2023 年 6 巻 4 号 p. 126-132
2023 年 6 巻 4 号 p. 126-132
The history of methylmercury (MeHg) toxicity research goes back to Minamata disease. In Japan, histopathological examination of patients afflicted with Minamata disease, alongside meticulous investigations at both in vitro and in vivo, were robustly undertaken to elucidate the deleterious effects of MeHg. It is not an overstatement to assert that Japanese investigations on MeHg toxicity have spearheaded global advancements in this field. Nevertheless, more than half a century has passed since the onset of Minamata disease, yet the intricacies of MeHg toxicity remains enigmatic. Moreover, environmental pollution of mercury and toxic metals is a global problem that needs to be solved. Therefore, our research has focused on, "toxicology of MeHg" and "remediation of mercury and other toxic metals". This review discusses the molecular mechanisms underlying roles of autophagy in MeHg responses of mammalian cells, identification of an anti-MeHg natural product, analysis of bacterial mercury-resistant gene, and plant biotechnology using bacterial transporters for phytoremediation.
Mercury compounds have long been used for their useful chemical and physical properties. The uses of mercury have further expanded to include pharmaceuticals, pesticides, daily necessities, and chemical catalysts with the development of modern industry. An example of its use as a chemical catalyst in the chemical industry is mercury sulfate used in the production process of acetaldehyde. Methylmercury (MeHg), a byproduct of the production process, is the causative agent of Minamata disease. From 1932 to around 1971, approximately 150 tons of mercury compounds were dumped in Minamata Bay, resulting in the outbreak of Minamata disease.1) Similar mercury pollution also occurred in the Agano River basin. These tragedies in Japan were disregarded, and mercury pollution similar to that in Minamata Bay occurred in many parts of the world. In the Guiyang region of Guizhou Province, China, approximately 130 tons of mercury compounds were dumped over a 30 years from 1971 to 2000.2) Environmental pollution by high concentrations of mercury compounds from gold mining and factory drainage continues in Brazil, Russia, Tanzania, and Southeast Asian regions.3) There are concerns regarding the spread of health hazards to humans in the future, and although more than half a century has passed since the outbreak of Minamata disease, there are still many unknowns regarding the mechanism of MeHg toxicity.
In this review, we focused on the "toxicology of MeHg" and "remediation of mercury and other toxic metals". In the section "MeHg Toxicology," we present our ongoing investigations regarding autophagy as a biological defense mechanism against MeHg, as well as the exploration of pharmaceutical interventions targeting MeHg. As for the segment focused on "remediation of mercury and other toxic metals", we introduce our research endeavors encompassing bacterial mercury-resistant genes, and plant genetic engineering utilizing mercury transporters for the remediation of mercury-contaminated environments.
MeHg causes serious damage to various organs in both animals and humans. Bioaccumulation of MeHg occurs through the food chain, and consumption of contaminated fish and other seafood is the primary source of exposure to humans. Although the effects of exposure to low levels of MeHg from dietary sources are unknown, there are reports of increased incidence of neurodegenerative brain diseases, such as Parkinson's disease (PD), Alzheimer's disease (AD), and amyotrophic lateral sclerosis (ALS).4) It is also assumed that the cytotoxicity of MeHg is not limited to the developing nervous system and may be a risk for diseases after neural maturation.5)
Autophagy plays a role in maintaining cellular homeostasis by degrading intracellular degenerated proteins and aggregates. We showed that autophagy is activated at low concentrations of MeHg and autophagy-deficient cells lacking the core ATG gene, ATG5, are sensitive to MeHg.6) In addition, in the autophagy-deficient cells, MeHg stress enhanced multinucleation and DNA damage,7) suggesting that autophagy is a defense mechanism against MeHg toxicity. We also showed that ubiquitinated proteins, which are substrates for autophagic degradation in cells, were increased following MeHg exposure.5) p62/Sequestosome1 (p62) is an autophagic cargo adaptor that selectively recognizes ubiquitinated proteins and recruits them to the autophagosome. p62 also binds directly to LC3-II to facilitate the degradation of ubiquitinated proteins by autophagy. Accordingly, p62-deficient (p62KO) MEF cells displayed an increase in ubiquitinated proteins following MeHg exposure8) and enhanced sensitivity to MeHg. In addition, p62 functions to alleviate MeHg-induced endoplasmic reticulum (ER) stress.9) Furthermore, we identified neural precursor expressed developmentally down-regulated protein 4 (NEDD4) as a binding molecule for p62 and showed that the binding of p62 to NEDD4 may function to alleviate MeHg toxicity.10) These results suggest that p62 is a key molecule in cellular processes for MeHg toxicity mitigation. In addition to autophagy, the proteasome was shown to reduce MeHg toxicity.11) Recently, we found that p62-deficient cells showed reduced activation of nuclear factor-erythroid 2-related factor (Nrf2) and higher intracellular Hg concentrations after MeHg exposure, compared to wild-type cells (unpublished data).
