2023 年 6 巻 1 号 p. 16-20
2023 年 6 巻 1 号 p. 16-20
Astrocytes obtained from the cerebral hemisphere were maintained in serum-free defined medium containing dibutyryl cyclic AMP (dbcAMP) for 4 h, at which time almost all astrocytes showed a process-bearing stellate shape. They were then exposed to methylmercury (MeHg) at 1–3 μM or solvent alone (control) for up to 24 h. Astrocytes showed a flat polygonal shape after MeHg exposure at 3 µM for 1 h or at 2 µM for 3 h, whereas the shape was not affected after exposure at less than 1 µM for 24 h. Mercury accumulation levels were similar after MeHg exposure at 3 µM for 1 h and at 2 µM for 3 h, while the level after exposure at 1 µM did not reach those levels. The shape of astrocytes exposed to MeHg at 2 µM returned to stellate from polygonal after at least 24 h, although it remained polygonal in astrocytes exposed to MeHg at 3 µM. The viable cell number was significantly lower than in the control culture only in the culture exposed to MeHg at 3 µM for 24 h. In addition, genistein, a tyrosine kinase inhibitor, inhibited the morphological changes (spreading) induced by MeHg at 2–3 µM. These results suggest that a dose-dependent difference is observed in shape changes and cell death caused by MeHg in astrocytes, and that the responses to MeHg correlate to its accumulation levels, especially for the first few hours. They also suggest that tyrosine phosphorylation may play an important role in MeHg-induced spreading in astrocytes.
It is well known that methylmercury (MeHg) passes through the blood-brain barrier and damages the central nervous system (CNS).1) However, the mechanism of neuronal cell degeneration induced by MeHg has not been well established, although apoptosis is observed in the neuronal cells of some brain regions in vivo and in vitro.2–4) Since the uptake of glutamate (a major excitatory transmitter), which can induce neuronal cell damage,5,6) into astrocytes is well known to be inhibited by MeHg,7,8) it has been thought that glutamate may be involved in MeHg neurotoxicity. It has been demonstrated that MK-801, a non-competitive antagonist of the N-methyl-D-aspartate (NMDA) receptor, suppresses MeHg-induced neuronal cell damage, particularly in the cerebral cortex in vivo9) and in vitro.10) Accordingly, astrocyte dysfunction could be a pivotal step in MeHg-induced neuronal cell damage.
The morphology of astrocytes is known to change from a flat polygonal shape to a process-bearing stellate shape with an elevation in cellular cAMP levels,11,12) and to a polygonal from a stellate shape with lysophosphatidic acid (LPA)13–15) and endothelins.16,17) In addition, LPA inhibits dibutyryl cyclic AMP (dbcAMP)-induced stellation and a loss of stress fiber in astrocytes.18) Since the glutamate uptake and expression level of its transporter (GLAST) are lower in endothelin-induced polygonal astrocytes than in dbcAMP-stimulated stellate astrocytes,17) astrocyte shape change should correlate with neuronal cell damage induced by glutamate. We recently found that MeHg at 3 μM caused the morphology of cerebral hemisphere astrocytes to change to a polygonal shape from a stellate shape after being maintained in a serum-free medium with 0.5 mM dbcAMP.15) These findings led us to speculate that the spreading of astrocytes caused by MeHg could enhance their neurotoxicity through disordered regulation of the extracellular concentration of glutamate, at least in the cerebrum. However, the time- and dose-dependent effects of MeHg on the morphology of astrocytes remain unclear, although it is reported that 1 µM of MeHg did not affect the shape in short term experiments.15)
In the present study, astrocytes prepared from the cerebral hemisphere maintained in a serum-free medium containing dbcAMP were exposed to MeHg, and then the time- and dose-dependent changes in the morphology were investigated. The results are discussed from the viewpoints of the relationship between cell morphology and mercury accumulation. We also investigate the effect of genistein, a tyrosine kinase inhibitor that inhibits stress fiber formation,19) on the MeHg-induced spreading of astrocytes.
Wistar rats obtained from CLEA Japan Co. (Tokyo, Japan) were maintained at 23.5 ± 1.5°C and 55 ± 10% relative humidity under a 12-h light/dark cycle, and were given standard laboratory chow and tap water ad libitum. Pregnant rats were prepared as previously described,20,21) and housed individually until birth. The protocol for animal experiments was approved by the animal experimentation committee of Chiba Institute of Science. All care and the experiment procedures were carried out according to the fundamental guidelines of the Ministry of Education, Culture, Sports, Science and Technology, Japan (Notice No. 71 of 2006).
