2016 Volume 80 Issue 10 Pages 2221-2226
2016 Volume 80 Issue 10 Pages 2221-2226
Background: The effects of β-adrenergic blockers on the fetus are not well understood. We analyzed the maternal and neonatal outcomes of β-adrenergic blocker treatment during pregnancy to identify the risk of fetal growth restriction (FGR).
Methods and Results: We retrospectively reviewed 158 pregnancies in women with cardiovascular disease at a single center. Maternal and neonatal outcomes were analyzed in 3 categories: the carvedilol (α/β-adrenergic blocker; α/β group, n=13); β-adrenergic blocker (β group, n=45), and control groups (n=100). Maternal outcome was not significantly different between the groups. FGR occurred in 1 patient (7%) in the α/β group, in 12 (26%) in the β group, and in 3 (3%) in the control group; there was a significant difference between the incidence of FGR between the β group and control group (P<0.05). The β group included propranolol (n=22), metoprolol (n=12), atenolol (n=6), and bisoprolol (n=5), and the individual incidence of FGR with these medications was 36%, 17%, 33%, and 0%, respectively.
Conclusions: As a group, β-adrenergic blockers were significantly associated with FGR, although the incidence of FGR varied with individual β-blocker. Carvedilol, an α/β-adrenergic blocker, had no association with FGR. More controlled studies are needed to fully establish such associations. (Circ J 2016; 80: 2221–2226)
Pregnant women with cardiovascular disease may need drug treatment, depending on the disease severity and type. Drug treatment during pregnancy should be individualized based on the maternal-fetal risk and benefit. Pregnancy increases the circulating blood volume and cardiac output by increasing the ventricular rate and stroke volume and decreasing peripheral vascular resistance. Circulating blood volume increases more rapidly after 20 gestational weeks, and the blood volume at 28–32 gestational weeks is 40–45% greater than the non-pregnancy volume. Therefore, in some pregnancies in women with cardiovascular disease, drug treatment needs to be initiated during pregnancy.1–4
For many years, β-adrenergic blockers have been used to treat hypertension in pregnant women.5–7 In addition, α/β-adrenergic blockers and β-adrenergic blockers are very often used to control arrhythmias and ventricular dysfunction in pregnant women with cardiovascular disease. It has been suggested, however, that β-adrenergic blockers are associated with fetal growth restriction (FGR).8,9 The US Food and Drug Administration (FDA) classifies β-adrenergic blockers as pregnancy category C drugs during the first and second trimesters and as pregnancy category D drugs during the third trimester. The FDA has classified the fetal risk of gestational prescription drug use into 5 categories: A, B, C, D, and X. Category C drugs carry risks that cannot be ruled out, but well-controlled clinical or animal studies showing a fetal risk have not been reported. Fetal damage is likely if these drugs are used during pregnancy, but their potential benefits may exceed their potential risks. Category D drugs carry evidence of risk. For these drugs, human studies conducted during pre- or post-marketing investigations demonstrated a fetal risk, but the potential benefits of these drugs may outweigh their potential risk.10
In a 1990 study, Butters et al examined pregnant women with hypertension. They compared patients treated with a β-adrenergic blocker, atenolol, with patients who did not receive medication and found a significantly higher rate of FGR in the atenolol group compared with the no-medication group.8
The fetal effects of α/β- and β-adrenergic blockers clearly merit further research. The aim of the present study was therefore to analyze the maternal and neonatal outcomes of pregnancy in women with cardiovascular disease who were treated with an α/β- or β-adrenergic blocker. The broader goal was to clarify the effects of these drugs in order to improve the drug treatment options for pregnant women with cardiovascular disease.
