2023 年 6 巻 2 号 p. 68-75
2023 年 6 巻 2 号 p. 68-75
The Bacterial Endotoxins Test (BET) uses Limulus amoebocyte lysate (LAL) reagents derived from the blood of horseshoe crabs for detection and quantification of endotoxins from Gram-negative bacteria in parenteral drug products and medical devices. Two types of recombinant reagents using genes cloned from the horseshoe crab genome have become available from several suppliers. One is a recombinant Factor C reagent (rFC), containing only recombinant Factor C, and the second is a recombinant Cascade Reagent (rCR) containing recombinant factor C, recombinant factor B and recombinant proclotting enzyme. Implementation of these recombinant reagents for BET requires validation that demonstrates results equal to or better than those determined by LAL reagents. Previous studies have shown that the PyroSmart NextGen® rCR meets the analytical performance requirements for both the plate and tube reader testing methods and provides equivalent results when testing samples containing autochthonous endotoxin. This study directly compares PyroSmart NextGen® to LAL reagent performance when testing a parenteral drug, which is a critical step for end-user implementation of alternative methods. It is the first published demonstration of an approach to the validation of alternative reagents that includes testing of a specific parenteral drug sample, and the data indicates that PyroSmart NextGen® is more precise when compared to LAL reagents. Relative recovery, linear regression, and Bland-Altman plot analyses also illustrate that PyroSmart NextGen® results are equal to or better than those determined by naturally sourced LAL reagents. This indicates that PyroSmart NextGen® is a useful alternative method for quantifying bacterial endotoxins in parenteral drugs.
In 1977, the United States Food and Drug Administration (FDA) announced the licensing of Limulus amoebocyte lysate (LAL) reagents derived from the hemolymph of living horseshoe crabs for the detection of potentially fatal endotoxins in biological products and medical devices. Over time, LAL became a compendial reagent for the Bacterial Endotoxins Test (BET) in the United States Pharmacopeia (USP), the European Pharmacopeia (Ph. Eur.) and the Japanese Pharmacopeia (JP).1,2) More recently, the horseshoe crab genome has been used to produce recombinant zymogen proteases, which are key components of recombinant Factor C reagents (rFC) and recombinant cascade reagents (rCR) such as PyroSmart NextGen®. These alternative reagents have the advantages of eliminating lot-to-lot variability and the false positives caused by triggering of the (1→3)-ß-D-glucan coagulation pathway which can occur when using naturally sourced LAL reagents.1) Additionally, recombinant reagents promote sustainable endotoxin testing by meeting the replacement, reduction, and refinement principles for animal welfare.3,4)
In the United States, recombinant reagents are considered alternative to compendial LAL reagents, therefore their use requires full method validation with results equal to or better than compendial LAL methods.5,6) The Ph. Eur. has introduced a chapter on testing for bacterial endotoxins using rFC, although product-specific validation including demonstration of equivalency is still required to be performed by the end-user.7-9) To comply with all applicable regulations, the analytical performance of PyroSmart NextGen® using both the plate and tube reader testing methods has been validated, which allows for comprehensive equivalency analysis with LAL reagents. A previous study testing samples containing autochthonous endotoxin established the methods for evaluating this equivalency against in-house criteria.10,11)
This study assesses the analytical performance of PyroSmart NextGen®, Pyrochrome® (chromogenic LAL), and Pyrotell®-T (turbidimetric LAL) according to in-house acceptance criteria and directly compares the results to demonstrate equivalency. Analytical performance includes analysis of linearity, accuracy, precision, range, quantitation limit, and specificity according to USP <1225> and the ICH Q2 guideline.12,13) Three lots of a parenteral drug sample were also tested using PyroSmart NextGen® and both LAL reagents for direct comparison of method suitability results according to USP <1085> and USP <85>.14,15) This approach aligns with case study examples outlined in a recent FDA presentation. It specifies that non-product-specific analytical performance of alternative reagents should be evaluated by the manufacturer and the results when testing three lots of a specific product should be assessed for precision and accuracy by the end-user. This testing should be performed using three lots of the alternative reagent and three lots of a reference LAL reagent to demonstrate results equal to or better than LAL.16) For this study, the product-specific data constitutes a demonstration of method suitability and was assessed for linearity, accuracy, precision, range, quantitation limit, specificity, and robustness according to USP <1085>, USP <85>, USP <1225> and the ICH Q2 guideline (quantitative test for impurities).12-15) Additional comparison analysis was performed according to previously established methods to further illustrate the non-inferiority of PyroSmart NextGen®.11) This is the first direct equivalency analysis of rCR and LAL reagent data, which demonstrates a non-product-specific method validation combined with product-specific method suitability. This study can be used as a general example for end-users implementing alternative reagents for BET if the assay details are optimized for specific needs.