A series of our studies focusing on p62 provided new insight into the cellular mechanisms for mitigating MeHg toxicity (Fig. 1). Further functional elucidation of p62 in response to MeHg exposure may provide clues on how the cells mitigate MeHg toxicity. It is thought that p62 associates with neurodegenerative brain diseases. An analysis of mice lacking p62 suggests that p62-mediated autophagy is involved in the onset and progression of dementia by degrading the tau protein, which accumulates in the brain diseases, such as AD.12) Further researches using in vitro and in vivo model systems are being conducted to elucidate the function of p62 as a key molecule that controls the onset and progression of MeHg-induced neurodegenerative brain diseases.
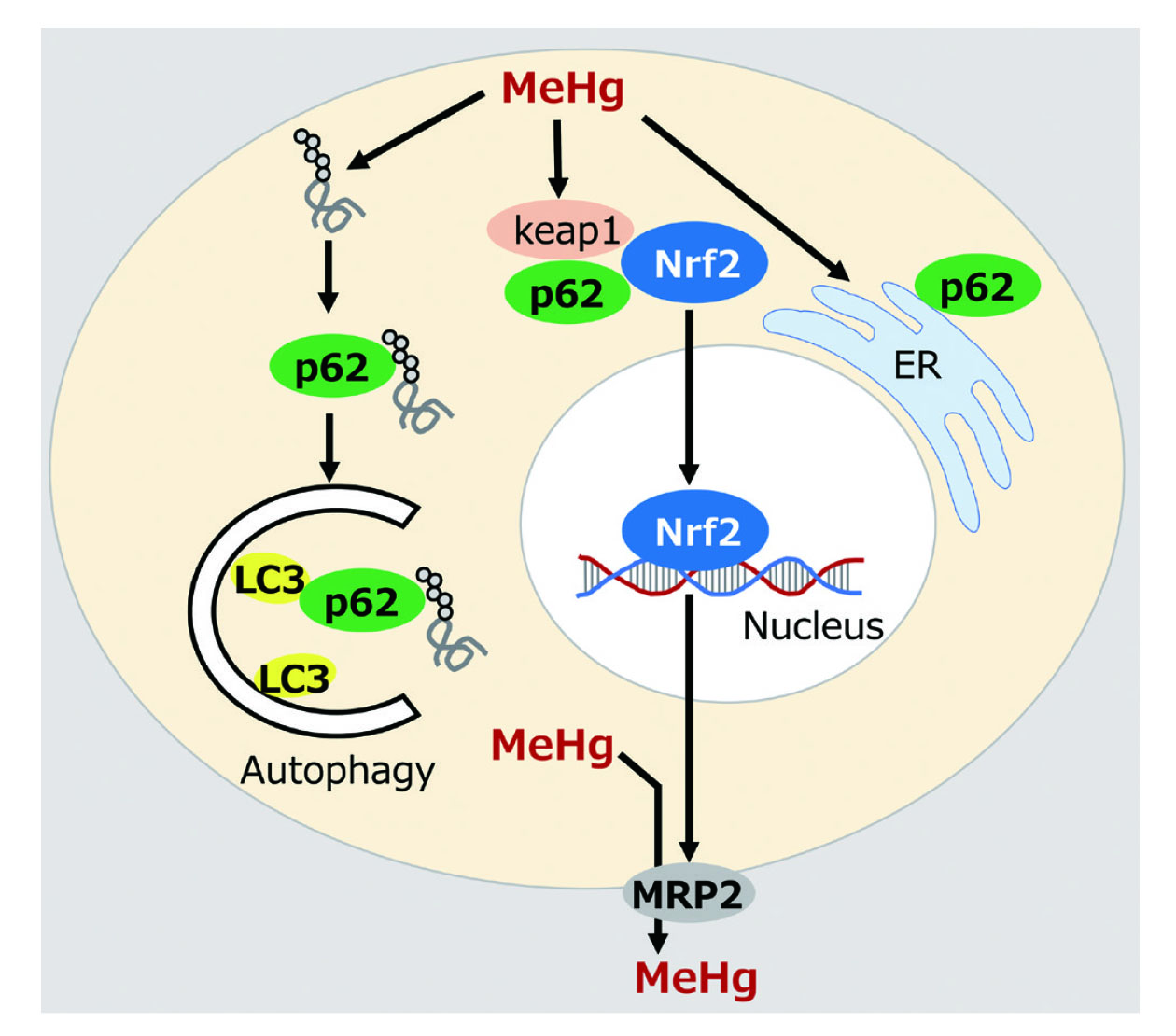
p62-Mediated Signaling Pathways Against MeHg Exposure.
p62 binds with MeHg-induced ubiquitinated proteins and degrades them via autophagy. p62 also interacts with keap1 and activates the Nrf2 pathway that regulates intracellular mercury level. p62 plays an important role for mitigation of ER stress by MeHg.
From the perspective of studying the characteristics of autophagy and MeHg toxicity, we have revealed compounds that inhibit autophagy.13,14) Furthermore, we demonstrated the adverse effects of gadolinium toxicity,15,16) and the correlation between allergies and MeHg.17) Additionally, we investigated the influence of highly unsaturated fatty acids on the toxicity of MeHg,18) as well as the impact of MeHg on adipocyte differentiation19) and the toxicity resulting from trace inorganic Hg converted from MeHg,20) in order to unravel the nature of MeHg toxicity and the cellular protective response against MeHg toxicity. Subsequent investigations are warranted to elucidate the specific molecular responses triggered by MeHg exposure.