We retrospectively reviewed a series of 689 pregnant women with cardiovascular disease who delivered infants at the National Cerebral and Cardiovascular Center in Osaka, Japan between 2000 and 2010. In this series, we identified 58 women with singleton pregnancies who were treated with an oral α/β- or β-adrenergic blocker for at least 2 weeks before delivery. For the control group, we randomly identified 100 women with singleton pregnancies who were not treated with an oral α/β- or β-adrenergic blocker over the same period. Thus, we examined 3 groups of patients: those treated with an α/β-adrenergic blocker (the α/β group), those treated with a β-adrenergic blocker (the β group), and the control group.
Patient data were collected from their medical records and included each patient’s age, parity, smoking and drinking habits during pregnancy, medication(s) used other than β-blockers, New York Heart Association (NYHA) functional class before pregnancy, echocardiographic measurement of cardiac function before pregnancy, complications other than cardiovascular disease, obstetric complications other than FGR, and underlying maternal cardiovascular disease.
Maternal outcomes were examined, including gestational age, delivery mode, cardiovascular events, and deterioration of NYHA class. Cardiovascular events were defined as new-onset or worsening of arrhythmia and heart failure. Neonatal outcomes examined included birth weight, umbilical artery pH, Apgar score at 5 min, congenital disease, and FGR.
Gestational AgeGestational age was determined based on the date of the patient’s last menstrual period if gestational age based on fetal crown-rump length (CRL) differed by <7 days at 8–11 weeks of pregnancy. If the difference in the CRL-based gestational age was >8 days at 8–11 weeks, then gestational age was determined based on CRL. If the patient had undergone fertility treatment, gestational age was calculated depending on the type of treatment.
Cardiovascular Disease GroupsThe underlying maternal cardiovascular diseases were divided into 6 groups based on the European Society of Cardiology (ESC) guidelines for the management of cardiovascular disease during pregnancy:11 (1) congenital heart disease and pulmonary hypertension; (2) aortic disease (including Marfan syndrome); (3) valvular heart disease; (4) coronary artery disease and acute coronary syndrome; (5) cardiomyopathy and heart failure; and (6) arrhythmia.
Light for Gestational Age and FGRBirth weight was evaluated using the birth size standards by gestational age for Japanese neonates, issued by Japan’s Health and Welfare Ministry.12 These birth size standards were determined in 1995 from birth size data of 1,133 infants at 22–41 gestational weeks whose gestational age was confirmed using the date of the mother’s last menstrual period and early gestational ultrasound examinations; the study involved the cooperation of 21 major medical centers throughout Japan. These standards were generally used from 1998 to 2010 in Japan. In the present study, “light for gestational age” was defined as birth weight <10th percentile for gestational age based on the Japanese standards.
FGR was defined as less growth than the anticipated fetal body weight for each gestational age, for any reason. Generally, FGR is determined on ultrasound during pregnancy. In the present study, however, light for gestational age was considered equivalent to FGR.
Statistical AnalysisWe conducted univariate analysis using the chi-squared test, Mann-Whitney U-test, and multiple logistic regression analysis. P<0.05 was considered significant.
A total of 158 pregnancies were included in the analysis. Of those, 13 pregnancies were in the α/β group, 45 were in the β group, and 100 were in the control group. Maternal background data are listed in Table 1. There were no significant differences in maternal background between the 3 groups. All pregnancies resulted in live births.