Cell CultureAstrocyte cultures were prepared as previously described15,20) with minor modifications. In brief, the cerebral hemispheres of newborn rats (within 24 h after birth) were excised under pentobarbital anesthesia, and then the meninges were carefully dissected off in minimum essential medium (MEM). After being washed in Ca2+, Mg2+-free Hanks’ balanced salt solution [HBSS (-)] or Ca2+, Mg2+-free phosphate-buffered saline [PBS (-)], the tissues of the brain region were treated with 0.1% trypsin at 37°C for 10 min. The cells obtained were grown in Basal Medium Eagle with Earle’s salts supplemented with 15% fetal calf serum (FCS; Invitrogen Co., Carlsbad, CA, USA), 0.1% L-glutamine, 0.6% D-glucose, antibiotics (Penicillin-Streptomycin; Invitrogen Co.), and an antimycotic (Fungizon; Invitrogen Co.) in culture flasks (25 cm2; BD Bioscience; Billerica, MA, USA) at 37°C in 6% CO2 in a humidified atmosphere. After cells reached confluence, the culture flasks were vigorously shaken by hand to remove small cells on the protoplasmic cell layer. Monolayer cells (astrocytes) were trypsinized and re-suspended with fresh 15% FCS-containing medium. Cells were then plated on poly-L-lysine (PLL; Sigma, St. Louis, MO, USA)-coated culture plates (BD Bioscience).
TreatmentAfter the cells reached 85–95% confluence, the 15% FCS-containing medium was changed to a serum-free defined medium (SFDM), the composition of which was previously described,20) containing 0.5 mM dbcAMP (Sigma). Four hours after the medium change, some cultures were exposed to methylmercuric chloride (Tokyo Chemical Industry Co., Tokyo, Japan) at final concentrations of 1–3 µM, according to the method previously described.20) Control cultures were added to solvent (ethanol) alone at that time. Before the exposure, other cultures were pretreated with genistein (Calbiochem, San Diego, CA, USA) at 50 µg/ml as a final concentration or vehicle (dimethylsulfoxide, DMSO) for 15 min, 3.75 h after the medium change.
Mercury AnalysisLysates of cells on 6-well plates were prepared using 0.1% sodium dodecylsulfate (SDS) in PBS (-), and the mercury content in each cell lysate was measured by the oxygen combustion-gold amalgamation method22) using a Rigaku Mercury Analyzer MA-2 (Nippon Instruments Co., Tokyo, Japan) and expressed as total mercury (µg) per whole protein (mg) as previously described.15,20)
Cell Viability AnalysisCells on 24-well plates were fixed with glutaraldehyde (1% as a final concentration), and viable cell numbers were determined spectrophotometrically using 0.1% crystal violet in 0.2 M 2-[N-morpholino]ethanesulfonic acid (pH 6.8) as previously described.21)
Statistical AnalysisSignificant differences between individual means were determined by one-way analysis of variance (ANOVA) followed by Duncan’s new multiple range test or by Student’s t-test. Differences were considered significant at p < 0.05.
Figure 1 shows time- and dose-dependent changes in morphology of astrocytes after an exposure of MeHg (1–3 µM) or ethanol (control). After being maintained in a SFDM containing 0.5 mM dbcAMP for 4 h, almost all astrocytes showed a process-bearing stellate shape (data not shown), and control cells continued to have that shape up to 24 h (Fig. 1A). In contrast, astrocytes showed a flat polygonal shape after exposure to MeHg at 3 µM for 1 h or at 2 µM for 3 h (Fig 1A). After the shape changed to polygonal with MeHg exposure at 2 µM, the astrocyte shape started to return to stellate after at least 6 h (Fig. 1B). The proportion of the cells with stellate shape increased with time (Fig. 1B), and almost all cells showed a stellate shape at 24 h (Fig. 1A). However, astrocyte shape remained polygonal up to 24 h after MeHg exposure at 3 µM (Fig. 1A). Similar to the control cells, the astrocyte shape was not affected after exposure to MeHg at 1 µM (Fig. 1A).

Time- and Dose-Dependent Change in Cell Morphology in Cultured Astrocytes After MeHg Exposure
(A) Cells were exposed to MeHg (1–3 µM) or ethanol (control) for 1, 3 or 24 h. (B) Cells were exposed to MeHg at 2 µM for 6 or 12 h. Bar = 50 µm.
