2023 年 6 巻 5 号 p. 166-171
2023 年 6 巻 5 号 p. 166-171
Tranexamic acid exerts various effects on living bodies; however, its effects on asthenopia remain unknown. In this study, an asthenopia-like model was developed and used to investigate the effects of tranexamic acid on asthenopia. Mice were placed in special cages constructed for the test, and continuous irradiation with blue light was applied for 20 days. The tranexamic acid-treated group was orally administered tranexamic acid daily during the test period. Motor activity was measured for 10 days after irradiation, and reactive oxygen species (ROS), plasmin, and transforming growth factor (TGF)-β levels in the ciliary muscle of the mice were measured on the last day. Blue light irradiation induced asthenopia and increased ROS, plasmin, and TGF-β levels. In contrast, tranexamic acid administration improved asthenopia and significantly decreased plasmin and TGF-β levels compared to blue light irradiation alone; however, ROS levels remained unchanged. The study results indicate that blue light irradiation induces asthenopia by activating the ROS/plasmin/TGF-β pathways and that tranexamic acid improves asthenopia by suppressing plasmin production.
Asthenopia symptoms include eye pain, blurred vision, and bloodshot eyes. These symptoms can be alleviated by resting; however, asthenopia is a state in which symptoms cannot be alleviated. Furthermore, asthenopia causes headaches, shoulder stiffness, and nausea.1-4)
Digitalization has progressed with the development of science, and asthenopia (digital eye strain)-induced complications have increased due to the long-term use of desktop laptops, mobile phones, tablets, e-readers, and storage devices.3-5) Furthermore, the spread of COVID-19 has increased the use of these digital devices. Digital eye strain is mainly caused by three factors. The first is dry eye, which is caused by decreased blinking6,7); stress overlap distorts autonomic nerve balances, and the sympathetic nerves become dominant, consequently decreasing parasympathetic nerve-modulated tear secretion and inducing dry eye. The second factor is related to the accommodation function of the eye. For example, blurred vision after computer use and difficulty refocusing when moving the eyes from one distance to another.6-8) The third factor is extraocular symptoms, which include musculoskeletal symptoms such as headache, neck, and shoulder pain.9)
Blue light is a type of visible light abundantly contained in light-emitting diodes (LED) often used in the liquid crystal screens of personal computers and smartphones. Blue light has a short wavelength (380-500 nm) and can not be absorbed by the eye cornea and lens; therefore, it easily reaches the retina. Blue light induces changes, such as asthenopia and sleep disturbance in the life rhythm of the human body. Similar to ultraviolet rays, it damages the eye retina and macula and causes retinal diseases and age-related macular degeneration.10) However, studies have reported that blocking blue light from computer screens does not improve asthenopia symptoms.11) Therefore, in this study, blue light irradiation, which is more suitable for computer and mobile phone screens, was applied and used to investigate the relationship between blue light and asthenopia.
Moreover, we have confirmed that tranexamic acid ameliorates various symptoms.12-14) Therefore, the possible ameliorating effect of tranexamic acid blue light-induced asthenopia was investigated.
Specific pathogen-free (SPF), 8-week-old Institute of Cancer Research (ICR) male mice (SLC, Hamamatsu, Shizuoka, Japan) were used for the experiments. All mice were individually housed in cages at a controlled temperature of 23 ± 1°C under SPF conditions with a 12-h light/dark cycle.
Experiment 1. The mice were randomly divided into six groups, with six mice in each group. They were placed in a scattered reflection room (Fig. 1) with blue or white light as the ceiling. The upper and lower halves of the sidewalls were covered with mirrors to reflect light and painted black to absorb and eliminate light, respectively. A fluorescent lamp was used as the light source with LED blue light (wavelength:380-500 nm, 47.5 W/m2, ISLM-150X150-BB, CCS Inc., Kamikyo-ku, Kyoto, Japan) or LED white light (12.2 W/m2, ISLM-150X150-HWW, CCS Inc.). Blue or white light was applied for 2 h daily. The irradiation method was a 10 minutes-on, 10 s-off irradiation (irradiating each light source for 10 min and stopping the irradiation for 10 s), which was repeated 12 times (Fig. 2). Mice were divided into six groups (non-irradiation group [control], blue light irradiation group for 1 d, blue light irradiation group for five consecutive days, blue light irradiation group for 10 consecutive days, blue light irradiation group for 20 consecutive days, and white light irradiation group for 20 consecutive days, with six animals in each group), and motor activity was measured over time for 10 days after irradiation.