USP Reference Standard Endotoxin (USP-RSE) was purchased from the United States Pharmaceutical Convention (MD, USA).
Sodium Citrate for InjectionThree lots of the parenteral drug Sodium Citrate for Injection were obtained from Seikagaku Corporation (Tokyo, Japan).
LAL ReagentsThree lots of Pyrochrome®, three lots of Pyrotell®-T, and one lot of Glucashield® buffer were obtained from Associates of Cape Cod, Inc. (MA, USA).
Recombinant ReagentThree lots of PyroSmart NextGen® were obtained from Associates of Cape Cod, Inc. (MA, USA).
Endotoxin AssaysEndotoxin was quantified using PyroSmart NextGen® and both LAL reagents according to their respective Instructions for Use. The onset time assay mode was used to measure the time required to reach a specific optical density threshold. The endotoxin concentration in samples was determined using the standard curve, which was constructed by plotting the log-converted onset time (Y-axis) against the log-converted standard concentration (X-axis).
Analytical Performance of PyroSmart NextGen® (Non-Product-Specific Method Validation Testing)Three lots of PyroSmart NextGen® were used by three different analysts to perform a total of six plate reader assays and six tube reader assays over multiple days. The assays included a ten-fold standard curve series of USP-RSE specific to each method (10, 1, 0.1, 0.01 EU/mL for the plate reader and 1, 0.1, 0.01, 0.001 EU/mL for the tube reader) tested in triplicate. The linearity, accuracy, precision, range, and quantitation limit aspects of the analytical performance of PyroSmart NextGen® were evaluated using these standard curve concentrations according to USP <1225> and the ICH Q2 guideline.12,13) Three separately prepared USP-RSE concentrations with high, medium, and low levels of endotoxin were incorporated (5, 0.5, 0.05 EU/mL for the plate reader and 0.3, 0.03, 0.003 EU/mL for the tube reader) and assessed for accuracy and precision. Although specificity for detecting endotoxin in the presence of (1→3)-ß-D-glucan was evaluated in previous studies not shown here, additional analysis when testing one lot of Sodium Citrate for Injection was included to provide an example using a sample matrix other than water.10) Based on previous data, this testing was performed using the maximum valid dilution (MVD), the MVD/2, and the MVD/5 with corresponding positive product controls (PPCs) at a final concentration in the middle of each standard curve. These sample results were evaluated for suitable precision (repeatability), valid PPC recoveries, and detectable endotoxin. The ICH guideline M10 and previous studies were referenced for all acceptance criteria.10,11,17)
Method Suitability of PyroSmart NextGen® (Product-Specific Testing)Three lots of the parenteral drug Sodium Citrate for Injection were tested at the previously-determined MVD/5 dilution with PPCs equivalent to the high, medium and low USP-RSE concentrations for the plate and tube reader testing methods. These results were assessed for linearity, accuracy, precision (repeatability), range, quantitation limit, specificity, and robustness to establish product-specific method suitability according to USP <1085>, USP <85>, USP <1225> and the ICH Q2 guideline (quantitative test for impurities).12-15)
Equivalency Testing of PyroSmart NextGen® and LAL ReagentsThe same assay setup (standard curve, sample dilutions, and USP-RSE concentrations) for PyroSmart NextGen® was tested using Pyrochrome® in a plate reader and Pyrotell®-T in a tube reader. Both LAL reagents were reconstituted with glucan-blocking buffer to eliminate the possibility of false positives caused by (1→3)-ß-D-glucan contamination. Using in-house acceptance criteria, the analytical performance and method suitability results of each LAL reagent were compared to those of PyroSmart NextGen® to demonstrate equivalency.10,11) The coefficient of variation results of the two methods were then compared utilizing the standard curve concentrations, the three separate USP-RSE concentrations, and the samples PPCs to further demonstrate precision equivalency. Additional analysis was performed according to the methods and criteria described in a previous study.11) The “relative recovery” of each USP-RSE concentration and all Sodium Citrate for Injection sample PPCs were evaluated by calculating the sample endotoxin concentration determined by PyroSmart NextGen® as a percentage of the endotoxin detected in the same sample determined by an LAL reagent.18) Linear regression analysis comparing the endotoxin concentration in the sample PPCs and the three separate USP-RSE concentrations results determined by PyroSmart NextGen® on the Y-axis and the LAL reagent on the X-axis was included in the equivalency analysis. Bland-Altman plots of the same data were generated to illustrate any significant differences between PyroSmart NextGen® and the LAL reagents.10,19)
All standard curve concentrations and the three separately prepared USP-RSE concentrations quantified using PyroSmart NextGen® meet the linearity, accuracy, precision, range, and quantitation limit specifications (Tables 1 and 2). Therefore, PyroSmart NextGen® analytical performance as defined by USP <1225> and the ICH Q2 guideline has been demonstrated.12,13) Additional analysis of specificity when testing a Sodium Citrate for Injection sample matrix also meets the precision, accuracy, and endotoxin detection criteria.