Severe neurological disorders occur in victims of MeHg poisoning. Several studies have evaluated the effects of toxic or subtoxic doses of MeHg in vitro and in vivo. However, the molecular responses and effects of low doses of MeHg to which humans are exposed through daily food intake are not well-understood, and there is a demand for anti-MeHg medicines especially during pregnancy. Therefore, we evaluated the efficacy of various synthetic oleanane-type saponin derivatives in alleviating MeHg toxicity, using in vitro and in vivo models. We synthesized a saponin derivative in which one glucose molecule was bound to the oleanolic acid (OA) skeleton, i.e., a simplified onjisaponin structure (Fig. 2). The synthetic OA-3-glucoside (OA3Glu), in which glucose was bound to the C3 position of OA, suppressed MeHg-induced cell death in Caco-2 colon carcinoma cells and mercury accumulation in the liver, kidney, and brain of MeHg-exposed mice. These results suggested that OA3Glu would be a potential anti-MeHg toxicity compound.21,22) Recently, we exposed mice to MeHg at a higher concentration than the previous reports and verified the effect of OA3Glu, as an anti-MeHg toxicity compound especially focusing on dynamic weight bearing test and electrophysiology in MeHg-exposed mice. OA3Glu can alleviate MeHg-induced Purkinje cell death and synaptic damage (unpublished data). Therefore, we propose OA3Glu as a candidate agent against MeHg toxicity. Currently, further research is in progress to unveil pharmaceutical interventions targeting MeHg, in order to improve the overall quality of life.