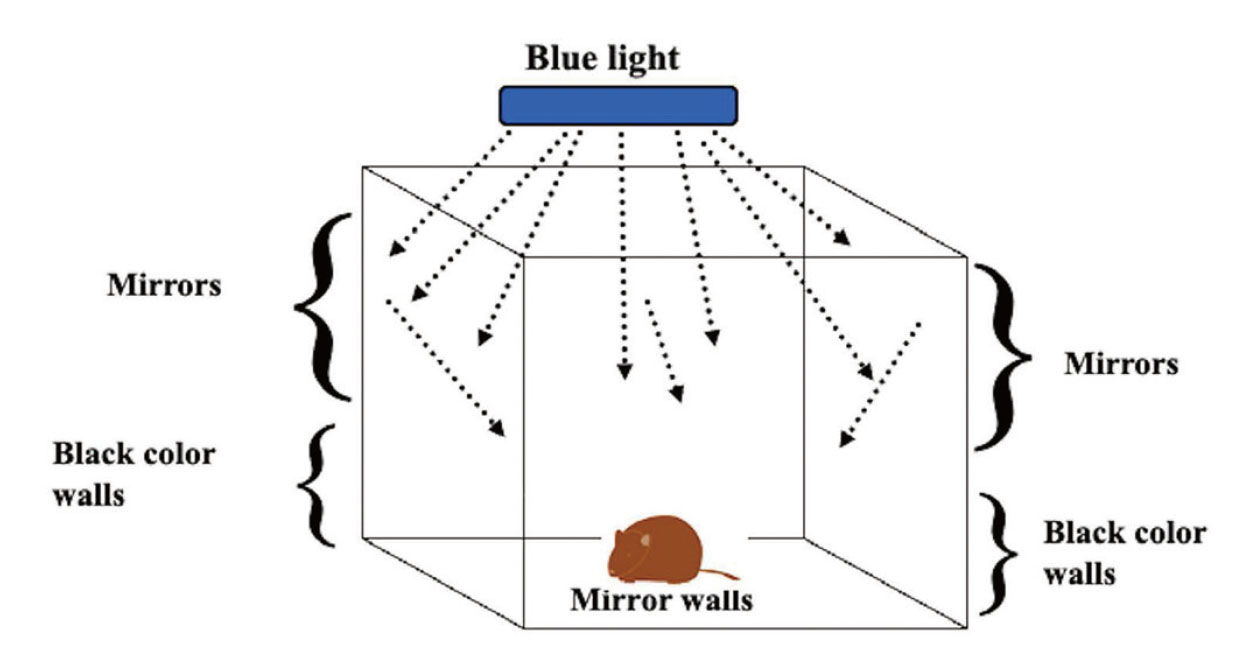
Special Device to Irradiate Animals with Blue Reflection Light
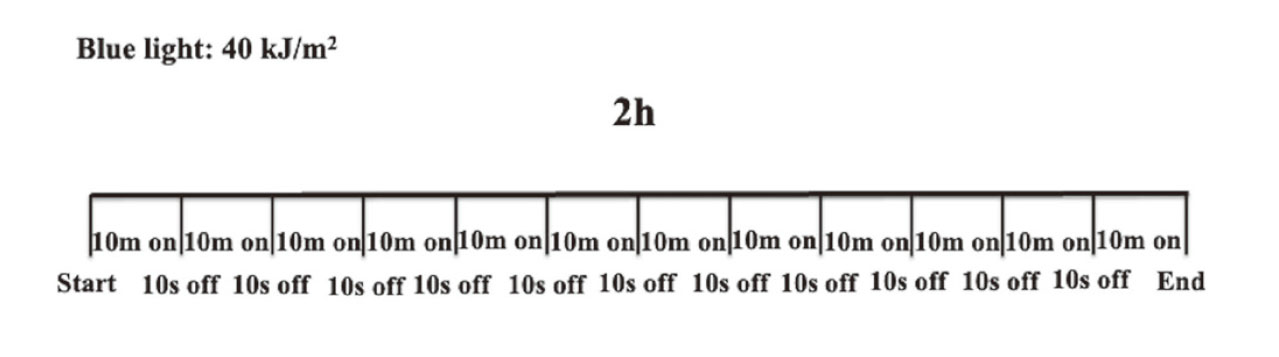
Schematic of the Blue Light Irradiation Method
10m: 10 minutes, 10s: 10 seconds.