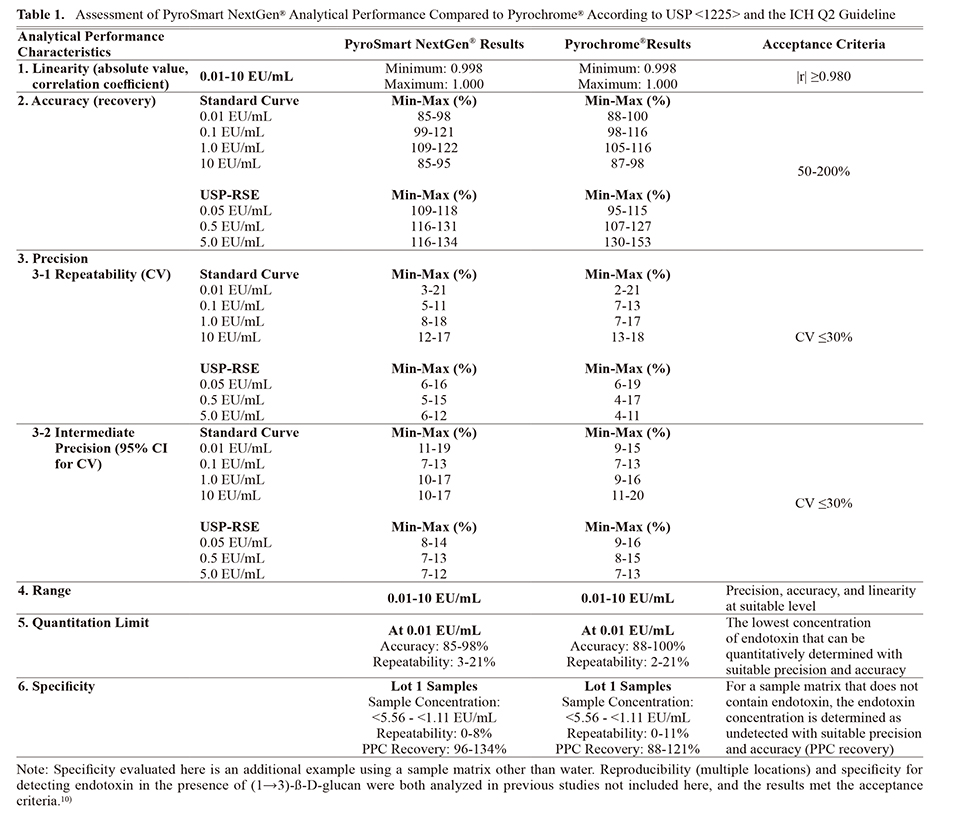
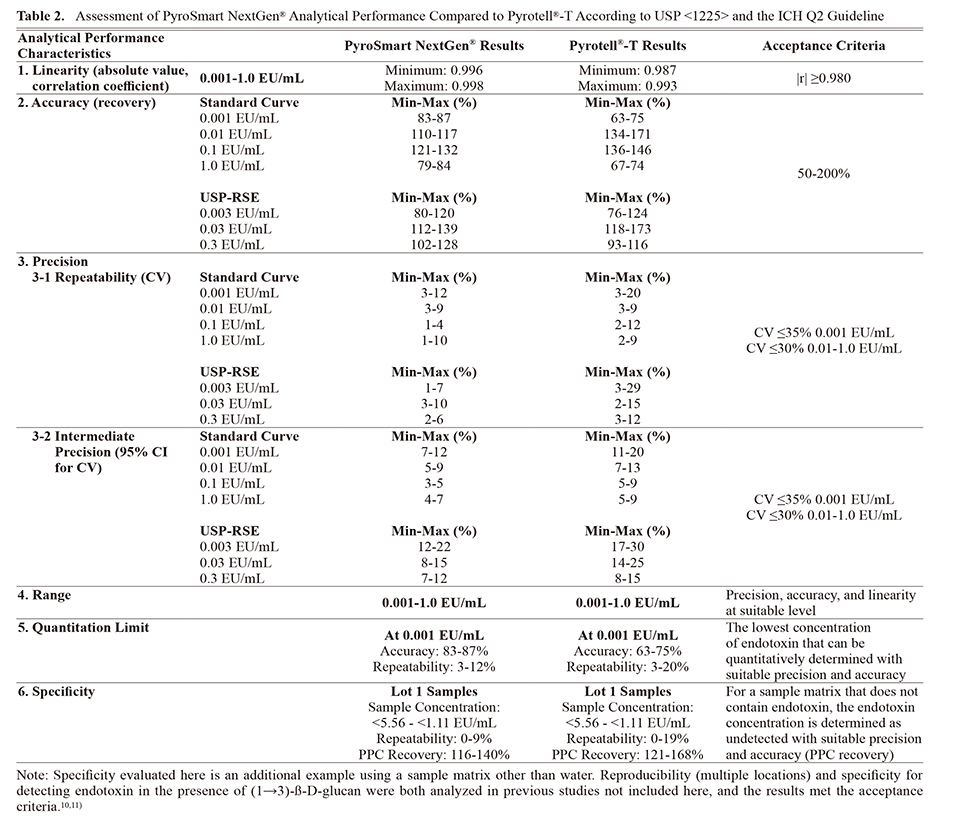 Method Suitability of PyroSmart NextGen® (Product-Specific Testing)
Method Suitability of PyroSmart NextGen® (Product-Specific Testing)
The three lots of Sodium Citrate for Injection tested with three PPC concentrations satisfy the linearity, accuracy, precision, range, quantitation limit, specificity, and robustness criteria (Tables 3 and 4). According to USP <1085>, USP <85>, USP <1225> and the ICH Q2 guideline (quantitative test for impurities), product-specific method suitability of PyroSmart NextGen® has been demonstrated.12-15)
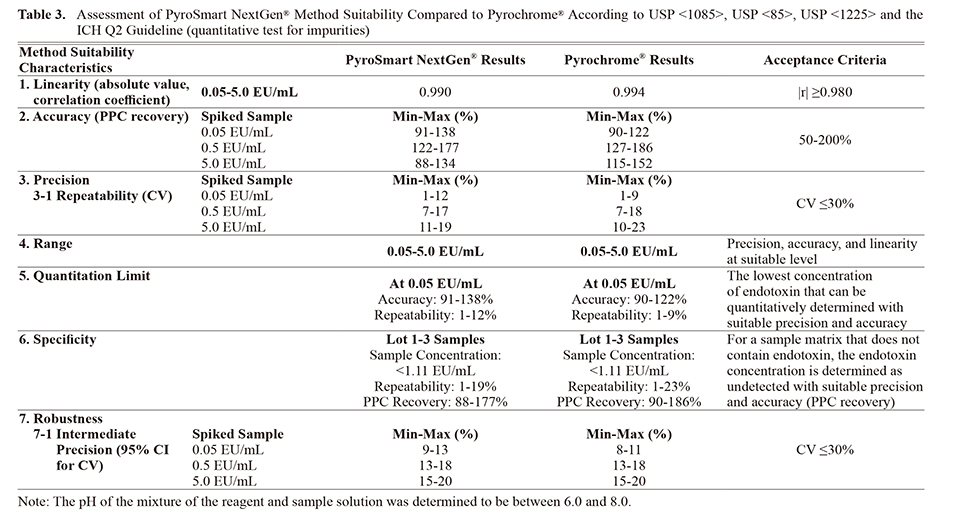
 Equivalency Testing of PyroSmart NextGen® and LAL Reagents
Equivalency Testing of PyroSmart NextGen® and LAL Reagents
Both the Pyrochrome® and Pyrotell®-T LAL reagents produce results that meet the in-house acceptance criteria for analytical performance and method suitability. Direct comparison of the LAL reagents to PyroSmart NextGen® illustrates that PyroSmart NextGen® has equal or better results for all parameters (Tables 1-4). Additional precision analysis demonstrates that the maximum coefficient of variation seen with PyroSmart NextGen® is 20.89% for plate and tube reader testing, whereas Pyrochrome® has a maximum of 23.22% and Pyrotell®-T has a maximum of 29.24% (Fig. 1). All samples containing detectable (in this case, added) endotoxin (three separately prepared USP-RSE concentrations and Sodium Citrate for Injection sample PPCs) have relative recovery results within 50-200% (Fig. 2). Before linear regression and Bland-Altman plot analysis, a normality test was completed, which determined that the three separately prepared USP-RSE concentration and sample PPC data should be transformed logarithmically. The subsequent linear regression analysis of the plate reader data results in slope of 0.9532 and a 95% confidence interval (CI) of 0.9418 to 0.9645. The tube reader linear regression has a slope of 1.023 and a 95% CI of 1.000 to 1.046 (Fig. 3). The Bland-Altman plate reader testing bias is -2.899 and 97% of data points are within the 95% upper and lower limits of agreement (LOA) whereas the tube reader has a bias of 5.301 and the same percentage of samples (97%) within the LOA (Fig. 4).
