2024 年 7 巻 1 号 p. 1-6
2024 年 7 巻 1 号 p. 1-6
Objective: Platinum- and anthracycline-based chemotherapy regimens cause nausea and vomiting in clinical practice, resulting in the deterioration of patients’ QoL, discontinuation of chemotherapy, and reduced therapeutic outcomes. Using pica behavior as an indicator, we aimed to clarify whether anamorelin, an orally active ghrelin receptor agonist, exerts antiemetic effects against cisplatin-induced nausea and vomiting in rats. Materials and Methods: Sprague–Dawley rats were treated with cisplatin (5 mg/kg, i.p.), three-drug or four-drug antiemetics (granisetron [0.3 mg/kg, p.o.], dexamethasone [1.5 mg/kg, p.o.], fosaprepitant [12.5 mg/kg, i.p.], with or without anamorelin [30 mg/kg, p.o.]). Data on kaolin intake, normal food intake, and spontaneous motor activity (SMA) were recorded 1 d before and 5 d after cisplatin administration. Body weight (BW) was measured daily, and the percentage change in BW from baseline was calculated. Results: At the primary endpoint, kaolin intake was significantly higher in the cisplatin-only group than in the pretreatment and vehicle groups (p < 0.05). Additionally, kaolin food intake was not significantly low in cisplatin-treated mice treated with three-drug antiemetics with or without anamorelin. At the secondary endpoints, normal food intake, SMA, and percentage change in BW were significantly lower in the cisplatin-only group than in the vehicle group. Conclusion: Our findings suggest that the prophylactic administration of standard three-drug antiemetics, besides anamorelin, may not improve cisplatin-induced nausea and vomiting. Further studies using methods suitable for evaluating anamorelin levels are required.
Cancer treatment encompasses a multifaceted approach, with chemotherapy, radiotherapy, and surgery being the primary modalities. Among these, platinum- and anthracycline-based chemotherapeutic regimens have proven highly effective in combating cancer; however, these can cause distressing side effects of nausea and vomiting.1) Among these regimens, cisplatin is a chemotherapeutic agent with a high emetic risk, with over 90% of patients treated with cisplatin without antiemetic prescriptions experiencing severe nausea and vomiting.2) Chemotherapy-induced nausea and vomiting (CINV) deteriorates patients’ QoL, often resulting in the discontinuation of chemotherapy and diminished therapeutic outcomes. Consequently, effective use of appropriate antiemetics is critical. The Japanese Society of Clinical Oncology, the American Society of Clinical Oncology, and National Comprehensive Cancer Network guidelines recommend prescribing three-drug or four-drug antiemetics comprising a 5-hydroxytriptamine receptor antagonist, neurokinin 1 receptor antagonist, and dexamethasone, with or without olanzapine, for patients receiving cisplatin.2-5) Olanzapine has recently been approved in Japan as an antiemetic for CINV. Nevertheless, olanzapine cannot be administered to patients with diabetes mellitus due to its propensity to induce hyperglycemia.6) Furthermore, olanzapine-induced somnolence remains a significant clinical concern, impacting the patient’s overall QoL. A systematic review reported that somnolence likely occurs when olanzapine is used in combination with standard antiemetic therapies,7) stressing the need to diversify antiemetics in many patients. However, recent outcomes from the pivotal phase III trials of olanzapine plus conventional antiemetic therapy showed a complete response rate of 67–79% (defined as no emesis and no use of rescue medication) in the delayed phase.8-11) This suggests that there are also instances where some patients cannot tolerate olanzapine, experience discomfort due to olanzapine-induced somnolence, or require novel antiemetic therapies.