Upon examination of the time-dependent change in mercury accumulation in astrocytes after exposure to MeHg at 1 µM, at which dose the astrocyte shape was not affected by MeHg (Fig. 1A), it was found that the mercury concentration had increased and peaked at approximately 2–4 h, and then decreased and reached a plateau after at least 12 h (Fig. 2). One hour after exposure to MeHg, the mercury concentration was significantly lower in astrocytes exposed at 2 μM than in those exposed at 3 µM (Table 1). The mercury concentration in astrocytes exposed at 2 µM significantly increased from 1 to 3 h, and reached a similar level to those exposed at 3 µM for 1 h, although the concentration was significantly lower than in those exposed at 3 µM for 3 h (Table 1). However, the concentration in astrocytes exposed at 1 µM did not reach those levels even at 3 h (Table 1), when the mercury accumulation was the highest (Fig. 2).
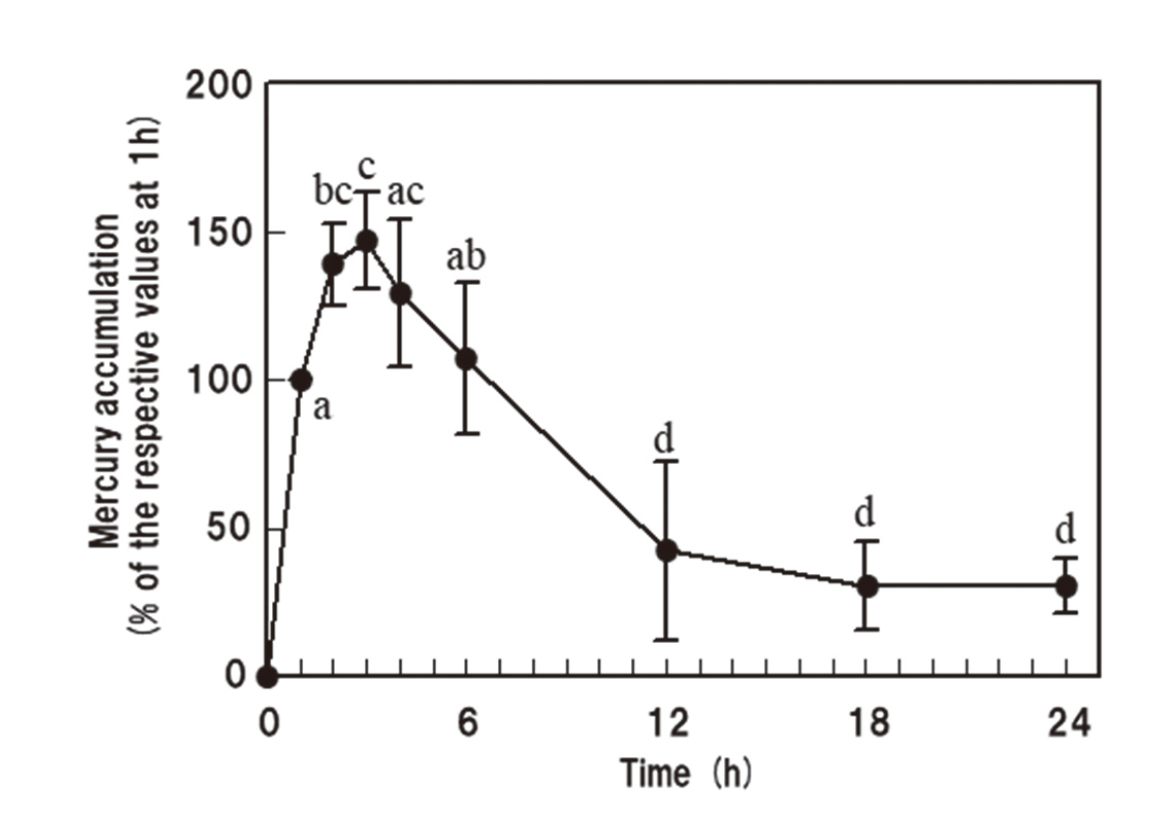
Time-Dependent Change in Mercury Accumulation in Cultured Astrocytes After MeHg Exposure
Cells were exposed to MeHg at 1 µM for up to 24 h. Values represent the mean ± S.D. of the percentages in relation to the mercury concentration at 1 h obtained from 3–5 independent determinations in triplicate. Values with different letters (a–d) are significantly different (P < 0.05).
| MeHg (μM) | Mercury accumulation (μg Hg/mg protein) | |||||
|---|---|---|---|---|---|---|
| Time (h) | ||||||
| 1 | 3 | |||||
| 1 | ND | 1.25 | ± | 0.14a | ||
| 2 | 2.13 | ± | 0.10b | 2.78 | ± | 0.03c |
| 3 | 2.82 | ± | 0.11c | 3.93 | ± | 0.18d |
ND: not determined.
Cells were exposed to MeHg at 1–3 μM for 1 or 3 h.
Values represent the mean ± S.D. obtained from 3 determinations.
Values with different letters (a–d) are significantly different (P < 0.05).
