2023 年 6 巻 6 号 p. 189-192
2023 年 6 巻 6 号 p. 189-192
Xanthine oxidase (XO) produces reactive oxygen species (ROS) and has been associated with vascular endothelial dysfunction. While the effects of xanthine oxidoreductase (XOR) inhibitors on inhibiting the generation of uric acid from xanthine have been reported, much less is known about their effects on XO-induced ROS. The mechanisms of action of each XOR inhibitor vary, but it is not known whether XOR inhibitors’ effects on oxidative stress also vary. The purpose of this study is to compare the effects of different XOR inhibitors on XO-induced ROS. We used an in vitro chemiluminescence assay with clinically relevant doses of XOR inhibitors (allopurinol, oxypurinol, febuxostat, and topiroxostat) to investigate their effects on circulating XO-derived ROS. All XOR inhibitors significantly inhibited ROS production, with febuxostat and topiroxostat showing strong effects. These results confirm differences in the effects at clinical did among XOR inhibitors on XO, with topiroxostat demonstrating a strong suppression of ROS production. This study should help guide clinical practice in using XOR inhibitors to improve patient care and management.
In patients with diabetes, there is an increase in oxidative stress and an associated overproduction of reactive oxygen species (ROS) that are thought to aggravate vascular disorders.1) Vascular endothelial dysfunction is observed from the early stage of impaired glucose tolerance and is deeply involved in the development and progression of macrovascular disorders.2) There are many possible causes of vascular endothelial dysfunction in diabetes, one of which is xanthine oxidase (XO).3)
Xanthine oxidoreductase (XOR) catalyzes the metabolism of hypoxanthine to xanthine and xanthine to uric acid in the purine metabolic pathway.4) There are two types of XOR, xanthine dehydrogenase (XDH) and XO. If the electron acceptor is nicotinamide adenine dinucleotide (NAD+), the XOR is XDH. If the electron acceptor is oxygen, then the XOR is XO. XO produces ROS, such as superoxide and hydrogen peroxide along with uric acid, which have been shown to cause tissue damage.5) Thus, it is important to suppress XO-induced oxidative stress to prevent this damage.
XOR inhibitors, such as allopurinol, febuxostat, and topiroxostat are used for the management of hyperuricemia or gout. Although allopurinol administration improves vascular endothelial function in humans, this improvement is not specifically related with the drug’s uric acid-lowering effects.6) Furthermore, it was found that stimulating uric acid excretion does not improve vascular endothelial function.7)
While the effects of XOR inhibitors on inhibiting the generation of uric acid from xanthine have been reported,8) much less is known about their effects on XO-induced ROS. Furthermore, the mechanisms of action of each XOR inhibitor vary, but it is not known whether XOR inhibitors’ effects on oxidative stress also vary.9-11) Therefore, the purpose of this study is to compare the effects of different XOR inhibitors on XO-induced ROS.
Hypoxanthine and XO (from cow’s milk) were purchased from Sigma Aldrich (St. Louis, MO, USA). Allopurinol, oxypurinol (the major metabolite of allopurinol), superoxide dismutase (SOD; from bovine erythrocytes), and 8-amino-5-chloro7-phenylpyrido [3,4-d] pyridazine-1,4-(2H,3H) dione sodium salt (L-012) were purchased from Wako Pure Chemical Industries (Osaka, Japan). Febuxostat was purchased from Toronto Research Chemicals (North York, ON, Canada). Topiroxostat was provided by Sanwa Kagaku Kenkyusyo (Inabe, Japan). All other reagents used were commercially available.
Chemiluminescence Measurement of Superoxide Generated by the Hypoxanthine–Xanthine Oxidase SystemTo study the effect of XOR inhibitors on superoxide production, the hypoxanthine–xanthine oxidase system was applied. A reaction mixture containing a solution of L-01212) (100 μM), ethylene diamine tetra acetic acid (EDTA) (171.4 μM) in Tris–HCl buffer (57.14 mM, pH 7.6), XO (1 mU/mL) in an EDTA solution, and an inhibitor of XO or SOD (200 U/mL) was dispensed into each well of a 96-well microplate. The group to which no drug was added was used as a vehicle.