A randomized phase II study reported improvements in appetite, nausea, and vomiting in patients undergoing cancer chemotherapy when Rikkunshi-to, a traditional Japanese herbal medicine (Kampo medicine), was administered with regular antiemetic treatment.12) Rikkunshi-to exhibits effects similar to ghrelin.13) Additionally, CINV tends to occur more frequently in patients with low ghrelin levels as opposed to those with high levels.14) Accordingly, we focused on anamorelin, a ghrelin receptor agonist and appetite-stimulating drug approved for use in Japan in 2021. Ghrelin receptors are present in the pituitary gland and the hypothalamus, where they stimulate appetite. The pituitary gland releases growth hormones that cause the liver to secrete insulin-like growth factor 1,15) which contributes to muscle anabolism and muscular weight gain.16)
The primary objective of the present study was to determine whether anamorelin, a ghrelin receptor agonist, exerts antiemetic effects against cisplatin-induced nausea and vomiting. Currently, anamorelin is the only drug indicated for treating cancer cachexia. If anamorelin exhibits additional antiemetic effects compared with the standard three-drug antiemetic regimen in this study, it is anticipated to broaden its potential indications and facilitate the development of novel antiemetic therapies through drug repositioning. The experiments were conducted on rat models. Rodents, such as rats and mice, do not commonly exhibit vomiting tendencies.17) However, they may display pica behavior as a compensatory behavior, wherein they consume non-nutritive substances.18) When rodents are exposed to highly emetogenic anticancer drugs or radiation, they feed on kaolin, a food containing non-nutritive substances. Ondansetron, a 5-hydroxytriptamine receptor antagonist, reportedly alleviates this pica behavior.19) Based on the study findings, we aimed to elucidate whether standard three-drug antiemetics combined with anamorelin demonstrates antiemetic effectiveness against cisplatin-induced nausea and vomiting in rats, with pica behavior serving as a key indicator.
Male Sprague-Dawley rats aged 5–6 weeks (150–200 g) were obtained from Sankyo Laboratory Service Co. Ltd. (Shizuoka, Japan). They were individually housed in a wire-bottom room with a regular light/dark cycle (lights on 8:00 AM–8:00 PM), stable temperature (approximately 25°C), and stable humidity (approximately 50%). They were provided water, normal food pellets, and kaolin food pellets ad libitum.
Drugs and ReagentsCisplatin was purchased from Sigma–Aldrich (St. Louis, MO, USA). The fosaprepitant dimeglumine was obtained from Cayman Chemical Co. (Ann Arbor, MI, USA). Dexamethasone was obtained from Nacalai Tesque, Inc. (Kyoto, Japan). Granisetron hydrochloride was obtained from the Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Anamorelin hydrochloride was obtained from Toronto Research Chemicals, Inc. (Toronto, ON, Canada). Standard laboratory chow pellets (CE-2) were obtained from CLEA Japan Inc. (Tokyo, Japan). Kaolin pellets (K50001) containing 1% (w/w) acacia gum were obtained from Research Diets, Inc. (New Brunswick, NJ, USA).
Experimental ProcedureCisplatin and fosaprepitant dimeglumine were dissolved in saline immediately before administration and administered intraperitoneally at doses of 5 and 13 mg/kg, respectively. Dexamethasone, granisetron hydrochloride, and anamorelin hydrochloride were dissolved in distilled water and orally administered at doses of 1.5, 0.3, and 30 mg/kg, respectively. The cisplatin dose was determined in accordance with that used in a previous study.20) Doses of antiemetics were calculated using equivalent human doses. Based on a previous report, the experiment was conducted after an acclimatization period from Day -6 to 1 h before cisplatin administration and an observation period of 5 d after cisplatin administration.21) Figure 1 shows the experimental schedule. Day 1 spanned from 4:01 PM immediately following cisplatin administration to 4:00 PM the following day. The subsequent observation periods were designated as Days 2 to 5. Day 0 was defined as the day preceding cisplatin administration. Fosaprepitant dimeglumine (i.p.) and saline were administered at 3:00 PM on Day 0 (1 h before cisplatin administration). Granisetron hydrochloride (p.o.), dexamethasone (p.o.), anamorelin hydrochloride (p.o.), and distilled water (p.o.) were administered at 3:30 PM on day 0 (30 min before cisplatin administration). Cisplatin (i.p.) and saline were administered at 4:00 PM on day 0. The rats were divided into four groups, with 12 rats per group. They were defined as vehicle, cisplatin-only, three-drug regimen, and four-drug regimen.