Structure of Onjisaponin and Oleanolic Acid 3-Glucose in Which One Glucose Molecule Was Bound to the Oleanolic Acid Skeleton, i.e., a Simplified Onjisaponin Structure.
Mercury compounds have a strong affinity for biological components, especially the thiol groups of proteins, and thus exhibit strong cellar toxicity. However, in mercury-contaminated areas such as Minamata Bay, there are mercury-resistant bacteria that have survived the toxicity of mercury.23) Pseudomonas strain K-62 (P. strain K-62) is a mercury-resistant bacterium isolated from soil heavily contaminated with phenylmercury acetate by Tonomura et al., 24–27) and shows the strongest mercury-resistance among the bacteria isolated in the world so far. The mercury resistance mechanism of P. strain K-62 was analyzed genetically in detailed. First, we showed that P. strain K-62 harbors six plasmids (82, 68, 56, 31, 26, and 8.5-kb), of which the 26-kb (pMR26) and 68-kb (pMR68) plasmids are responsible for mercury resistance in this strain28). Next, we cloned the mercury-resistant (mer) operons on pMR26 and pMR68 and analyzed their structure and function. pMR26 was found to contain two sets of mer operons responsible for the degradation of organomercurial compounds, consisting of merR-o/p-merT-merP-merA-merG-merB1 and merR-o/p-merB2-merD.29,30) This mer operon consists of the regulator (MerR), which controls the expression of structural genes, the operator promoter (o/p), the mercury binding protein (MerP), which is involved in binding to mercury in the periplasm, the transporter (MerT),31,32) which is involved in membrane permeation of mercury, the phenylmercury uptake inhibitor protein (MerG),33) organomercurial lyases (MerB1, MerB2),34) which degrade organomercurials (R-Hg+) to mercuric ions (Hg2+), mercuric reductase (MerA), which reduces mercuric ions to metallic mercury (Hg0), and operon terminal promoter (MerD). Additionally, we found a mer operon on pMR68 consisting of three clusters: merR-orf4-orf5-o/p-merT1-merP1-merF-merA-merB1, merT2-merP2, and merB2.35)
Generally, mercury-resistance of bacteria is controlled by the mer operon present on the plasmid or transposon. Hg2+ outside the bacteria binds to MerP on the periplasm and is then taken up by MerT on the inner membrane of the bacteria. R-Hg+, including MeHg and phenylmercury, is degraded to Hg2+ by MerB, and then reduced by MerA to Hg0, which is released outside the bacteria to achieve mercury resistance. As described above, the mercury resistance genes on the pMR26 and pMR68 mer operons of P. strain K-62 were unique in sequence order compared to other resistant bacteria and were substantialiy greater in both variety and number. Notable among these is the harboring of multiple isozymes of the mercury transporter (MerT, MerP), and metabolic enzymes (MerB, MerA), which may support the high mercury resistance of this microorganism (Fig. 3).