Chemiluminescence was first measured for 10 min with a luminometer (Glomax®-Multi Detection System, Promega, Madison, WI, USA) to provide a baseline. A reaction was then initiated by adding hypoxanthine (50 μM), and the intensity of the chemiluminescent was continuously recorded for 30 min at 37°C. To determine the chemiluminescence of the sample, the baseline was subtracted from the chemiluminescent signal. The difference was then calculated as the amount of luminescence per minute and finally, the percentage relative to the vehicle was determined.
The concentrations of each drug were set to a range that could cover the maximum blood concentration in healthy adults on the basis of interview forms from recently conducted clinical trials.13-15) Allopurinol, oxypurinol, febuxostat, and topiroxostat were diluted to nine distinct concentrations.16) IC50 values were obtained using GraphPad Prism™ version 7.0 (GraphPad Software Inc. San Diego, CA, USA). Data are expressed as the mean ± standard deviation. Comparisons between groups were analyzed using one-way ANOVA followed by Dunnett’s multiple comparison tests.
All XOR inhibitors significantly inhibited ROS production, as indicated by chemiluminescence against the vehicle. The clinically relevant concentrations of allopurinol, oxypurinol, febuxostat, and topiroxostat were 300 nM, 3 μM, 10 nM, and 3 nM, respectively. These values corresponded to 1.10%, 11.00%, 0.12%, and 0.12% of the maximum concentration (Cmax) for each drug, respectively. (Fig. 1).
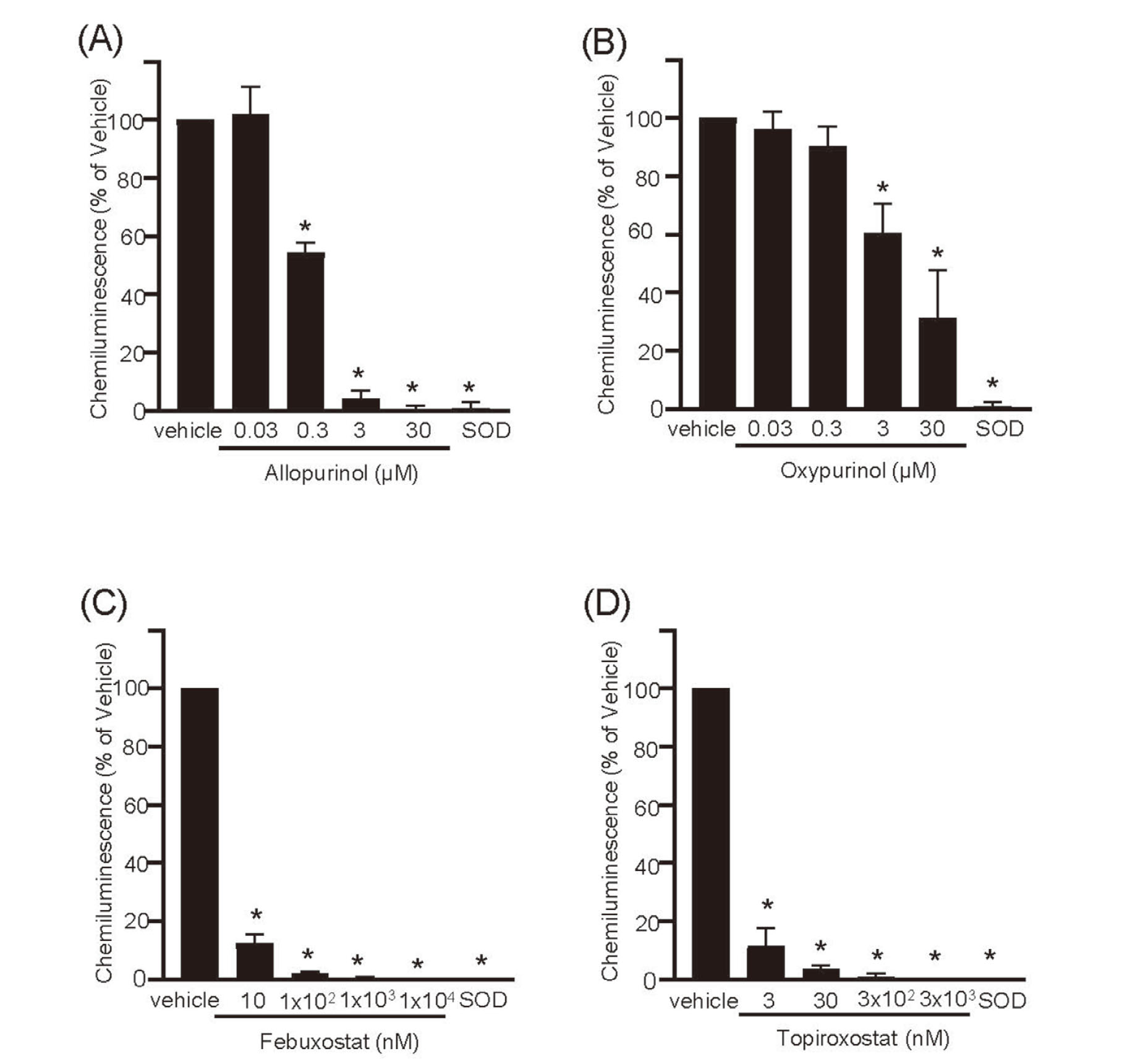
Effects of XOR Inhibitors at Clinical Doses on Reactive Oxygen Species Production by the Hypoxanthine–Xanthine Oxidase System
The evaluated drugs are: (A) allopurinol; (B) oxypurinol; (C) febuxostat; and (D) topiroxostat. Data are shown as the mean ± standard deviation, n = 6. *p < 0.001 vs. vehicle.