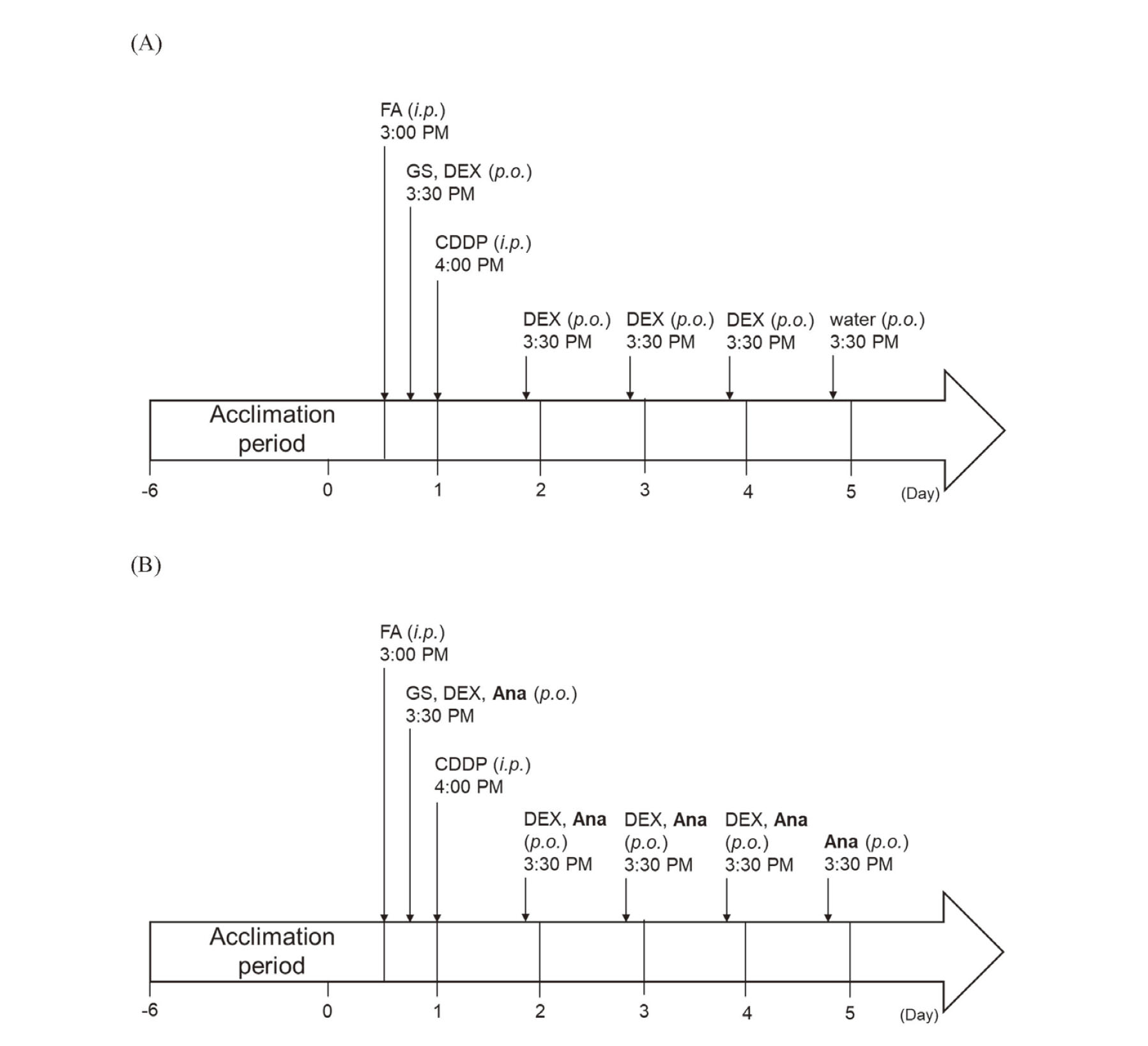
Experimental Schedule of Cisplatin Treatment Along with (A) Three-Drug and (B) Four-Drug Antiemetics
FA = fosaprepitant; GS = granisetron; DEX = dexamethasone; CDDP = cisplatin; Ana = anamorelin.