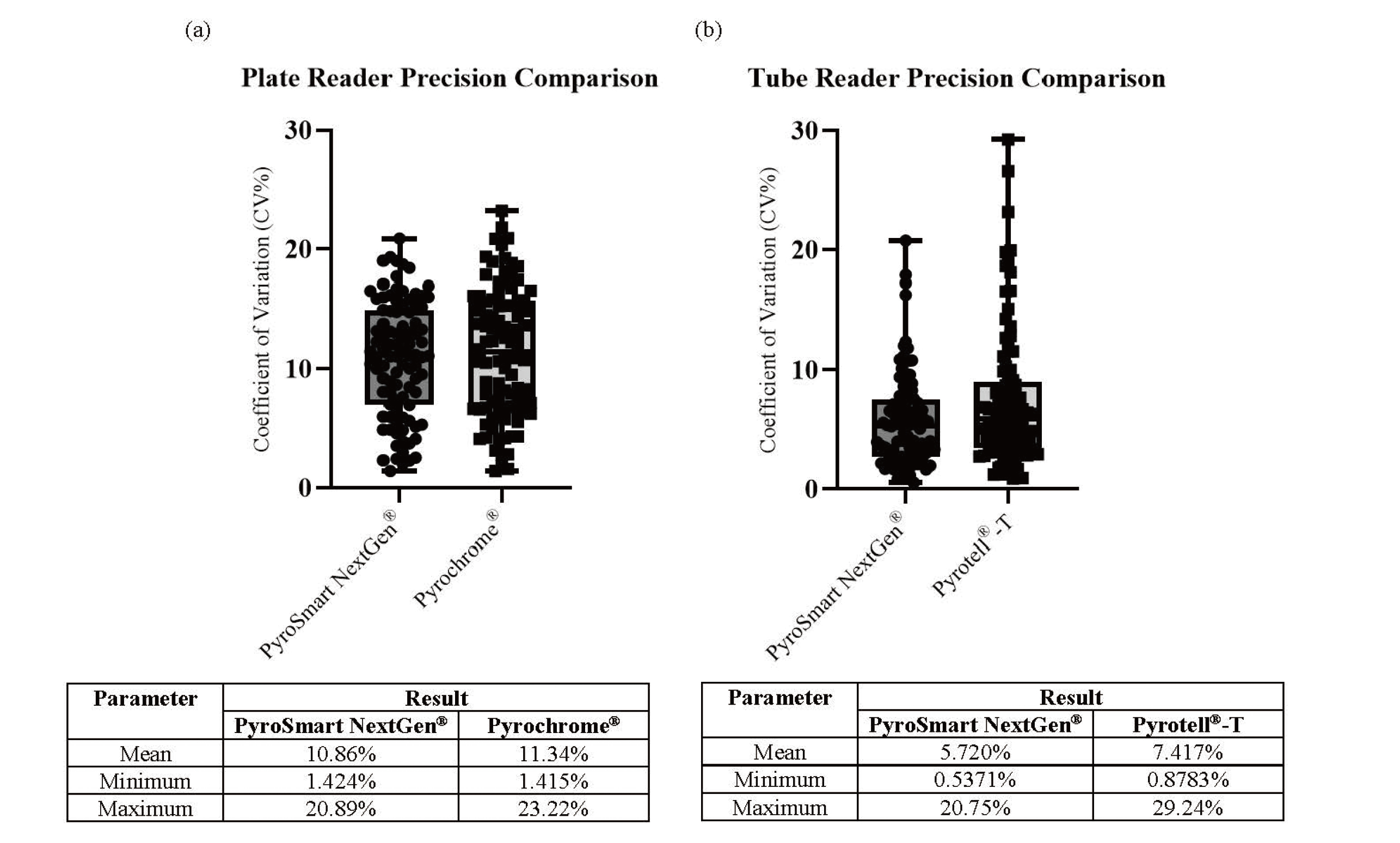
(a) Precision Comparison of the Endotoxin Concentrations Determined by PyroSmart NextGen® and Pyrochrome® Using a Plate Reader. (b) Precision Comparison of the Endotoxin Concentrations Determined by PyroSmart NextGen® and Pyrotell®-T Using a Tube Reader.
All results with calculable coefficient of variation values were included (the standard curve, the three separately prepared USP-RSE concentrations, and the sample PPCs).