Experiment 2. SPF, 8-weeks-old ICR male mice (SLC) were used for the experiment, which was performed using the same apparatuses and irradiation method as that in experiment 1. The mice were divided into three groups (non-irradiation group [control]; blue light irradiation group for 20 consecutive days; blue light irradiation group for 20 consecutive days + tranexamic acid administration group, with 6 animals in each group), and motor activity was measured over time for 10 days after irradiation.
Tranexamic Acid TreatmentThe mice were orally administered approximately 12 mg/kg of tranexamic acid (Daiichi Sankyo Healthcare Co., Ltd., Tokyo, Japan) in distilled water daily during the study period. The solvent-administered animals received distilled water (control group).15)
Open Field TestThe motor activities of the animals were quantified using an open field apparatus (50 cm [W] x 50cm [D] x 40 cm [H]) exposed to light with an intensity of 70 lx to record the total distance traveled by the mice. Data were analyzed for 10 min using a video-tracking system (Smart2 software, Panlab, Barcelona, Spain). The time 0 point was immediately after the final irradiation, and each locomotor activity was measured at 10 a.m., except for measurements 12 h after irradiation.
Measurement of Reactive Oxygen Species (ROS) and Transforming Growth Factor (TGF)-β LevelsPlasma and ciliary muscle samples were obtained on the last day of the experiment. Plasma was separated from blood samples by centrifugation at 3,000 x g for 10 min at 4°C, and the supernatant was obtained for analysis. For the ciliary muscle, 10 mg of ciliary muscle was rinsed in phosphate-buffered saline (PBS) to remove excess blood before homogenization. The samples were centrifuge homogenized at 1,500 x g for 15 min at 4°C, and the supernatant was obtained for assaying. The transforming growth factor (TGF)-β levels in the ciliary muscle and plasma were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits (MyBiosource, San Diego, CA, USA) according to the manufacturer’s instructions. Reactive oxygen species (ROS) levels in the ciliary muscle were also measured using an assay kit (OxiSelectTM In Vitro ROS/RNA Assay Kit, STA-347; Cell Biolabs Inc., San Diego, CA, USA) according to the manufacturer’s instructions. The optical density was measured using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Western Blotting Analysis of the Ciliary MuscleThe ciliary muscle samples were homogenized in a lysis buffer (Kurabo, Osaka, Japan). The homogenates were centrifuged, and the supernatants were obtained. Western blotting was performed as previously described.16) Briefly, the membranes were incubated at room temperature for 1 h with primary antibodies against plasmin (1:1000; GeneTex, Hsinchu City, Taiwan) or β-actin (1:5000; Sigma-Aldrich, St. Louis, MO, USA) as a loading control. The membranes were washed and incubated with horseradish peroxidase-conjugated secondary antibody (Novex, Frederick, MD, USA). Immune complexes were detected using ImmunoStar Zeta reagent (Wako, Osaka, Japan), and images were captured using Multi Gauge Software (Fujifilm, Greenwood, SC, USA).