At the end of the experiment, the rats were humanely euthanized using carbon dioxide inhalation. Each rat was evaluated after only one round of treatment and examination before euthanasia.
EthicsAll the experiments were approved by the Laboratory Animal Committee of Keio University (Approval No. A2022-261). All the experiments were performed in accordance with the Proper Conduct of Animal Experiments Guidelines issued by the Science Council of Japan.
EndpointsFigure 2 shows the automatic measurement system. The rats were allowed to acclimatize to the rearing environment for 7 d prior to cisplatin administration. They were housed in cages with dimensions 180 mm [width] × 240 mm [depth] × 200 mm [height], equipped with a food intake measurement device. Normal food intake, kaolin food intake, and spontaneous motor activity (SMA) were automatically measured on the day before and five consecutive days after cisplatin administration using a telemetry system for rats (FDM-700SW with a controller [cFDM-CTL], software [Feedam], and activity sensor [AS-10]; Melquest Ltd., Toyama, Japan). Normal and kaolin food intake was monitored every 1 h to the nearest 0.01 g, and the data were stored and analyzed using a laptop computer. The results are presented as cumulative daily amounts (g) for up to 5 d after cisplatin administration. Kaolin pellets were occasionally dropped outside the food box by the rats during feeding, which were collected and weighed. The SMA was monitored every 1 h to one count, and the data were stored and analyzed using a laptop computer. The results are presented as cumulative daily amounts (counts) for up to 5 d after cisplatin administration. Body weight (BW) was measured and subtracted from the measured data. The percentage change in BW was calculated as the weight on each day divided by BW on day 0.
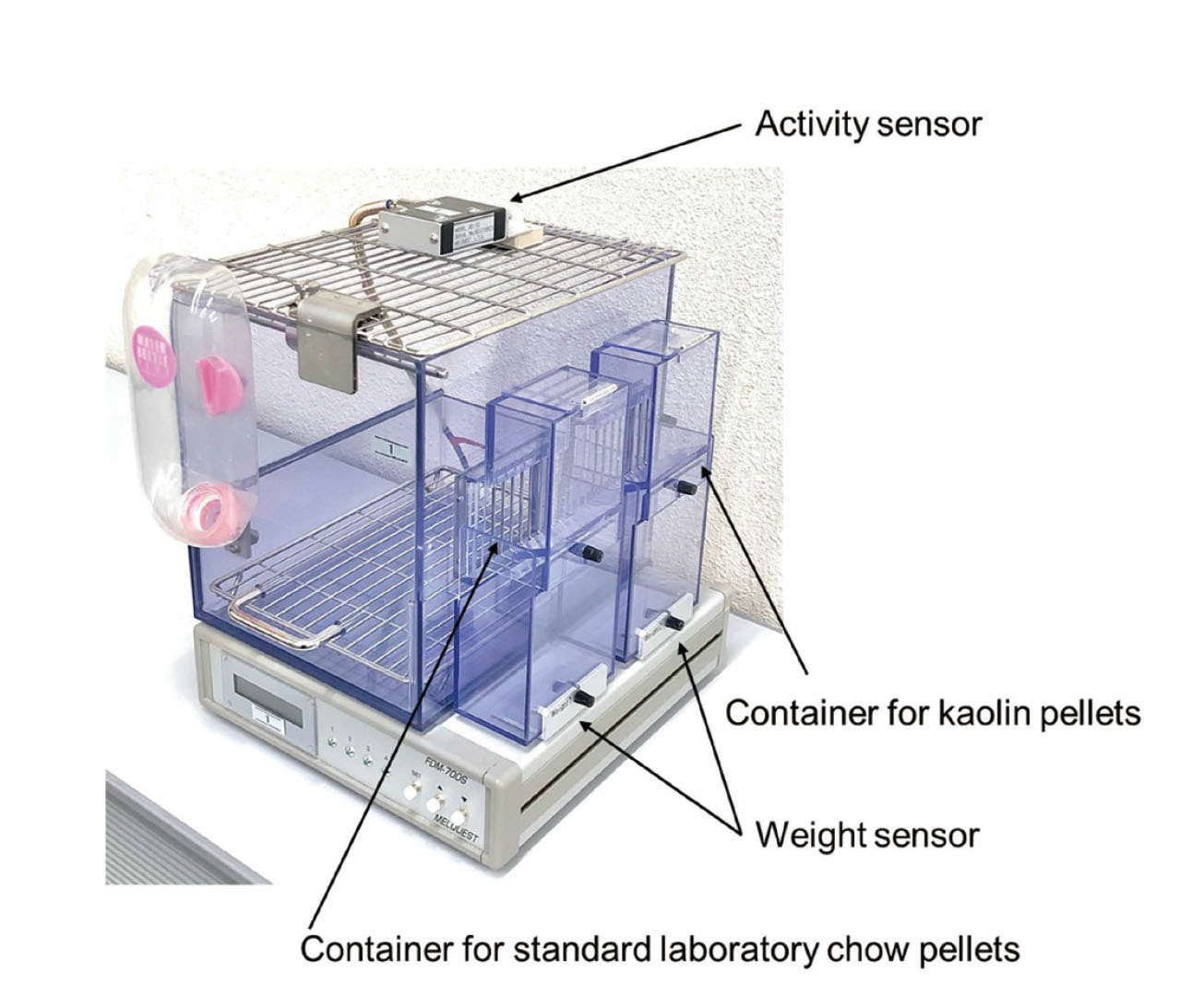
Automatic Measurement System for Rats
Two containers for kaolin and standard laboratory chow pellets, an activity sensor for SMA, and a controller equipped with two weight sensors. Kaolin and standard laboratory chow pellets were provided in their respective containers facing the housing cage.