| α/β-blocker group (n=13) |
β-blocker group (n=45) |
Control group (n=100) |
|
|---|---|---|---|
| Maternal age (years) | 32.6±4.2 | 30.2±5.1 | 31.1±4.5 |
| Primiparity | 9 (69) | 26 (58) | 46 (46) |
| Smoking during pregnancy | 0 (0) | 0 (0) | 0 (0) |
| Alcohol drinking during pregnancy | 0 (0) | 0 (0) | 0 (0) |
| Maternal BMI | 20.7±2.8 | 19.2±2.3 | 19.9±2.4 |
| Medication other than α/β-blocker or β-blocker | 5 (38) | 18 (42) | 38 (38) |
| β-adrenergic blocker dosing period (days) | 205±65.4 | 189±91.1 | – |
| NYHA class before pregnancy | |||
| Class I | 12 (93) | 37 (82) | 93 (93) |
| Class II | 0 (0) | 4 (9) | 5 (5) |
| Class III | 1 (7) | 1 (2) | 2 (2) |
| Class IV | 0 (0) | 0 (0) | 0 (0) |
| Cardiac function (EF <40%) | 0 (0) | 0 (0) | 1 (1) |
| Maternal complications other than CVD | |||
| Chronic hypertension | 0 (0) | 0 (0) | 4 (4) |
| Thyroid disease | 1 (7) | 3 (7) | 5 (5) |
| Other | 0 (0) | 1 (2) | 3 (3) |
| Obstetric complications other than FGR | |||
| Gestational DM | 1 (7) | 2 (4) | 2 (2) |
| PIH | 0 (0) | 1 (2) | 6 (6) |
| Other | 0 (0) | 0 (0) | 6 (6) |
Data given as n (%) or mean±SD. BMI, body mass index; CVD, cardiovascular disease; DM, diabetes mellitus; EF, ejection fraction; FGR, fetal growth restriction; NYHA, New York Heart Association; PIH, pregnancy-induced hypertension.
Carvedilol (2.5–20 mg/day) was the only α/β-adrenergic blocker used (the α/β group), and none of the patients was treated with labetalol. The β-adrenergic blockers (and dose range) used in the β group were atenolol (25–50 mg/day), propranolol (15–60 mg/day), bisoprolol (5–10 mg/day), and metoprolol (20–120 mg/day). None of the women used more than 1 type of β-blocker.
Other drugs used during pregnancy were digoxin, furosemide, verapamil, disopyramide, mexiletine, flecainide, cibenzoline, propylthiouracil, thiamazole, and levothyroxine. The most frequently used drug was digoxin (n=10), followed by verapamil (n=6). Four patients each in the α/β and control groups were treated with digoxin, as were 2 in the β group. Verapamil was used only for 6 patients in the β group; none of the patients in the α/β or control groups received this drug.
Smoking and alcohol consumption during pregnancy, which are associated with FGR, were not observed in any of the patients.
Most of the women were NYHA class I or II. Only 1 woman in the control group had reduced left ventricular ejection fraction (<40%).
None of the 58 patients had coexisting chronic hypertension; therefore, none was taking a β-blocker for blood pressure control during this study. Significant differences in FGR-associated obstetric complications (eg, gestational diabetes mellitus and pregnancy-induced hypertension) were not seen between the 3 patient groups.
The cardiovascular disease subgroups are listed in Table 2. Cardiomyopathy was the most common cardiovascular disease in the α/β group (47%), whereas arrhythmia was the most common in the β group (53%). There were significant differences in the disease subgroups between the α/β and β groups and between the β and control groups.
| α/β-blocker group (n=13) |
β-blocker group (n=45) |
Control group (n=100) |
|
|---|---|---|---|
| CHD and PH | 2 (15) | 7 (16) | 32 (32)* |
| Aortic disease (including Marfan syndrome) | 3 (23) | 3 (7) | 5 (5) |
| Valvular heart disease | 0 (0) | 1 (2) | 17 (17)* |
| CAD and ACS | 2 (15) | 0 (0) | 5 (5) |
| Cardiomyopathy and HF | 6 (47) | 10 (22) | 3 (3)* |
| Arrhythmia | 0 (0) | 24 (53) | 38 (38) |
Data given as n (%). *P<0.05. ACS, acute coronary syndrome; CAD, coronary artery disease; CHD, congenital heart disease; HF, heart failure; PH, pulmonary hypertension.