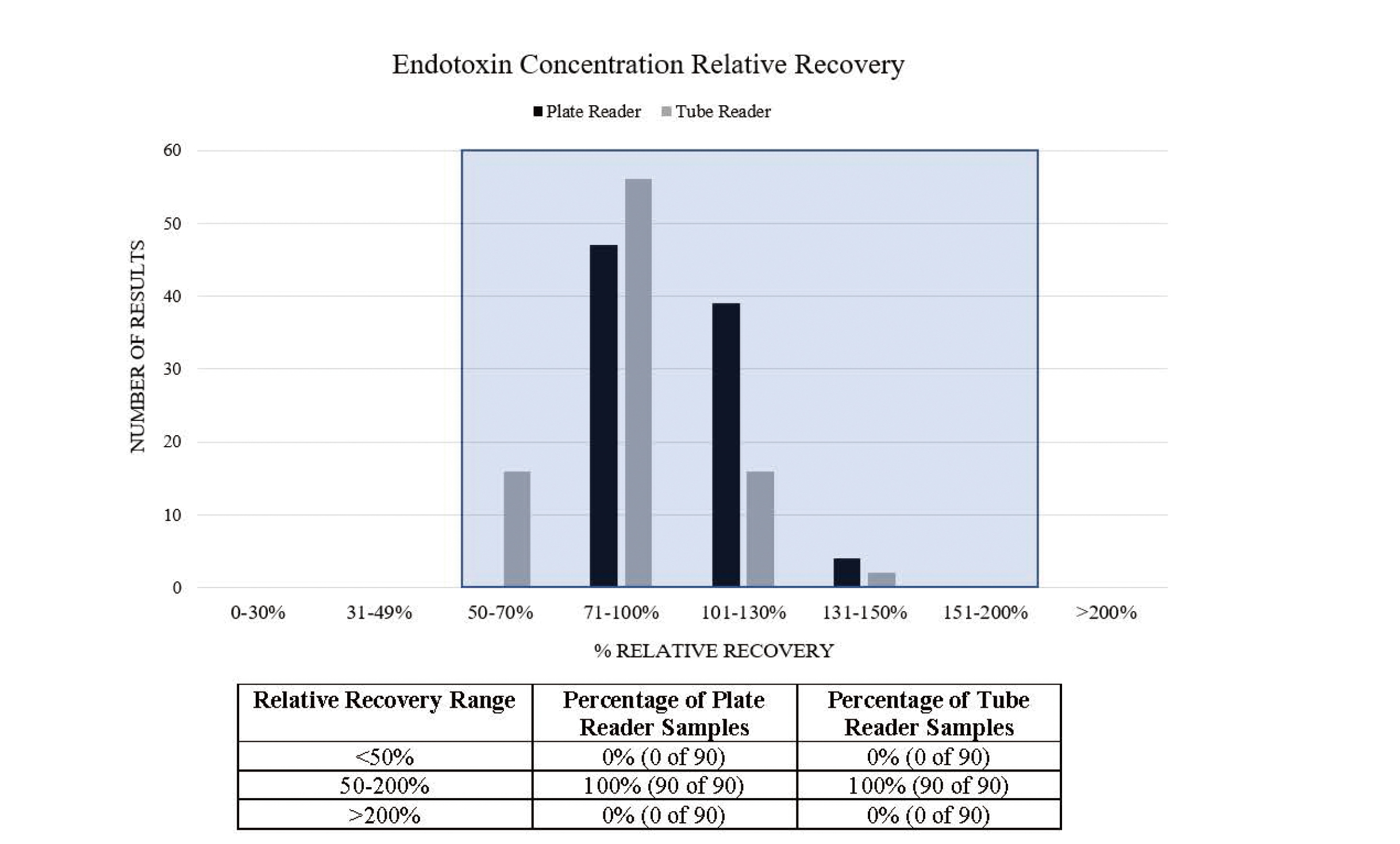
Relative Recovery Analysis Summary for the Endotoxin Concentration in Sample PPCs and the Three Separately Prepared USP-RSE Concentrations Determined Using the Plate and Tube Reader Methods.
Each column in the graph represents the number of results that have relative recoveries within each defined range. The shaded box highlights all results within 50-200%.