Statistical AnalysisAll data are presented as mean ± standard deviation (SD) values. Microsoft Excel 2010 (Microsoft Corp., Redmond, WA, USA) was used to analyze the statistical significance of the data, with one-way analysis of variance (ANOVA), followed by Tukey’s post-hoc test using statistical package for the social sciences (SPSS) version 20 (SPSS Inc., Chicago, IL, USA). Results with p values *< 0.05 and **< 0.01 were considered significant.
Using the test device and irradiation method, no recovery of motor activity was observed for 10 days after continuous blue light irradiation for 20 days; however, no decrease in motor activity was observed after continuous white light irradiation for 20 days (Fig. 3). On the other hand, in the group irradiated with blue light once, there was no difference in locomotor activity compared to the non-irradiation group one day after irradiation. In the group irradiated with blue light for 5 consecutive days, there was no difference in locomotor activity compared to the non-irradiation group 2 days after irradiation. Furthermore, the difference between the group irradiated with blue light for 10 consecutive days and the non-irradiation group disappeared on the 5th day after irradiation.
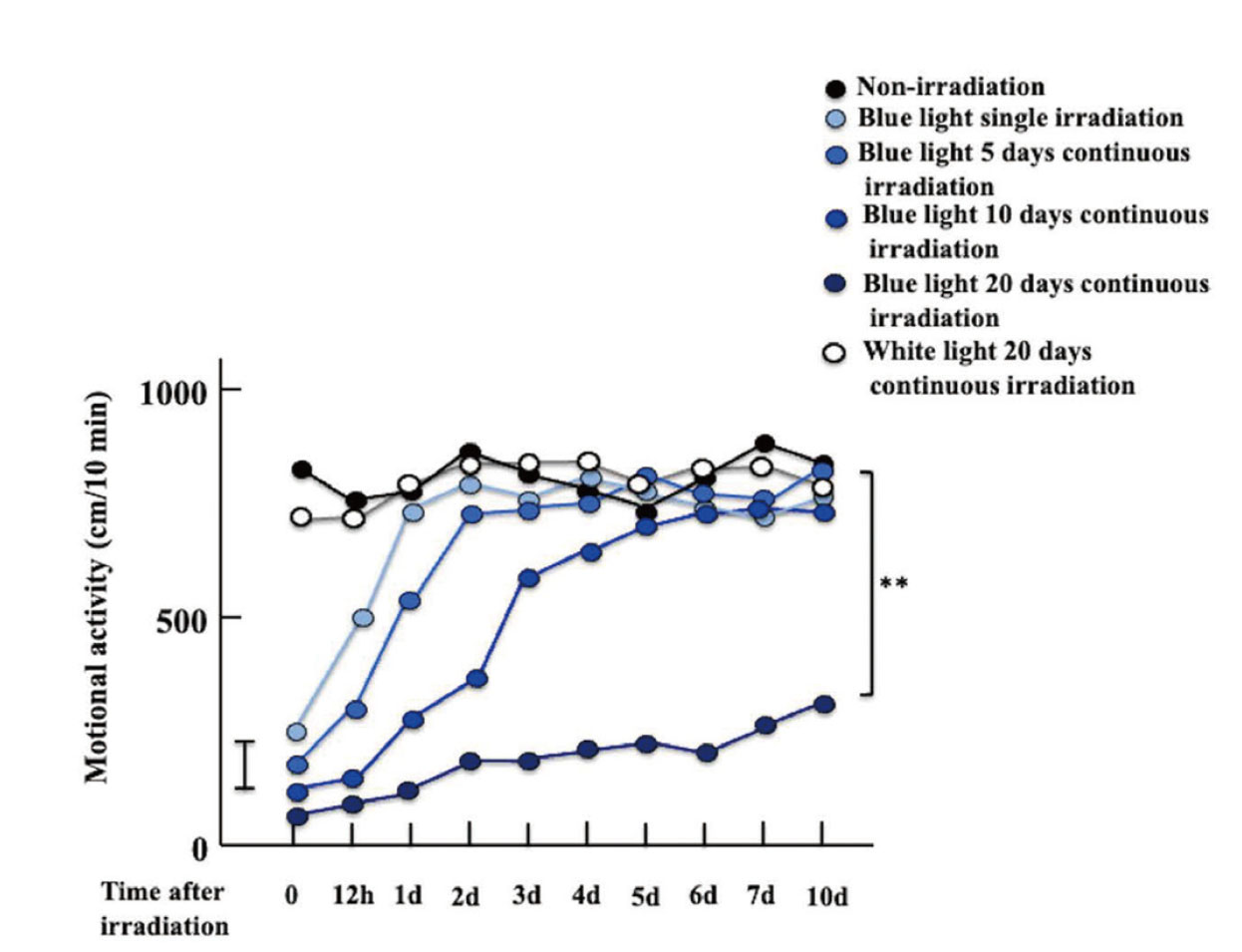
Effect of Blue Light Irradiation Method on Asthenopia
The values are presented as the mean of six animals. Vertical bars represent a pooled SEM of six animals per treatment. Significant differences were compared between the values of each group on the 10th day after irradiation. **p < 0.01. SEM, standard error of the mean.