Maternal outcome is given in Table 3. Mean±SD gestational age was 36.6±2.9 weeks in the α/β (carvedilol) group, 36.8±3.6 weeks in the β group, and 37.3±3.7 weeks in the control group, with no significant differences between the 3 groups. Cardiovascular events occurred in 54% of the α/β (carvedilol) group and in 66% of the β group; both were significantly different from the control group (30%). Most of the cardiovascular events were arrhythmias. Worsening of NYHA class during pregnancy occurred infrequently, and was not significantly different between the groups.
| α/β-blocker group (n=13) |
β-blocker group (n=45) |
Control group (n=100) |
|
|---|---|---|---|
| Gestational age (weeks) | 36.6±2.9 | 36.8±3.8 | 37.3±3.7 |
| Delivery mode | |||
| Vaginal | 2 (15) | 21 (47) | 52 (52) |
| Cesarean | 11 (85) | 24 (53) | 48 (48) |
| Cardiovascular events | 7 (54) | 30 (66) | 30 (30)* |
| Worsening of NYHA class during pregnancy | 1 (7) | 7 (16) | 14 (14) |
Data given as n (%) or mean±SD. *P<0.05. NYHA, New York Heart Association.
Neonatal outcome is summarized in Table 4. There were 4 preterm births (30.8%) in the α/β (carvedilol) group, 13 (31.0%) in the β group, and 18 (26.0%) in the control group. Low Apgar score (<6) was observed in 3 neonates in the β group, but this was due to preterm birth. All umbilical artery pH were higher than 7.1; acidosis was not observed.
| α/β-blocker group (n=13) |
β-blocker group (n=45) |
Control group (n=100) |
|
|---|---|---|---|
| Birth weight (g) | 2,636±637 | 2,411±609 | 2,713±678 |
| Apgar score (5 min) | 9.0±0.5 | 8.6±1.1 | 8.8±1.0 |
| Umbilical artery pH | 7.31±0.55 | 7.29±0.59 | 7.31±0.74 |
| Congenital disease | 0 (0) | 0 (0) | 3 (3)* |
| FGR | 1 (7) | 12 (26)* | 3 (3) |
Data given as n (%) or mean±SD. *P<0.05. FGR, fetal growth restriction.
No congenital disease was observed in the α/β (carvedilol) or β groups, but 3 cases (5%) were observed in the control group. There were no neonatal deaths in any of the groups.
There was 1 case (7%) of FGR in the α/β group, 12 (26%) in the β group, and 3 (3%) in the control group (Table 4). These differences were not significant between the α/β and β groups or between the α/β and control groups, but the β and control groups were significantly different. Women in the β group were more likely to give birth to an infant with FGR compared with the control group. On multiple logistic regression analysis to adjust for maternal age, parity, maternal body mass index, primiparity, smoking, alcohol consumption, hypertension, thyroid disease, gestational diabetes mellitus, and NYHA class, there were no significant differences between the α/β (carvedilol) and control groups (adjusted odds ratio aOR, 2.36; 95% CI: 0.23–21.91). There was, however, a significant difference between the β and control groups (aOR, 9.21; 95% CI: 2.34–320.53; Table 5). We also investigated the relationship between type of β-adrenergic blocker and incidence of FGR. As shown in Table 6, FGR occurred in pregnancies under atenolol, propranolol, or metoprolol treatment, but not in the 5 pregnancies in which bisoprolol was used. This indicates that the individual effect of drugs may differ, although they all belong to the β-blocker drug class. We also determined aOR for propranolol and metoprolol, because they had reasonable sample sizes. There was a statistically significant difference between the propranolol and control groups, but the difference between the metoprolol and the control group did not reach statistical significance (propranolol: aOR, 18.32; 95% CI: 4.35–77.32, P=0.001; metoprolol: aOR, 6.35; 95% CI: 0.95–43.3, P=0.088).
| FGR, n (%) | aOR† (95% CI) | P-value | |
|---|---|---|---|
| α/β-blocker group (n=13) | 1 (7) | 2.36 (0.23–21.91) | 0.82 |
| β-blocker group (n=45) | 12 (26) | 9.21 (2.34–320.53) | 0.031 |
| Control group (n=100) | 3 (3) | 1 | – |
†Adjusted for maternal age, parity, maternal BMI, primiparity, smoking, drinking, hypertension, thyroid disease, gestational DM, and NYHA class. aOR, adjusted odds ratio. Other abbreviations as in Table 1.