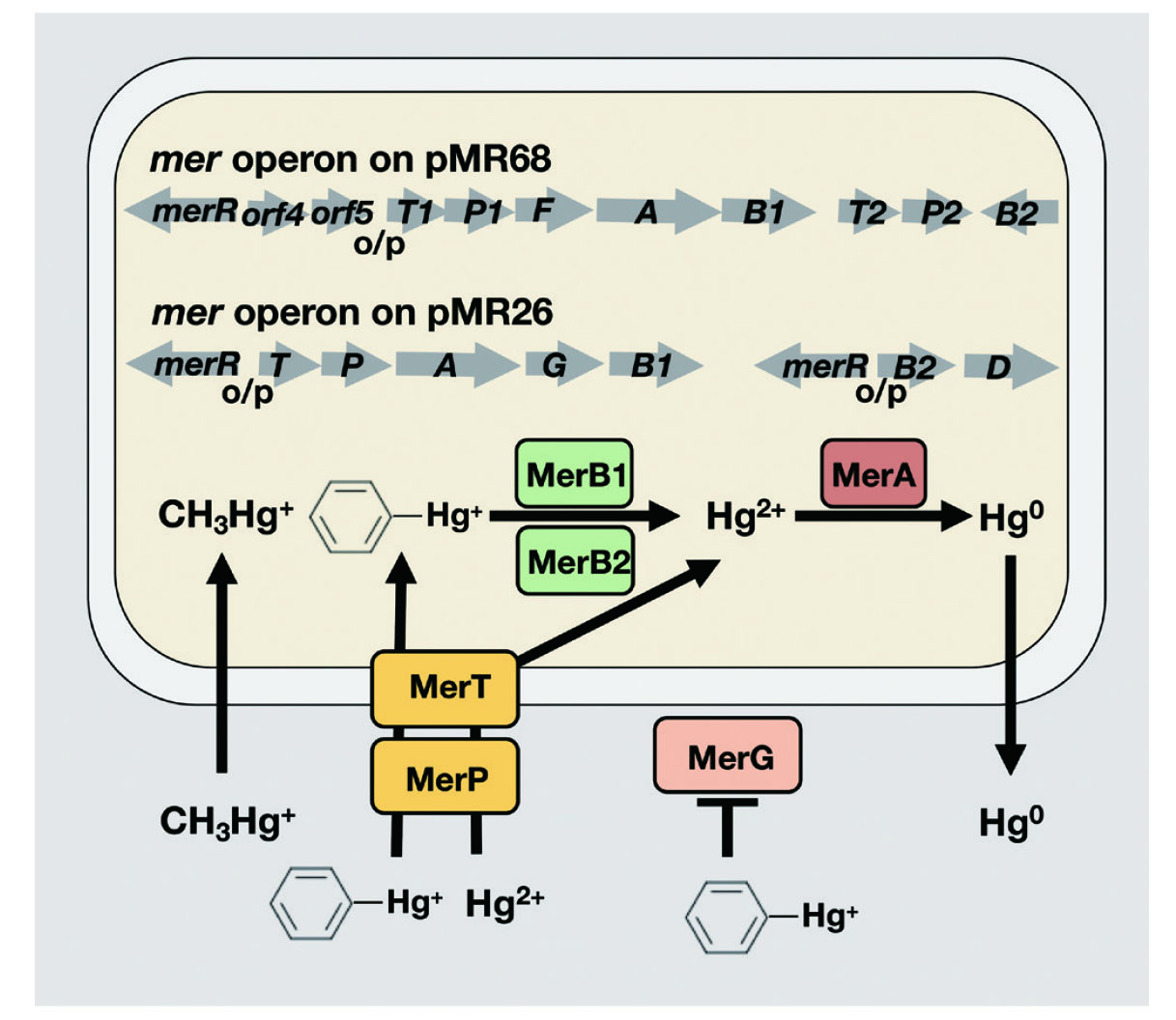
Organization of the Mer Operon on pMR68 and pMR26 and a Schematic Model of the Mercury-Resistant System in Pseudomonas Strain K-62.
MerA: mercuric reductase, MerB: organomercurial lyase, MerG: reducing in-cell permeability to phenylmercury, MerP: mercury-binding protein, MerT: mercury transporter.