The IC50 values were calculated to understand the characteristics of each drug in detail. The concentrations to significantly inhibit ROS production by 50% (IC50) were 0.258 μM for allopurinol, 1.80 μM for oxypurinol, 1.33 nM for febuxostat, and 0.495 nM for topiroxostat (Fig. 2).

IC50 Values of XOR Inhibitors on Reactive Oxygen Species Production by the Hypoxanthine–Xanthine Oxidase System
The evaluated drugs are: (A) allopurinol; (B) oxypurinol; (C) febuxostat; and (D) topiroxostat. Data are shown as the mean ± standard deviation, n = 6.
Previous studies have suggested that XOR inhibitors may inhibit the production of ROS.16) The common measurement for XOR activity is almost uric acid production.17) Although the electron spin resonance method can selectively detect superoxide,18) it is not widely used because of limitations in the detection equipment and the need to be skilled in waveform analysis. In this study, we measured superoxide production by chemiluminescence using L-012.
The results showed that febuxostat and topiroxostat had more potent suppression of ROS production compared with those observed with allopurinol and oxypurinol. These differences are likely the result of their mechanism of action. Allopurinol is metabolized to oxypurinol, which binds only to molybdenum (Mo) (IV) to inhibit XOR.9) Conversely, febuxostat forms strong complexes with both Mo (VI) and Mo (IV) forms of XOR.10) Topiroxostat is a hybrid-type inhibitor that exhibits both structure- and mechanism-based inhibition and has a high affinity for and stable binding to XOR.11) Thus, because electrons emitted from substrate might affect the intramolecular transfer across the three domains of the XOR structure, the higher affinity and stability of topiroxostat may explain its potent antioxidant activity in our study.
ROS generated during uric acid production causes rapid radical reactions in the environment, leading to vascular endothelial and organ damage.19) The differences in organoprotective effects of XOR inhibitors is an important clinical concern.20) In the present study, we confirmed these differences using clinically relevant doses among XOR inhibitors on XO, with topiroxostat demonstrating strong suppression of ROS production. This is supported by previous clinical reports suggesting organoprotective effects of topiroxostat.21-23) The results of our study will be useful when considering the administration of XOR inhibitors in gout and hyperuricemia, as well as their expansion to other diseases.
There are two limitations of this study. First, we could not determine the uric acid concentration of the reaction mixture in our in vitro assay. Second, we used bovine XO, so the effects of XOR inhibitors on ROS derived from human XO should be evaluated in vivo to determine the clinical relevance of our effects. However, the activity of XOR inhibitors was almost applied to purified XO enzymes from bovine milk.
In conclusion, our study demonstrates that XOR inhibitors suppress ROS production in our XO assay system with febuxostat and topiroxostat showing strong suppression.
We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Conflict of InterestChigusa Kikuchi and Tamihide Matsunaga received a research grant from Sanwa Kagaku Kenkyusho. Takashi Nakamura and Takayo Murase are employees of Sanwa Kagaku Kenkyusho. The remaining authors have no conflicts of interest.