1. Behavioral effects of tranexamic acid administration in blue light irradiated mice
According to the open field test, motor activity remained downregulated in blue light-irradiated mice for 10 d after irradiation; however, the tranexamic acid-administered mice recovered over time and became the same as the control mice (Fig. 4).

Effect of TA Treatment on Asthenopia in Blue Light Irradiated Mice
The values are presented as the mean of six animals. Vertical bars represent a pooled SEM of six animals per treatment. Significant differences were compared between the values of each group on the 10th day after irradiation. **p < 0.01. TA: tranexamic acid. SEM, standard error of the mean.
2. Effect of tranexamic acid administration on ROS levels and plasmin expression in ciliary muscle in blue light irradiated mice
ROS levels in the ciliary muscle were significantly higher in blue light-irradiated mice than in control mice. The blue light irradiation + tranexamic acid administration mice group was comparable to the blue light irradiation only mice group (Fig. 5a). Moreover, plasmin expression was increased by blue-light irradiation but decreased by tranexamic acid administration (Fig. 5b).
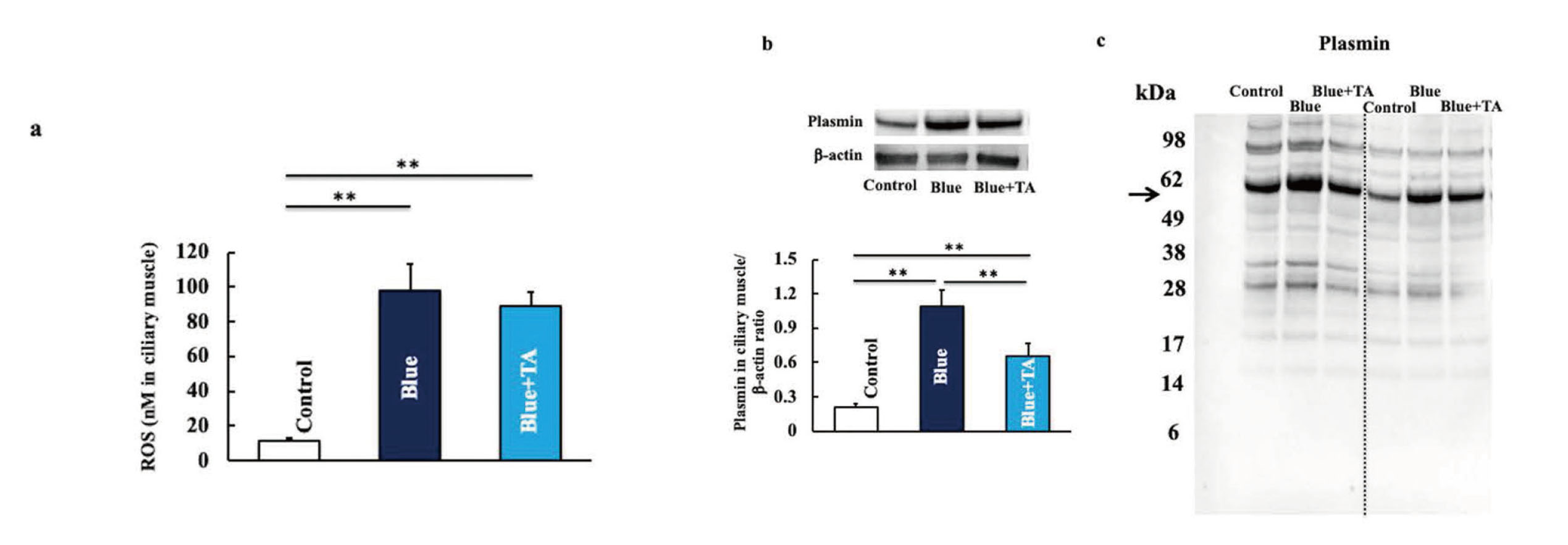
Effects of TA Treatment on ROS Level and Plasmin Expression in Ciliary Muscle in Blue Light Irradiation Mice
Ten days after irradiation, ROS level (a) and plasmin expression (b) were measured. Western blot diagram of plasmin with molecular weight markers (c). The values are presented as mean ± SD of six animals. **p < 0.01. TA, tranexamic acid; ROS, reactive oxygen species.
3. Effect of tranexamic acid administration on ciliary muscle and plasma TGF-β levels in blue light irradiated mice
Ciliary muscle and plasma TGF-β levels were examined; their TGF-β levels increased with blue light irradiation and decreased with tranexamic acid administration (Fig. 6). Furthermore, previous studies have shown that administration of TA alone has no effect on living organisms and shows the same tendency as the control group.14)

Effect of TA Treatment on Ciliary Muscle and Plasma TGF-β Levels in Blue Light Irradiated Mice
Ten days after irradiation, the levels of the ciliary muscle (a) and plasma (b) TGF-β were measured. Note that TGF-β is the total amount. The values are presented as mean ± SD of six animals. *p < 0.05, **p < 0.01. TA, tranexamic acid; TGF-β, transforming growth factor-β.