| Propranolol (n=22) |
Metoprolol (n=12) |
Atenolol (n=6) |
Bisoprolol (n=5) |
|
|---|---|---|---|---|
| FGR | 8 (36) | 2 (17) | 2 (33) | 0 (0) |
| aOR† (95% CI) | 18.32 (4.35–77.32) | 6.35 (0.95–43.3) | – | – |
| P-value | 0.001 | 0.088 | – | – |
Data given as n (%). †Adjusted for maternal age, parity, maternal BMI, primiparity, smoking, drinking, hypertension, thyroid disease, gestational DM, and NYHA class. Abbreviations as in Tables 1,5.
We also examined the relationship between FGR and timing of β-adrenergic blocker treatment in the β group (Figure 1). FGR was not observed at the beginning of the third trimester. We compared the treatment duration between women who gave birth to infants with FGR (215±18.9 days, n=12) and those who gave birth to non-FGR infants (134±16.4 days, n=29; Figure 2). There was a significant difference between these groups.

Rate of fetal growth restriction (FGR) vs. timing of β-blocker treatment. Those who did not receive treatment before pregnancy but during pregnancy were divided into first, second, and third trimesters based on the time at which treatment was started. The earlier the treatment was started, the greater the possibility of FGR.
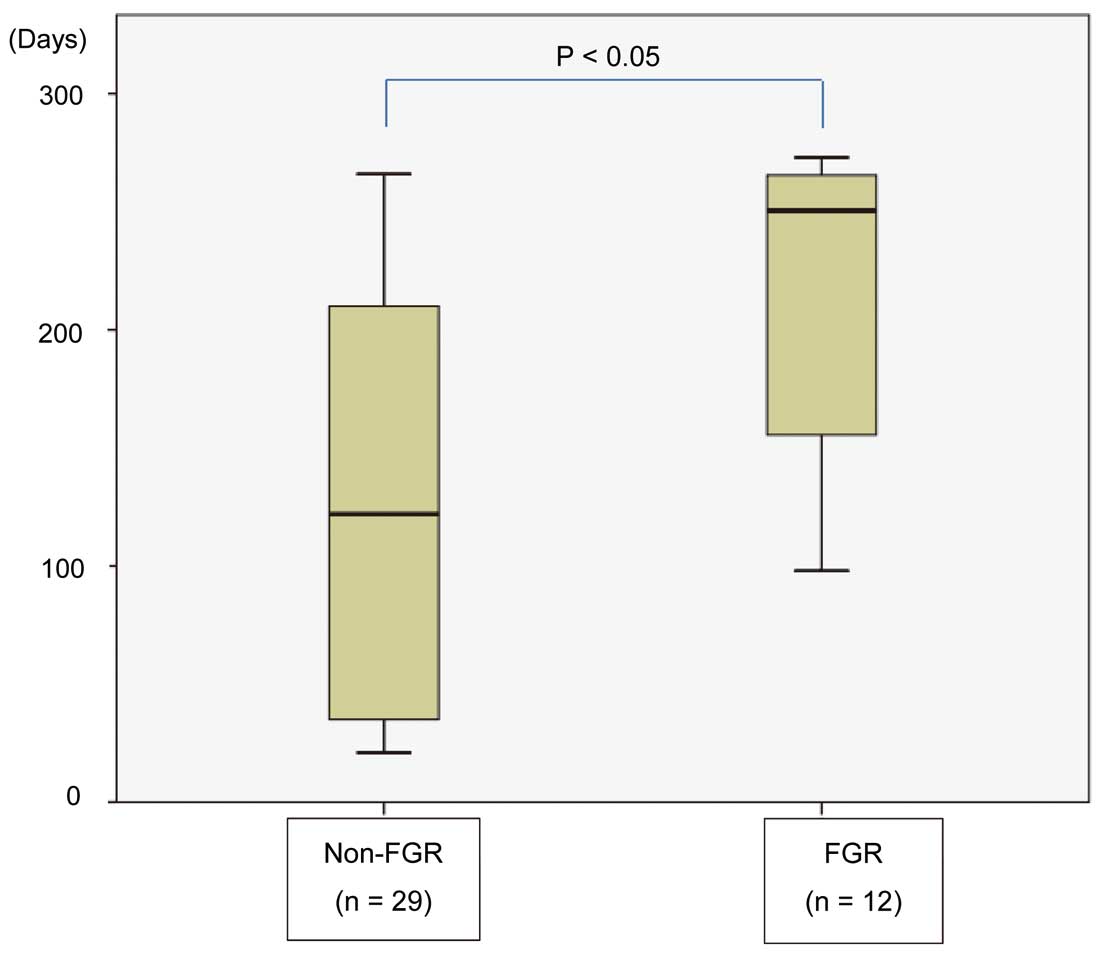
Duration of β-adrenergic blocker treatment in women with cardiovascular disease in pregnancy vs. occurrence of fetal growth restriction (FGR). FGR-complicated pregnancies had a significantly longer β-adrenergic blocker treatment period.
