCC-3'), and actin (forward, 5'-GCCCTGAGGCACTCTTCCA-3' and reverse, 5'-CGGATGTCCACGTCACACTTC-3') (Eurofins Genomics, Tokyo, Japan), as well as GeneAce SYBR qPCR Mix α Low ROX (Nippon Gene, Tokyo, Japan). RT-PCR was performed by using a CFX-384 Real-Time PCR Detection System (BioRad Laboratories, Hercules, CA, USA). The expression level of each gene was normalized to that of β-actin.
Statistical Analysis
Statistical analyses were conducted by using GraphPad Prism (version 9.3.1). Data are expressed as means ± S.D. For the results in Fig. 1, statistical analyses were performed using Dunnett’s method. For the results in Fig. 2 and Fig. 3, statistical analyses were performed using Tukey’s method. P-values lower than 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
nSP10 Suppressed CGB Expression in BeWo Cells During Syncytialization
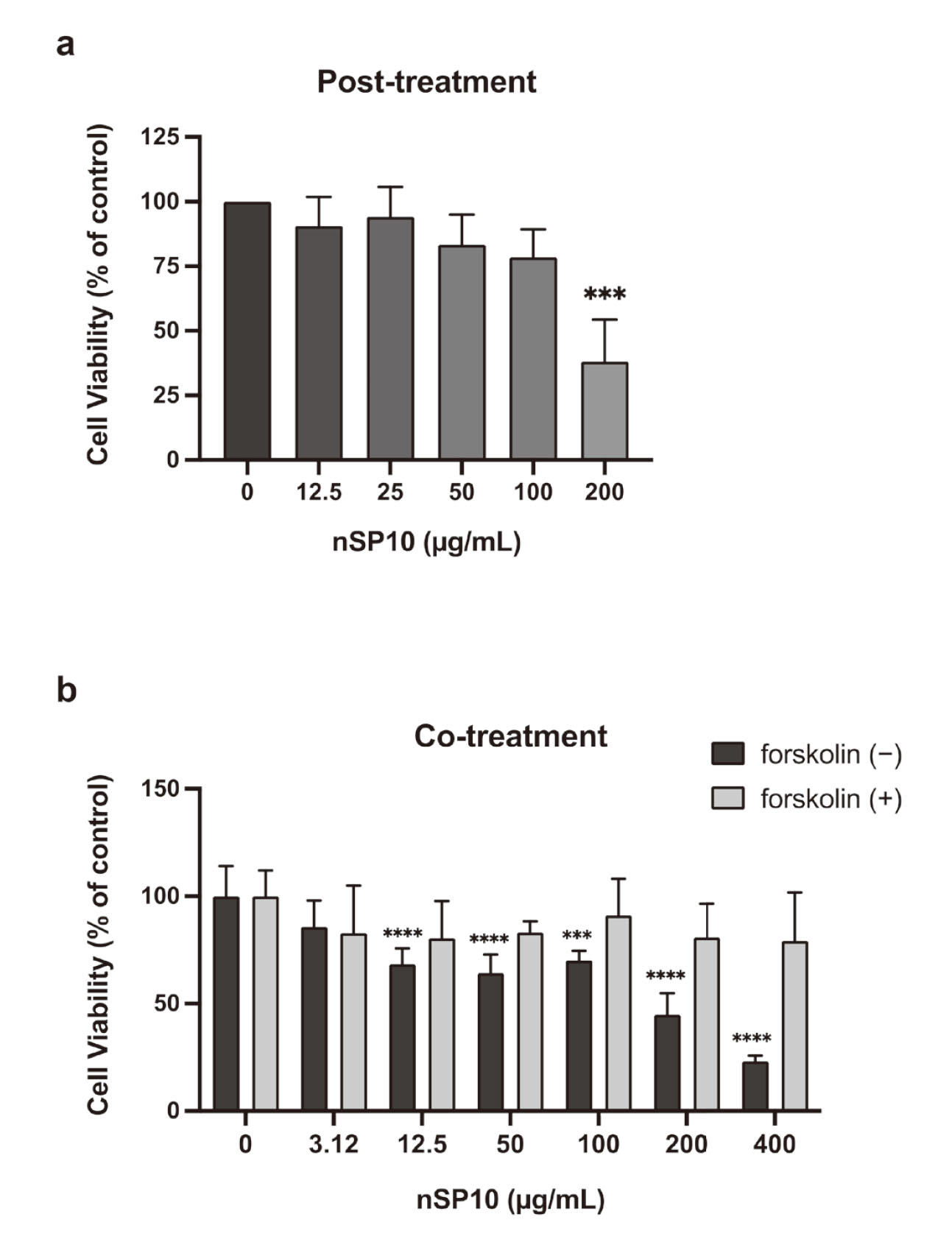
hCGβ, governed by the CGB gene, is a hormone mainly synthesized by syncytiotrophoblast cells in the placenta and plays a critical role in stimulating the production of progesterone, which is necessary for maintaining normal pregnancy in the early stage.11) For the in vitro model, we used BeWo trophoblasts cells, which are commonly used to study the dynamics of cytotrophoblast differentiation into syncytiotrophoblasts12) and as a cell model for hCGβ production by trophoblast cells.13) The cell viability assay showed that, at a dose of 50 µg/mL, nSP10 was not toxic to pre-syncytialized BeWo cells (Fig. 1a). In contrast, nSP10 co-treated with forskolin was not toxic to BeWo cells during the syncytialization process at this condition (Fig. 1b). We then analyzed the expression of CGB mRNA. RT-PCR analysis showed that there was no significant change in CGB expression level at each dose of