In this study, an asthenopia-like model was developed. Mice were placed in a newly devised irradiation cage, and a 10 min-on, 10 s-off irradiation was applied 2 h daily for 20 consecutive days; 10 days after irradiation, motor activity continued to decrease, and asthenopia was observed. Therefore, the effect of tranexamic acid on blue light irradiation-induced asthenopia was investigated.
Oxidative stress is induced by blue light irradiation. One type of blue light irradiation-induced oxidative stress is mitochondrial oxidative stress.17-19) Blue light induces lipofuscin accumulation in retinal pigment epithelium (RPE) cells, promoting ROS uptake in mitochondria.20) Furthermore, blue-light-induced RPE cell death is caused by the activation of caspase-3, a cell death-inducing factor.21) Moreover, excessive exposure of retinal cells to blue light causes the aggregation of s-opsin, a type of photoreceptor protein that captures blue light,22) and induces ROS from photoreceptor cells, stressing the cells.22)
ROS induces plasminogen activator-plasmin (PA-Plm) system activation.23,24) Plasmin increases in aged mice25) and with long-term exposure to natural light26) or ultraviolet rays.14) Plasmin is directly involved in macrophage activation. It generally activates M1 macrophages and secretes inflammatory cytokines, such as tumor necrosis factor-α and interleukin (IL)-6 and IL-12, to promote inflammation.27-29) In this study, macrophages activated by plasmin increased by blue light irradiation shifted to the M2 type, and as a result, an increase in TGF secreted by M2 macrophages was observed. This suggests that blue light irradiation may induce M1/M2 polarization conversion in macrophages. The mechanism by which blue light irradiation induces M1/M2 polarization conversion is unknown; however, it has been reported that the conversion mechanism of M1/M2 polarization is regulated by the lysosomal enzyme cathepsin E and its switch, cathepsin B.30,31) Cathepsin B activates nuclear factor kappa B (NF-kB) and shifts to injured microglia (M1 type), whereas cathepsin E exhibits the M2 type. In this study, cathepsin E levels in the ciliary muscle were increased by blue light irradiation, while cathepsin B levels remained unchanged (data not shown). Therefore, cathepsin E and B may regulate macrophages; however, the details are unclear, and further investigation is required. These results suggest that blue light activates the plasmin/M2 macrophage pathway, but administration of tranexamic acid inhibits macrophage activation by inhibiting plasmin, which in turn suppresses TGF-β production.