Among various toxic metal-resistant bacteria, no transporter has been found to actively uptake toxic metals into the bacteria except for the mer operons. For example, arsenic-, lead-, and silver-resistant bacteria have pumps that exude these toxic metals from the bacteria, and this is how they acquire resistance.36,37) The mechanism of mercury metabolism and volatilization or evaporation of various mercury forms into Hg0 is advantageous for the resistant bacteria and may have developed a different mechanism from that of other toxic metals.
Next, we focused on MerT, which is a unique molecule among various toxic metal-resistant bacteria that has the activity to transport mercury from the outside to the inside of the bacteria, and examined its potential use for environmental remediation. In addition to MerT, other mercury transporters identified in mercury-resistant bacteria include MerC, MerE, and MerF. We have previously shown that MerE is a mercury transporter and transports Hg2+ and MeHg,38–40) and MerT transports phenylmercury.32,41) However, the functions of these transporters remained unclear. In other words, it was not clear which transporter was most suitable for use in environmental remediation. Therefore, we investigated the transport activity of these mercury transporters MerC, MerE, MerF, and MerT for Hg2+ and phenylmercury. The results showed that MerE, MerF, MerT, and MerC all have Hg2+ and phenylmercury transport activity and could be used for their remediation.42) However, focusing on the transport of MeHg and toxic metals such as Cd, MerC was found to be the most active of the four mercury transporters from these microorganisms.43,44) These results indicate that MerC is the best transporter for its application for toxic metals remediation.
Physicochemical methods have been widely used for remediation of soil contaminated with toxic metals. For instance, soil dressing (removing contaminated soil, replacing it with non-contaminated soil) is one of the examples. While this method has the advantage of relatively rapid and radical decontamination, it has the disadvantage of high cost and damages to the target environment and ecosystems. Another issue of the soil dressing is disposal of the removed contaminated soil. Alternatively, a biological method called bioremediation has been attracting attention as a technology to compensate for the shortcomings of the physicochemical method. Bioremediation is a technology that generally utilizes biological functions to restore contaminated environments. This technology is considered to be relatively low-cost and effective for low-concentration and widespread contamination that is difficult to deal with by physicochemical treatment. Recently, phytoremediation technology that uses the physiological functions of plants has been attracting attention among these bioremediation technologies.45–48) While this method has advantages from economic and environmental aspects, it also has the disadvantage of a lengthy remediation time compared to the soil dressing. In order to overcome this disadvantage and to make the remediation technology of toxic heavy metals using biological functions more reliable, it is necessary to manipulate plant metal uptake and transport systems.
In the generation of plants suitable for phytoremediation of toxic metals, two important processes should be manipulated: (1) the uptake transport capacity of heavy metals in plant roots and (2) sequestration of cytosolic heavy metals into vacuoles, an acidic component of plant cells to reduce the toxicity of heavy metals in the cells. The advantage of this system is that the toxic metals absorbed from soils are sealed in the plant vacuoles, and there is little risk of re-contamination of the surrounding environments.
Based on this strategy, we attempted to develop a new environmental remediation technology by molecular breeding of plants expressing MerT and found that it has remediation activity for heavy metals, such as mercury and cadmium in soil as well as in water systems.49,50) We also reported phytoremediation of mercury using MerC and MerE, which have strong mercury transport activity.51–55) We have developed transgenic plants with two different concepts. First, a MerC was fused with a plant endogenous plasma membrane localization factor SYP121 to regulate subcellular localization of the heterilogously expressed bacterial transporter on the plant plasma membrane to facilitate plant heavy metal transporters (concept 1). Second, a fusion of MerC and a plant endogenous vacuolar membrane localization factor AtVAM3 is used to localize the transporter on the vacuolar membrane and facilitate the sequestration of heavy metals into the vacuole (concept 2). Five MerC transgenic Arabidopsis plants, four MerC-SYP121 transgenic plants, and three MerC-AtVAM3 transgenic plants were established.53) Representative lines C10 (MerC transgenic plant), CS17 (MerC-SYP121 transgenic plant), and CV11 (MerC-AtVAM3 transgenic plant), which retained each target gene in the genome and stably expressed mRNA in the presence of Hg2+, were selected.55) C10, CS17, and CV11 showed higher resistance than wild type Arabidopsis plants, respectively. CS17 also showed higher resistance than C10 and CV11. As for mercury accumulation, at low concentrations, CS17 showed higher accumulation than the wild-type plants, and at high concentrations, C10, CS17, and CV11 showed higher accumulation than the wild-type plants.55) These results suggest that phytoremediation using AtVAM3 and SYP121 is a suitable technique for the retrieval of mercury compounds.