nSP10 in pre-syncytialized BeWo cells (Fig. 2a). However, co-treatment with forskolin and nSP10 (50 µg/mL) significantly decreased the forskolin-induced upregulation in CGB expression level (Fig. 2b). At this time, the expression of CGB was significantly decreased by H-89 (a protein kinase A inhibitor used as a positive control for inhibition of the syncytialization process).14) These results suggest that nSP10 has the potential to inhibit placental hormone production during syncytiotrophoblast formation.
One of the reasons for the decrease in CGB expression during co-treatment may be a difference in the uptake pathway. A previous study reported that trophoblasts actively uptake amino acids through activation of macropinocytosis as syncytialization progresses.15) Activation of this mechanism may make trophoblasts sensitive to foreign substances during syncytialization. In addition, it has been shown that some nanoparticles are taken up into cells by macropinocytosis.16) Indeed, in this study nSP10 induced strong cytotoxicity against BeWo cells that were undergoing syncytialization compared with previously syncytialized BeWo cells. Therefore, we are currently using endocytosis inhibitors to analyze the effect of differential nSP10 uptake into BeWo cells.
Since other placental hormones are produced by syncytiotrophoblasts along with hCGβ, nSP10 may affect the production of these hormones during syncytialization. For instance, fms-like tyrosine kinase 1 promotes placental angiogenesis17) and human placental lactogen is involved in maternal glucose metabolism.18) It is necessary in the future to use these hormones as an index to evaluate the effects of nSP10 on syncytiotrophoblasts.
nSP10 Suppresses Forskolin-Induced BeWo Syncytialization