The primary endpoint was kaolin intake. The secondary endpoints were normal food intake, SMA, and changes in BW during the 5 d following cisplatin administration.
Statistical AnalysisData are shown as mean ± standard deviation. Comparisons with day 0 in the same group and the following day were performed using one-way ANOVA followed by Dunnett’s multiple comparison test. Intergroup comparisons on the same day were performed using a two-way ANOVA, followed by Tukey’s multiple comparison test. GraphPad Prism 9 (GraphPad Software Inc., San Diego, CA, USA) was used for the statistical analyses and graphing. All p-values were two-sided, and statistical significance was set at a p-value < 0.05.
As shown in Fig. 3 (A), kaolin intake was significantly higher in the cisplatin-only group than in the vehicle-only group on days 1, 4, and 5. The three-drug regimen group showed a significant increase in kaolin food intake on day 1 compared to the vehicle group, and the differences faded after Day 2. In contrast, similar to the three-drug regimen group, the four-drug regimen group significantly increased kaolin food intake compared to the vehicle group on day 1 alone.

Effects of Treatment with Vehicle, Cisplatin-Only, and Three-Drug and Four-Drug Antiemetics on (A) Kaolin Intake, (B) Normal Food Intake, (C) SMA, and (D) Change in BW in Rats on the Day Before and Five Consecutive Days After Cisplatin Administration
Data are expressed as mean ± standard deviation (n = 12 per group). *p < 0.05 and **p < 0.01 for the comparison with day 0 in the same group (one-way ANOVA followed by Dunnett’s multiple comparisons test). †p < 0.05, ‡ p < 0.01, #p < 0.05, and ##p < 0.01 for the comparison with the vehicle group and the cisplatin only group respectively (two-way ANOVA followed by Tukey’s multiple comparisons test). 3-drug, granisetron, fosaprepitant, and dexamethasone; 4-drug, granisetron, fosaprepitant, dexamethasone, and anamorelin; BW, body weight; SMA, spontaneous motor activity
The three-drug and four-drug regimen groups showed significantly decreased kaolin food intake on days 4 and 5 compared to the cisplatin-only group (p < 0.05). Differences in kaolin food intake were not observed between the three-drug and four-drug regimen groups.