These MerC-expressing plants have used ubiquitous strong expression promoters, but recently, we have been working on the application of Arabidopsis cell type-specific promoters to achieve advanced regulation of MerC expression in the plant56–58) (Fig. 4). Specifically, MerC-SYP121 expression was restricted to the plasma membrane of epidermal or endodermal cells by using a promoter specific to epidermal (pEpi) or endodermal (pSCR) cells, which are important cell types in metal absorption by the root. As a result, although the expression of MerC-SYP121 in the whole root was low, the absorption efficiency of mercury was increased to the same level as in the ubiquitous expression system, and there was also promotion of the transfer of mercury to the aboveground tissue.56,57) Furthermore, expression of MerC-AtVAM3 on the vacuolar membrane, which functions in vacuolar sequestration of cytosolic mercury was targeted by using a mesophyll cell-specific promoter (pRBCS1A). As a result, there was a success in conferring mercury tolerance while avoiding the trapping of mercury in vacuoles in root cells.58) We are currently testing the applicability of these cell-type-specific MerC-expressing plants to cadmium59) and arsenic pollution, and are working to enhance environmental remediation efficiency by pyramiding these different cell-type-specific MerC expression systems.
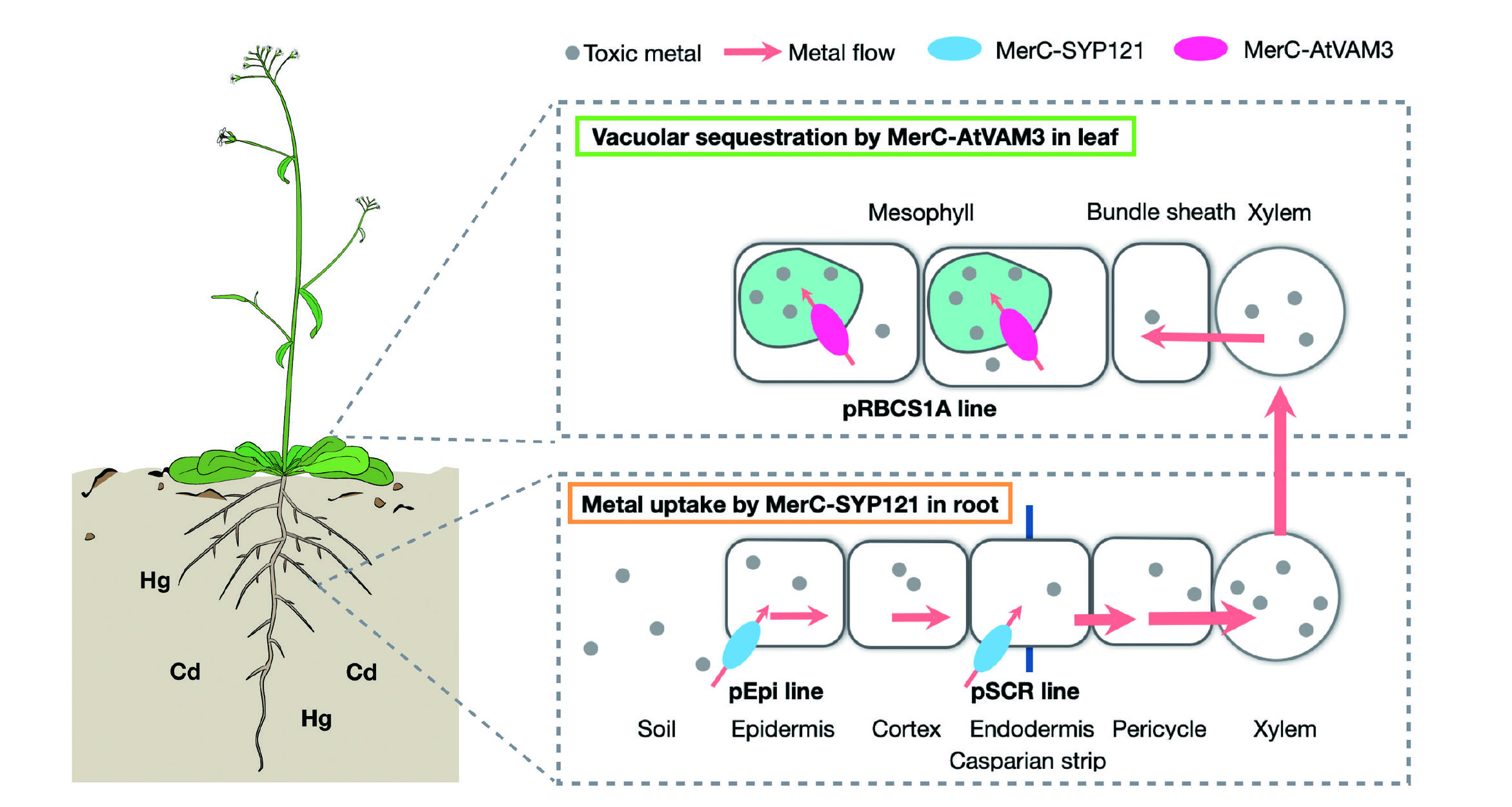
Schematic of the Engineering of Plant Toxic Metal Transport Using the Bacterial Mercury Transporter MerC.
In roots, MerC is expressed as a fusion protein with SYP121, a plant plasma-membrane resident syntaxin, and is localized to the plasma-membrane to facilitate toxic metal uptake by roots. An epidermis-specific promoter (pEpi) and an endodermis specific promoter (pSCR) are used to regulate the MerC-SYP121 