In the α/β-adrenergic blocker group, the only agent used was carvedilol; none of the patients receiving carvedilol had fetal bradycardia. In the carvedilol group, neonatal hypoglycemia was observed in 2 patients (15%); this was defined as blood glucose <45 mg/dl within 2 h after birth. Within this period, neonatal blood glucose is at its lowest; thus, at the present Center, an i.v. line containing 10% glucose is placed in all low-birth-weight and preterm newborns soon after birth. In the present study, 4 newborns received such an i.v. line, and none was hypoglycemic during the first 2 h after birth. This suggests that the neonatal hypoglycemia rate might not be accurate.
We evaluated maternal and neonatal outcomes of pregnancy in women with cardiovascular disease who were treated with an α/β-adrenergic blocker (carvedilol) or a β-adrenergic blocker (propranolol, metoprolol, atenolol, or bisoprolol), and we identified 3 important clinical issues. First, as a group, the use of β-adrenergic blockers was associated with an elevated risk of FGR, whereas the use of an α/β-adrenergic blocker (carvedilol) was not. Second, in the β group, a longer treatment duration increased the probability of giving birth to an infant with FGR. And third, among β-adrenergic blockers, propranolol and atenolol were associated with the highest incidences of FGR. This highlights the fact that different β-blocking drugs can have a different effect on FGR. Particularly, in 5 patients on bisoprolol, no instances of FGR were noted, while FGR was found in 36%, 17%, and 33% of women treated with propranolol, metoprolol, and atenolol, respectively.
In the present study, the incidence of FGR differed significantly between the β (all 4 drugs together) and control groups, as shown by the aOR. Other factors that may affect the risk of FGR did not differ significantly among the 3 groups. The reason why β-adrenergic blockers can cause FGR may be based on their ability to increase vascular resistance in both the mother and fetus.13 The increase in vascular resistance is caused by 2 main mechanisms: β2-blocking activity and relative α-stimulant activity. Therefore, β-adrenergic blockers possessing intrinsic sympathomimetic activity (ISA) are less likely to cause FGR, because ISA is antagonistic to these 2 mechanisms. In the present study, none of the drugs had ISA. α/β-adrenergic blockers, however, counteract the relative α-stimulant activity. Because data were not collected on pulsatility index or other indices of uterine and umbilical artery activity, increases in maternal and fetal vascular resistance could not be documented in the FGR cases in the present study. Previous studies, however, reported that β-adrenergic blockers increased maternal and fetal vascular resistance and decreased uteroplacental blood flow.14,15 Based on the results of those reports, decreased placental flow could have been related to the FGR.