Secondary EndpointAs shown in Fig. 3 (B), all groups consumed approximately 20–23 g of normal food at baseline. Cisplatin administration significantly decreased normal food intake uniformly in the cisplatin-only, three-drug, and four-drug regimen groups compared to day 0 and the vehicle group. This phenomenon persisted throughout the entire 5 d observation period, and no restoration of normal food intake occurred.
Figure 3 (C) illustrates the SMA for all the groups. All groups exhibited between 16,000 and 18,000 counts of SMA on day 0. Vehicle administration significantly inhibited SMA on day 1 compared with day 0. In contrast, the decreased counts recovered after day 1. Cisplatin administration significantly inhibited SMA on day 1 compared with that on day 0 and showed an upward trend starting on day 2, but not to the same extent as that in the vehicle group. The three-drug regimen group showed a decrease in SMA counts on day 1, which significantly diminished from day 3. The four-drug regimen group showed significant inhibition of SMA from day 1 compared to day 0. Furthermore, the decrease was significant from day 3 in the four-drug group compared to that in the vehicle group (p < 0.05).
Figure 3 (D) illustrates the percentage change in BW. The BW of the vehicle group increased significantly on day 2 compared to that on day 0 (p < 0.01). BW in the cisplatin-only group remained unchanged, and weight gain was significantly inhibited compared to that in the vehicle group. Both the three-drug and four-drug regimen groups showed a significant decrease in BW compared to that on day 0 and the cisplatin-only group.
Platinum- and anthracycline-based chemotherapy regimens cause nausea and vomiting in clinical practice, resulting in the deterioration of patients’ QoL, discontinuation of chemotherapy, and reduced therapeutic outcomes. Ghrelin or agents that enhance the effects of ghrelin may enhance its antiemetic effect, and anamorelin, a ghrelin receptor agonist, may have an antiemetic effect. Although the indications for anamorelin are expected to expand through drug repositioning, the present study did not find anamorelin to be effective when used in combination with a standard three-drug antiemetic regimen in cisplatin-induced CINV in rats. These results do not support our hypothesis based on prior clinical evidence. Additionally, no improvements in food intake, SMA, and BW were observed with or without anamorelin in this study. Particularly, our data showed a worsening of the SMA. To the best of our knowledge, this is the first drug repositioning study to investigate the use of anamorelin as a novel antiemetic.
The antiemetic effect was evaluated based on a decrease in pica behavior in rats. Cisplatin administration at 5 mg/kg increased kaolin food intake compared with that in the vehicle group. We found that the increase or decrease in kaolin intake over time was similar to that previously reported.20) Notably, however, compared to previous studies, the intake of kaolin-containing foods was relatively low. The low kaolin intake observed in the present study may be due to individual differences. Several rats consumed little kaolin despite the administration of cisplatin. Furthermore, oral administration may have affected feeding behavior owing to pharyngeal stimulation caused by gavage. Standard three-drug antiemetic regimen (granisetron, fosaprepitant, and dexamethasone) tend to decrease kaolin food intake. This decrease was particularly pronounced on Day 4 in our study. However, given the lack of improvement on Day 1 in both the three-drug and four-drug regimens, the dose of granisetron, which is expected to be effective in the acute phase (within 24 h), might be insufficient. In the four-drug regimen group, kaolin food intake remained nearly unchanged compared to the three-drug group, suggesting that anamorelin did not improve cisplatin-induced CINV. Collectively, these findings suggested that anamorelin had no additional antiemetic effects.