expression to epidermis and endodermis, respectively. Metal uptake and translocation to the shoot are facilitated by the MerC-SYP121 expression. In leaves, MerC-AtVAM3, which is targeted to the vacuolar membrane is expressed under the control of mesophyll specific promoter pRBCS1A. MerC-AtVAM3 sweeps out cytosolic toxic metals into the vacuole, an acidic storage organelle to protect the cells. Vector graphics illustration of an Arabidopsis plant is adapted, with permission, from Figshare (Bouché, 2018).
In addition to the phytoremediation research, we have been working on the topics of toxic metal toxicity in plants from a wide angle: mercury accumulation in plants in mercury-polluted areas in Guizhou, China,60) analysis of cadmium hyperaccumulation and tolerance mechanisms in plants,61–64) and toxic metals and plant phytochelatins65–67) have also been examined.
We would like to further develop the functional analysis of p62 as a defense factor against MeHg and drug discovery research for anti-MeHg drugs for public health. The analysis of the function of mercury transporters and the development of technologies for the phytoremediation of toxic metals can contribute to environmental health. These researches are expected to contribute to the development of environmental hygiene and human public health.
We would like to express our sincere gratitude to the graduate students and postgraduates of the Department of Public Health, School of Pharmaceutical Sciences, Kitasato University, who provided invaluable cooperation in the course of this study. We thank Dr. Hidemitsu Pan-Hou, Professor Emeritus, Faculty of Pharmaceutical Sciences, Setsunan University, and Dr. Naoto Oku, Professor, Teikyo University, for their guidance and support.
Conflict of interestThe authors declare no conflict of interest.