The viable cell numbers were not influenced by the 3 h-exposures to MeHg even at 3 µM compared to the control culture exposed to solvent alone (Fig. 3A). Twenty-four hours after the MeHg exposure, the viable cell number was significantly lower in the culture exposed at 3 µM than in the control culture, whereas the numbers were not affected by the MeHg exposures at 1–2 µM (Fig. 3B). Similar results were previously observed in astrocyte cultures exposed to MeHg immediately after changing the medium to a SFDM.20)

Viable Cell Number in Astrocyte Cultures After MeHg Exposure
■, Control; ▤, MeHg 1 µM; ▥, MeHg 2 µM; ▦, MeHg 3 µM. Cells were exposed to MeHg (1–3 µM) or ethanol (control) for 3 (A) or 24 h (B). Values represent the mean ± S.D. obtained from 3 determinations. Values with different letters (a, b) are significantly different at the specified time (P < 0.05).
To elucidate the involvement of tyrosine phosphorylation on MeHg-induced shape change, the effect of genistein, a tyrosine kinase inhibitor, was investigated. Pretreatment with genistein inhibited the MeHg-induced spreading of astrocytes, although the shape changed from stellate to polygonal after exposure to MeHg at 2–3 µM for 3 h (Fig. 4). Thus, the shape was similar in genistein-pretreated astrocytes with or without MeHg (Fig. 4).

Effect of Genistein on MeHg-Induced Morphological Changes in Cultured Astrocytes
Cells were pretreated with genistein (50 µg/mL) or vehicle (DMSO) alone for 15 min, and then exposed to MeHg (2–3 µM) or ethanol for 3 h. Bar = 50 µm.
In the present study, the morphology of astrocytes changed with dbcAMP stimulation from a stellate shape to a polygonal shape within 1 and 3 h after MeHg exposures at 3 and 2 µM, respectively (Fig. 1A). The MeHg exposure at 1 µM did not affect the shape (Fig. 1A), and only exposure at 3 µM decreased the viable cell numbers within 24 h (Fig. 3B). These results suggest that the effects of MeHg become severer in a dose-dependent manner. We previously suggested that the accumulations of mercury compounds, including MeHg, up to 3 h strongly correlates with susceptibility to them, at least when maintained under serum-free conditions.20) Accordingly, shape changes as well as cell death in astrocytes might also depend on the accumulation of MeHg, particularly for the first few hours, when its accumulation peaked (Fig. 2). It should be noted that mercury concentrations are similar in the cells exposed to MeHg at 3 µM for 1 h and at 2 µM for 3 h (Table 1), when astrocyte shape is observed to change from stellate to polygonal (Fig. 1A). The mercury concentration did not reach those levels after exposure at 1 µM (Table 1), at which the astrocyte shape did not change (Fig. 1A). Therefore, mercury concentrations must reach a level that is effective for MeHg-induced spreading of astrocytes. In addition, after the exposure to MeHg at 2 µM, the astrocyte morphology reversed from polygonal to stellate with time, probably along with a decrement in the mercury concentration, and almost all astrocytes showed a stellate shape after at least 24 h (Fig. 1A and B). Thus, there would seem to be a close relationship between the shape changes and MeHg accumulation in cultured astrocytes.
Protein kinases play central roles in the regulation of cell growth, division, and differentiation. In cultured astrocytes, an inhibition of tyrosine kinase caused by genistein has been demonstrated to result in a change of the morphology to stellate from polygonal, suggesting that tyrosine kinase contributes to maintenance of the polygonal shape.23) In addition, genistein inhibits LPA-induced stress fiber formation.19) Since the spreading of astrocytes induced by LPA13,14) and MeHg15) is reportedly accompanied by the formation of stress fibers, it is possible that these shape changes are affected by genistein. The present result showed that pretreatment with genistein inhibited the shape change from stellate to polygonal in astrocytes induced by MeHg exposure at 2–3 µM for 3 h (Fig. 4). Our preliminary experiment revealed that genistein did not affect the mercury accumulation in astrocytes at either dose (data not shown). Therefore, its inhibitory effect is likely due to the inhibition of tyrosine kinase rather than the difference in accumulation of MeHg. These findings suggest the importance of tyrosine phosphorylation in MeHg-induced spreading of astrocytes, and would contribute to elucidate the mechanism, including signal transduction pathways.
In conclusion, the morphology of cerebral hemisphere astrocytes maintained in dbcAMP-containing serum-free medium changes from stellate to polygonal within 3 h after exposure to MeHg at more than 2 µM, and this morphological change is closely related to both MeHg accumulation and tyrosine phosphorylation.
Conflict of interestThe authors declare no conflict of interest.