We also investigated the SMA, changes in BW, and the intake of normal food and kaolin. Similar to the results for kaolin intake, anamorelin did not lead to improvements in any of the secondary endpoints. A decreased food intake generally indicates anorexia. Consistent with our findings, several studies have reported a decrease in normal food intake in rats following cisplatin administration.20-22) When anamorelin was orally administered to rats daily, normal food intake increased in a dose-dependent manner.16) However, in the present study, anamorelin (30 mg/kg) did not increase food intake, suggesting that the appetite-stimulating effect of anamorelin could not exceed the appetite-suppressing effect of cisplatin. Similarly, anamorelin administration did not affect changes in BW. The finding that the three-drug regimen administration reduced BW is consistent with the findings of a previous study wherein rats were treated with dexamethasone.23) This result suggests that anamorelin did not exhibit a discernible drug effect, while dexamethasone appeared to influence weight loss. Cisplatin administration causes behavioral inhibition in rats at night.24) In this study, anamorelin did not appear to improve the cisplatin-induced decrease in SMA, suggesting that anamorelin did not ameliorate cisplatin-induced behavioral inhibition in rats.
This experiment was conducted in accordance with a previous mouse study,25) with the key differences being the use of rats and the combination of oral and intraperitoneal administration in the present. To the best of our knowledge, this is the first study examining pica behavior wherein oral and intraperitoneal administrations were combined, more accurately reflecting the dosing regimen used in clinical practice compared to other studies. Although neurokinin 1 receptor antagonists are marketed as two types of formulations–injection and capsule–their oral administration is complicated because the doses differ before and after chemotherapy initiation. Therefore, we administered the injections only once before starting chemotherapy. Dexamethasone can be administered either as an injection or orally, and daily intraperitoneal administration is physically painful for rats; therefore, we administered the dexamethasone administration method orally throughout the experimental period.
This study had several limitations. First, individual rats exhibited considerable variability, and kaolin food intake varied among them. As we used four instruments in the present study, our experiment was conducted with four animals per term (the same group). Therefore, individual differences could not be completely eliminated. Some rats displayed reduced food consumption, leading to lower kaolin food intake after cisplatin administration in this study than in previous studies. Second, the appetite-stimulating effects of anamorelin may have affected kaolin intake. A previous study examined the effect of the ghrelin agonist HM0126) in the Suncus murinus animal model. Unlike rats and mice, S. murinus is prone to vomiting. As a result, the administration of HM01 exhibited dose-dependent antiemetic effects in their models. Considering their findings, the present study, which evaluated pica behavior, may not be the best method for evaluating the antiemetic effects of anamorelin.
ConclusionThis study evaluated whether anamorelin plus standard antiemetic therapy improved cisplatin-induced pica behavior, normal food intake, SMA, and changes in BW over 5 d using a drug repositioning method. Our results indicated that anamorelin did not affect cisplatin-induced CINV. However, to the best of our knowledge, this is the first study to report the use of anamorelin as an antiemetic. Addressing our study limitations through additional research would enable a more comprehensive evaluation of our findings. Consequently, further studies consist of vomiting animals such as S. murinus, are required to evaluate the antiemetic effects of anamorelin.
AcknowledgmentsWe would like to thank Editage (www.editage.com) for the English language editing.
FundingThis study was supported in part by JSPS KAKENHI, Grant Number 19K07230 (Grant-in-Aid for Scientific Research (C), 2019-2021). The funding bodies had no role in the design of the study, collection, analysis, and interpretation of the data, writing of the manuscript, or the decision to submit the manuscript for publication.
Conflict of interestHitoshi Kawazoe received research funding outside the submitted work from Eli Lilly. Tomonori Nakamura received research funding outside the submitted work from Chugai, Daiichi Sankyo, Kyowa Kirin, Otsuka Pharmaceutical, Sanofi, and Shionogi. The other authors declare no conflict of interest.