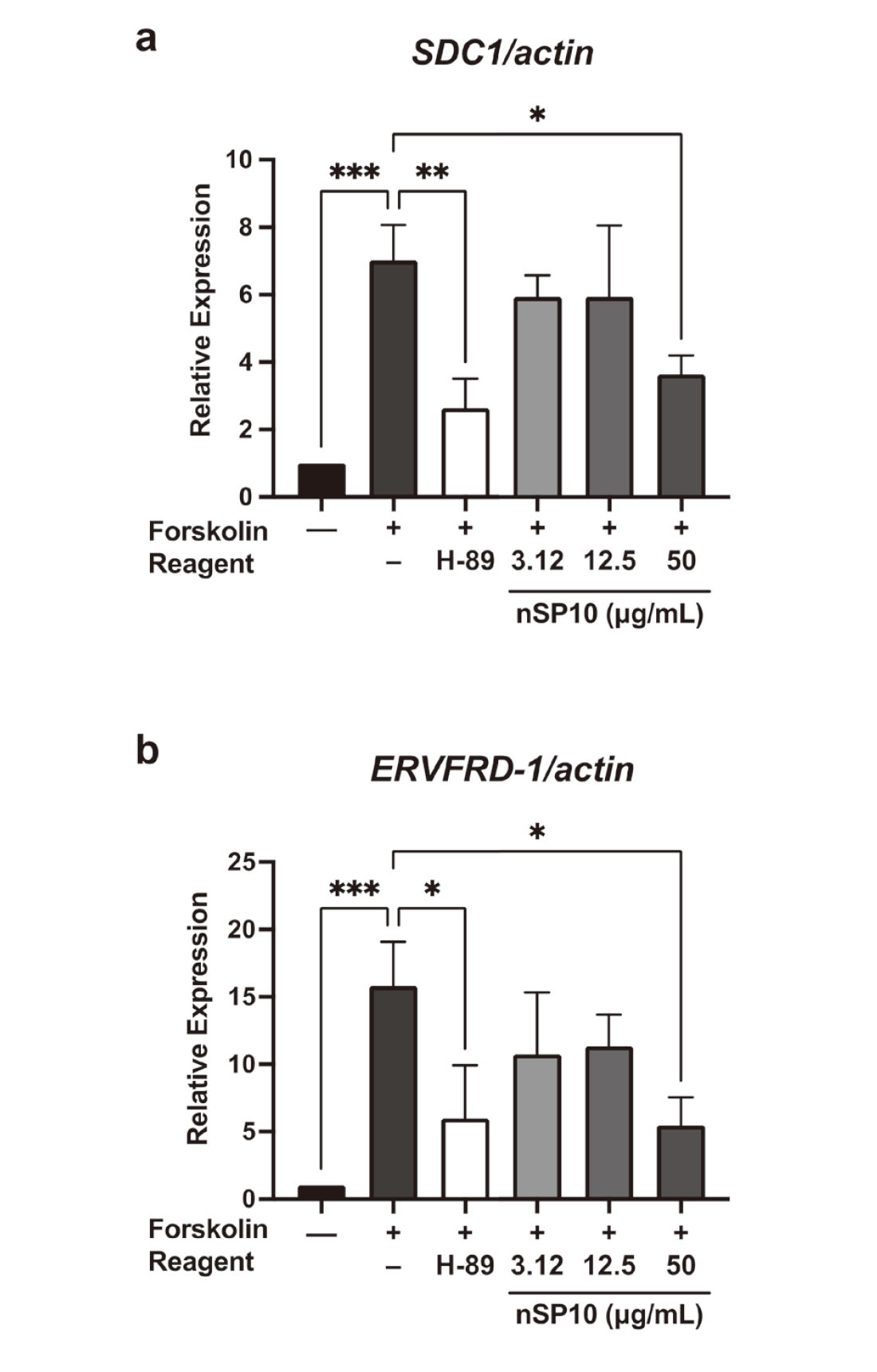
Given that many of the functions in the placenta are maintained mainly by syncytiotrophoblasts, the process of syncytiotrophoblast formation, in which cytotrophoblast cells fuse and differentiate into multinucleated syncytiotrophoblast cells, is important throughout pregnancy.19,20) Thus, to examine the effect of nSP10 on forskolin-induced syncytialization in BeWo cells, we evaluated the mRNA expression of ERVFRD-1 (which encodes syncytin-2) and SDC1 (which encodes syndecan-1), because both genes play important roles in cell fusion and signaling during the trophoblast syncytialization process.21) Actually, we confirmed that the forskolin-induced elevation level of both genes was significantly decreased by H-89. RT-PCR analysis showed that co-treatment with nSP10 (50 µg/mL) and forskolin resulted in a significant decreased SDC1 (Fig. 3a) and ERVFRD-1 mRNA levels (Fig. 3b). These results suggest that nSP10 decreased the expression of ERVFRD-1 and SDC1 during trophoblast formation, thus suppressing the production of hCGβ in BeWo cells during syncytialization.
Syncytin-2 is highly expressed in mononuclear trophoblast cells and plays important roles in each step of the mononuclear trophoblast cell fusion process.22) Thus, syncytin-2 is essential for placental and fetal development in early pregnancy. Aberrant expression of the mediating gene adversely affects the pregnancy outcome, as evidenced by embryonic lethality of all fetuses in syncytin-A knockout mice.23) Our result suggests that nSP10 could suppress the mononuclear fusion process via downregulation of ERVFRD-1 expression. Inadequate syncytialization can also affect pregnancy outcomes; therefore, we are currently other studies to examine the effects of nSP10 on pregnant mice.