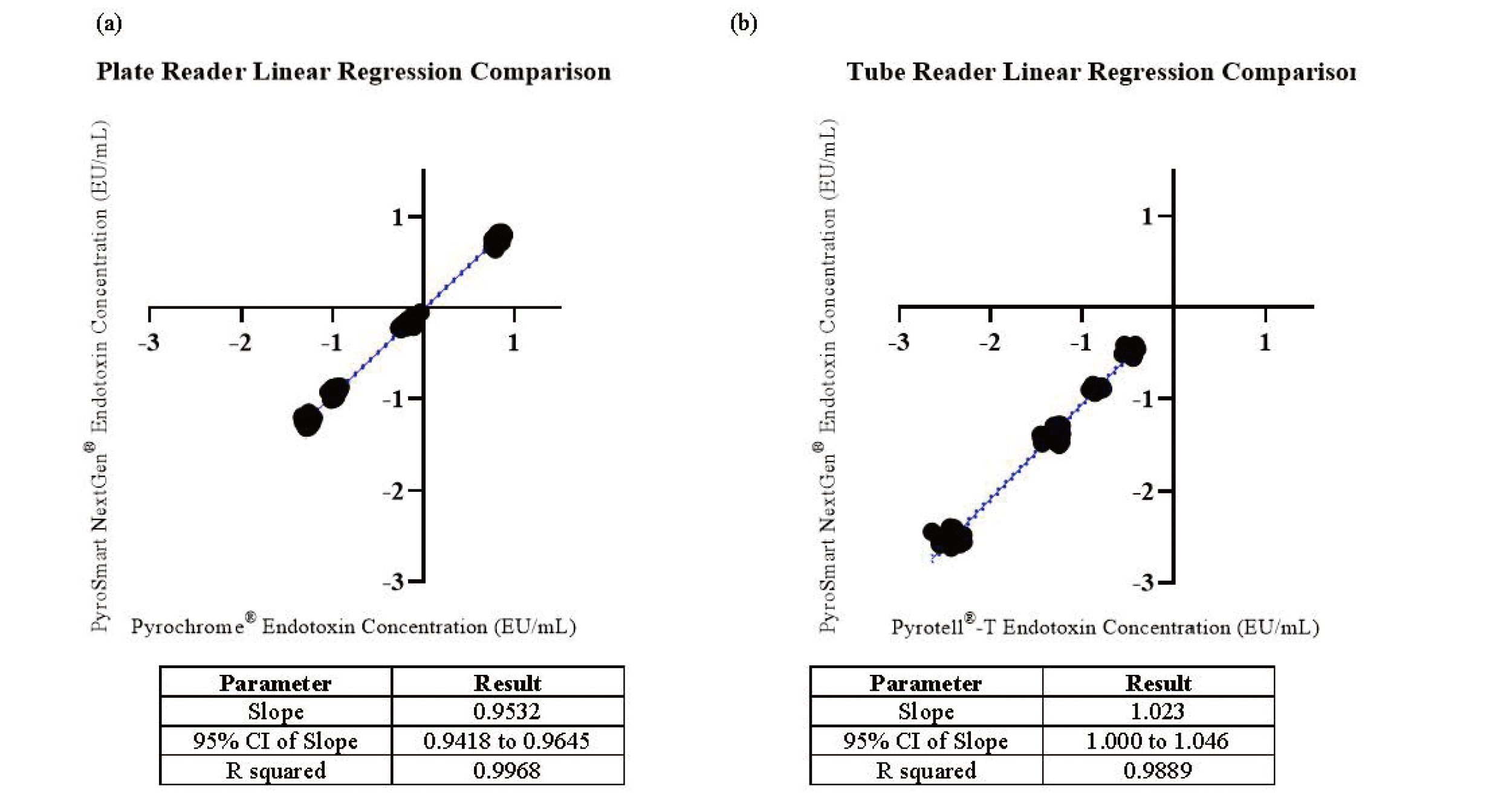
(a) Linear Regression Analysis of the Samples Containing Added Endotoxin and the Three Separately Prepared USP-RSE Concentrations Tested with Pyrochrome® Compared to Those Tested with PyroSmart NextGen®. (b) Linear Regression Analysis of the Samples Containing Added Endotoxin and the Three Separately Prepared USP-RSE Concentrations Tested with Pyrotell®-T Compared to Those Tested with PyroSmart NextGen®.
The solid line depicts the slope, and the dotted lines are the 95% confidence interval (CI) of the slope.

(a) Bland-Altman Plot Analysis of the Three Separately Prepared USP-RSE Concentrations and the Samples with Added Endotoxin Determined by PyroSmart NextGen® and by Pyrochrome® Tested Using a Plate Reader. (b) Bland-Altman Plot Analysis of the Three Separately Prepared USP-RSE Concentrations and the Samples with Added Endotoxin Determined by PyroSmart NextGen® and by Pyrotell®-T Tested Using a Tube Reader.