TGF-β expression is in parallel with fatigue, as fatigue increases with elevated TGF-β levels in the brain.32-34) In this study, TGF-β increased in the ciliary muscle and plasma, indicating the possible fatiguing of the ciliary muscle; this fatigue may reduce the lens accommodative function of the ciliary muscle and induce asthenopia and general fatigue. However, details of TGF-β function in the ciliary muscle are known; therefore, further investigation is required.
In order to examine whether the measured TGF-β was produced by ocular-derived macrophages, we compared a group of mice in which only the eyes were irradiated with blue light and a group in which blue light was irradiated to areas other than the eyes. Blue light irradiation was carried out for 20 days. As a result, a significant increase in blood TGF-β was observed in the group in which only the eyes were irradiated with blue light (Supplementary Fig. 1). This suggested that the TGF-β increased in this study may be of ocular origin. However, more detailed testing is considered necessary.
On the other hand, it has been reported that long-term blue light irradiation increases IL-10 secretion due to a shift to M2 macrophages.35) IL-10 is an anti-inflammatory cytokine and is involved in immunosuppression. Immunosuppression plays an important role in fatigue induced by UV irradiation.36,37) This suggests that IL-10, which increases during blue light irradiation, may cause eye strain. Therefore, it is necessary to consider details regarding IL-10.
In addition, generally, the generation mechanism of plasmin occurs by activating the serine protease plasminogen activator from plasminogen and inducing the conversion of plasminogen to plasmin. In this study, the mechanism by which tranexamic acid reduces plasmin is thought to be as follows. It is thought that blue light induces an increase in plasminogen, but tranexamic acid's inhibitory effect on plasminogen activator may suppress plasmin production.12) However, the effects of blue light on plasminogen and plasminogen activators need to be studied in detail.
It is known that stress and UV irradiation activate the proopiomelanocortin (POMC) pathway and cause fatigue.38,39) The hypothalamo-pituitary-adrenal (HPA) axis derived from POMC is stimulated to secrete adrenocorticotropin hormone (ACTH), which causes the adrenal cortex to secrete corticosteroids. Corticosterone induces immunosuppression and induces fatigue. Irradiation with blue light also increases corticosterone, which may be involved in motor activity (Supplementary Fig. 2). However, it is thought that corticosterone secretion is not sustainable and the state of fatigue (low motor activity) does not continue.36)
The above results can be summarized as follows. Blue light exposure causes oxidative stress in the ciliary body. ROS induced by oxidative stress causes an increase in plasmin. Plasmin is directly involved in macrophage activation. There are M1 and M2 types of macrophages, and upon blue light irradiation, cathepsin E, which is involved in the conversion of M1/M2 polarity, increases and induces M2 macrophages. M2 macrophages showed the possibility of inducing eye fatigue by increasing the fatigue substance TGF-β. It has been suggested that tranexamic acid may reduce TGF-β and improve eye fatigue by suppressing the increase in plasmin involved in macrophage activation (Fig. 7).

Flowchart Showing the Mechanism of the Blue Light Irradiation on Skin Cancer
Overall, the study results suggest that blue light irradiation induces asthenopia via ROS/plasmin/M2 macrophages/TGF-β pathway activation. Moreover, tranexamic acid was found to improve asthenopia by suppressing plasmin production.
This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI program (Grant No. 23K06074).
Conflict of interestThe authors declare no conflict of interest.