Syndecan-1 is a transmembrane proteoglycan with increased expression during syncytialization and is responsible for cell signaling through binding to growth factors such as transforming growth factor-β and through interaction with the extracellular matrix.24) Therefore, alterations in SDC1 expression are known to lead to abnormal placental function, and indeed decreased levels of syndecan-1 in the serum of preeclampsia patients have been reported.25) Previous studies in the BeWo model have also shown that hCGβ production is reduced when syndecan-1 is silenced by siRNA.26) From the above, nSP10 may induce decreased placental function by inhibiting hCGβ production via downregulation of SDC1.
Collectively, we revealed that nSP10 could suppress trophoblast cell fusion, thus inhibiting the production of hCG in syncytialized BeWo cells. Our current findings suggest that nSP10 during syncytialization process may induce a decline in placental function. Therefore, further studies are needed to understand the extent to which nSP10-induced hCGβ reduction affects pregnancy outcome.
Acknowledgments
This study was supported by a Health Labor Sciences Research Grant from the Ministry of Health, Labour and Welfare of Japan (no. 21KD1002 to Y.T.); and by Support for Pioneering Research Initiated by the Next Generation from the Japan Science and Technology Agency (no. JPMJSP2138 to Y.S.). This research was partially supported by the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)) from the Japan Agency for Medical Research and Development under Grant Number JP21am0101084.
Conflict of interest
The authors declare no conflict of interest.
REFERENCES
- 1) Cindrova-Davies T, Sferruzzi-Perri AN. Human placental development and function. Semin. Cell Dev. Biol., 131, 66–77 (2022).
- 2) Cooke LDF, Tumbarello DA, Harvey NC, Sethi JK, Lewis RM, Cleal JK. Endocytosis in the placenta: an undervalued mediator of placental transfer. Placenta, 113, 67–73 (2021).
- 3) Blundell C, Tess ER, Schanzer AS, Coutifaris C, Su EJ, Parry S, Huh D. A microphysiological model of the human placental barrier. Lab Chip, 16, 3065–3073 (2016).
- 4) Barjaktarovic M, Korevaar TIM, Jaddoe VWV, de Rijke YB, Peeters RP, Steegers EAP. Human chorionic gonadotropin and risk of pre-eclampsia: prospective population-based cohort study. Ultrasound Obstet. Gynecol., 54, 477–483 (2019).
- 5) Grillo R, Rosa AH, Fraceto LF. Engineered nanoparticles and organic matter: a review of the state-of-the-art. Chemosphere, 119, 608–619 (2015).
- 6) Vance ME, Kuiken T, Vejerano EP, McGinnis SP, Hochella MF Jr, Rejeski D, Hull MS. Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol., 6, 1769–1780 (2015).
- 7) Mackevica A, Foss Hansen S. Release of nanomaterials from solid nanocomposites and consumer exposure assessment - a forward-looking review. Nanotoxicology, 10, 641–653 (2016).
- 8) Higashisaka K. Health Effects and Safety Assurance of Nanoparticles in Vulnerable Generations. Biol. Pharm. Bull., 45, 806–812 (2022).
- 9) Yamashita K, Yoshioka Y, Higashisaka K, Mimura K, Morishita Y, Nozaki M, Yoshida T, Ogura T, Nabeshi H, Nagano K, Abe Y, Kamada H, Monobe Y, Imazawa T, Aoshima H, Shishido K, Kawai Y, Mayumi T, Tsunoda S, Itoh N, Yoshikawa T, Yanagihara I, Saito S, Tsutsumi Y. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat. Nanotechnol., 6, 321–328 (2011).
- 10) Higashisaka K, Nakashima A, Iwahara Y, Aoki A, Nakayama M, Yanagihara I, Lin Y, Nagano K, Tsunoda SI, Saito S, Yoshioka Y, Tsutsumi Y. Neutrophil depletion exacerbates pregnancy complications, including placental damage, induced by silica nanoparticles in mice. Front. Immunol., 9, 1850 (2018).