A bias (solid red line) of zero indicates that the results are identical, and the dotted red lines are the 95% confidence interval (CI) of the bias. The red data points are those outside of the 95% upper and lower limits of agreement (LOA, dotted black lines).
The USP General Notices and Requirements 6.30 and the 2012 FDA Guidance for Industry: Pyrogen and Endotoxins Testing state that alternative reagents must be validated with results equivalent to or better than the results of LAL reagents.4,5) A previous study has validated the analytical performance of PyroSmart NextGen® when using the plate reader in both the rate and onset assay modes. The capability of PyroSmart NextGen® to determine the potency of different bacterial strains, and to recover endotoxin from various interfering pharmaceuticals at a level equivalent to LAL reagents was successfully demonstrated.10) A second study validated PyroSmart NextGen® tube reader analytical performance and demonstrated result equivalency when testing water samples containing autochthonous endotoxin.11) This study provides further confirmation of PyroSmart NextGen® analytical performance in a plate and tube reader. It also demonstrates that the product-specific method suitability data meets the accuracy and precision requirements when testing three lots of Sodium Citrate for Injection.14,15,20) Additional analysis of linearity, range, quantitation limit, specificity, and robustness using the method suitability data was performed according to the USP <1225> and the ICH Q2 guideline quantitative test for impurities. The results meet all criteria and demonstrate the full analytical capability of PyroSmart NextGen® when testing a monograph product (Sodium Citrate for Injection) for the first time.12,13) In addition to evaluation of PyroSmart NextGen®, the same testing was performed using LAL reagents, and for consistent analysis of equivalency all analytical performance and method suitability data was assessed according to the same in-house acceptance criteria. The precision results of the endotoxin concentrations determined by all reagents were also compared. This is the first direct comparison of non-product specific and product-specific rCR test results to those obtained by LAL reagents. The approach used aligns with FDA case studies, which outline the validation of alternative reagents for BET within the framework of non-product-specific analytical performance and product-specific testing to demonstrate equivalency to LAL.16)
The non-inferiority of PyroSmart NextGen® is further supported in this study by additional equivalency assessment. Because there are not specific guidelines for comparison studies, the three separately prepared USP-RSE concentrations and samples containing added endotoxin were evaluated for equivalency according to the methods and in-house criteria defined in the large-scale water sample study. This includes calculating the relative recovery of the endotoxin concentration in samples for both the plate and tube reader methods with a criterion that 70% of samples should have results within 50-200%. Comparison of PyroSmart NextGen® and LAL reagents in a plate and tube reader were also evaluated using linear regression and Bland-Altman plot analysis. The original water study criteria stated that the linear regression slope must be between 0.7 and 1.3, and the Bland-Altman plots must have at least 95% of data points within the LOA.11) In this study, 100% of the endotoxin concentration results have relative recovery results within 50-200%. This greatly exceeds the original criterion, likely because known concentrations of USP-RSE were added to the highly purified parenteral drug sample, which also presumably does not contain any contaminants that would affect the assay. Linear regression analysis resulted in slopes of 0.9532 and 1.023, which meet the pre-determined criterion, and the Bland-Altman plots resulted in 97% of the data within the 95% LOA for both plate and tube reader method comparisons. Various publications involving different types of samples further support that PyroSmart NextGen® is comparable to LAL and other recombinant reagents including rFC.10,11,21-23)
As shown in this and two previous studies, PyroSmart NextGen® is a robust rCR that meets all analytical performance and method suitability criteria in both a plate and tube reader. Endotoxin concentration coefficient of variation analysis demonstrates that PyroSmart NextGen® is more precise when compared to LAL reagents. The development and manufacturing processes also meet the quality standards that are applied to FDA-licensed LAL reagents. The wide standard curve range and multiple test method options for PyroSmart NextGen® enable direct comparison to various LAL reagents, and optimization by the end-user to suit their particular needs. Assay specifics such as dilution factors, standard curve range, added endotoxin concentrations and acceptance criteria must be optimized for each end-user’s requirements. It is also important to note that this study was designed in accordance with the current regulatory and guideline documents at the time of publication, and future updates may change the requirements. Further research using various parenteral drugs and multiple endotoxin detection systems could be used to support the use of alternative methods for BET. This, and two previous studies illustrate an approach that can be used for recombinant reagent validation and implementation. They demonstrate that PyroSmart NextGen® meets all in-house criteria with results that are equivalent to or better than those determined by naturally sourced LAL reagents when testing multiple sample types.
Conflict of interestKelley, Stevens, Akiyoshi are employees of Associates of Cape Cod, Inc. and Jahngen is a consultant to Associates of Cape Cod, Inc., Oda is an employee of both Associates of Cape Cod, Inc. and Seikagaku Corporation.