- 11) Matsukawa H, Ikezaki M, Nishioka K, Iwahashi N, Fujimoto M, Nishitsuji K, Ihara Y, Ino K. Calnexin Is Involved in Forskolin-Induced Syncytialization in Cytotrophoblast Model BeWo Cells. Biomolecules, 12, 1050 (2022).
- 12) Jaju Bhattad G, Jeyarajah MJ, McGill MG, Dumeaux V, Okae H, Arima T, Lajoie P, Bérubé NG, Renaud SJ. Histone deacetylase 1 and 2 drive differentiation and fusion of progenitor cells in human placental trophoblasts. Cell Death Dis., 11, 311 (2020).
- 13) Hu R, Jin H, Zhou S, Yang P, Li X. Proteomic analysis of hypoxia-induced responses in the syncytialization of human placental cell line BeWo. Placenta, 28, 399–407 (2007).
- 14) Orendi K, Gauster M, Moser G, Meiri H, Huppertz B. The choriocarcinoma cell line BeWo: syncytial fusion and expression of syncytium-specific proteins. Reproduction, 140, 759–766 (2010).
- 15) Shao X, Cao G, Chen D, Liu J, Yu B, Liu M, Li YX, Cao B, Sadovsky Y, Wang YL. Placental trophoblast syncytialization potentiates macropinocytosis via mTOR signaling to adapt to reduced amino acid supply. Proc. Natl. Acad. Sci. USA, 118, 118 (2021).
- 16) Li YX, Pang HB. Macropinocytosis as a cell entry route for peptide-functionalized and bystander nanoparticles. J. Control. Release, 329, 1222–1230 (2021).
- 17) Sasagawa T, Nagamatsu T, Morita K, Mimura N, Iriyama T, Fujii T, Shibuya M. HIF-2α, but not HIF-1α, mediates hypoxia-induced up-regulation of Flt-1 gene expression in placental trophoblasts. Sci. Rep., 8, 17375 (2018).
- 18) Corbacho AM, Martínez De La Escalera G, Clapp C. Roles of prolactin and related members of the prolactin/growth hormone/placental lactogen family in angiogenesis. J. Endocrinol., 173, 219–238 (2002).
- 19) Bastida-Ruiz D, Yart L, Wuillemin C, Ribaux P, Morris N, Epiney M, Martinez de Tejada B, Cohen M. The fine-tuning of endoplasmic reticulum stress response and autophagy activation during trophoblast syncytialization. Cell Death Dis., 10, 651 (2019).
- 20) Renaud SJ, Jeyarajah MJ. How trophoblasts fuse: an in-depth look into placental syncytiotrophoblast formation. Cell. Mol. Life Sci., 79, 433 (2022).
- 21) Walker OS, Ragos R, Wong MK, Adam M, Cheung A, Raha S. Reactive oxygen species from mitochondria impacts trophoblast fusion and the production of endocrine hormones by syncytiotrophoblasts. PLoS One, 15, e0229332 (2020).
- 22) Vargas A, Moreau J, Landry S, LeBellego F, Toufaily C, Rassart E, Lafond J, Barbeau B. Syncytin-2 plays an important role in the fusion of human trophoblast cells. J. Mol. Biol., 392, 301–318 (2009).
- 23) Dupressoir A, Vernochet C, Bawa O, Harper F, Pierron G, Opolon P, Heidmann T. Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proc. Natl. Acad. Sci. USA, 106, 12127–12132 (2009).
- 24) Jokimaa V, Inki P, Kujari H, Hirvonen O, Ekholm E, Anttila L. Expression of syndecan-1 in human placenta and decidua. Placenta, 19, 157–163 (1998).
- 25) Prakash GJ, Suman P, Gupta SK. Relevance of syndecan-1 in the trophoblastic BeWo cell syncytialization. Am. J. Reprod. Immunol., 66, 385–393 (2011).
- 26) Szabo S, Xu Y, Romero R, Fule T, Karaszi K, Bhatti G, Varkonyi T, Varkonyi I, Krenacs T, Dong Z, Tarca AL, Chaiworapongsa T, Hassan SS, Papp Z, Kovalszky I, Than NG. Changes of placental syndecan-1 expression in preeclampsia and HELLP syndrome. Virchows Arch., 463, 445–458 (2013).