Other FGR mechanisms potentially associated with β-adrenergic blockers include fetal bradycardia and fetal hypoglycemia. In the present study, continuous fetal bradycardia was not observed. Fetal hypoglycemia could not be evaluated because of the absence of a procedure for measuring fetal blood glucose. In the present study, however, maternal hypoglycemia due to β-adrenergic blocker treatment and neonatal hypoglycemia at birth were not observed. Further studies are needed to determine the associations between fetal bradycardia and fetal hypoglycemia with FGR.
In the α/β-adrenergic blocker group, the only agent used was carvedilol. To the best of our knowledge, a review of carvedilol’s fetal effects has not been published; the present study is the first to report such effects. In contrast, several studies have examined the fetal effects of labetalol, and they were sharply divided regarding a link between labetalol and FGR.16–18
In the β group, a longer treatment duration resulted in a greater chance of giving birth to an infant with FGR, suggesting that a longer dosing period is a risk factor for FGR. This is logical, because the longer the treatment duration, the more effect the drug might have on both maternal and fetal circulation. Although a previous study reported that β-adrenergic blockers were associated with FGR,13 a Cochrane review examining mild-moderate hypertension during pregnancy suggested that the relative risk for FGR was low and did not reach statistical significance under the conditions tested; the only exception was 1 study that investigated high doses early in the pregnancy.19 In the present study, only 1 patient required a dosage higher than that recommended in the drug information. In this patient, 10 mg/day bisoprolol was used, but the patient’s infant did not have FGR. Because all patients except 1 received the recommended doses of β-adrenergic blockers, we evaluated the possible association of the length of treatment duration with FGR outcome.
Among the β-adrenergic blockers, propranolol and atenolol had the highest incidence of FGR, 36% and 33%, respectively. In the metoprolol group, FGR was observed in 17% of patients, while no instances of FGR were observed in the bisoprolol group. This suggests that different β-adrenergic blockers drugs confer a different risk of FGR. Therefore, we determined aOR for propranolol and metoprolol and found a significant difference between the propranolol and control groups. The pharmacological effect of β-adrenergic blockers may vary depending on whether the agent possesses ISA or β1-receptor selectivity and whether it crosses the placenta. Propranolol does not possess ISA and is categorized as a non-selective β-blocker; moreover, it readily crosses the placenta because of its lipophilicity. Therefore, propranolol might increase vascular resistance in both the mother and fetus, yielding a higher risk of FGR. Reports on propranolol in human pregnancy have not been published, but propranolol increases vascular resistance and reduces umbilical blood flow in ewes.20 In the present study, the rates of FGR associated with the use of non-selective and β1-selective β-blockers were 36.4% and 17.4% (aOR, 2.71; 95% CI: 0.68–10.83), respectively.
Atenolol is a β1-selective β-blocker that is also water soluble. It possesses weak β2-blocking action but is less able to cross the placenta.21 In the present study, however, atenolol was associated with a higher risk of FGR, despite similar β1-selective β-blockers (metoprolol and bisoprolol) not being associated with a higher risk of FGR. Based on pharmacokinetics and placental transmission characteristics, the reason why atenolol was associated with the highest rate of FGR among the 3 drugs is not yet clear. Clearly, this other cause of FGR is yet to be identified.
Several study limitations must be considered. First, the present study involved a retrospective design. Additionally, there were differences in type of cardiovascular disease between the groups, and the diseases were treated with different drugs. Pre-pregnancy NYHA functional class, however, was not significantly different between the groups. A factor that has been reported to affect neonatal outcome is the severity (ie, NYHA functional class) of the cardiovascular disease rather than its subgroup,22 and neonatal outcome in the present study would have been affected by this. Cardiovascular events were more frequent in the α/β and β groups, and we speculate that this was because the drug treatment was started when the cardiovascular events occurred. Second, the number of cases was small. Multivariate analysis might be suitable for demonstrating relationships between β-adrenergic blockers and FGR, but it cannot confirm the accuracy of the results in a small study. Therefore, an aOR approach was used as an alternative. Third, assessment of circulation and vascular resistance parameters was not possible in either the mother or the fetuses (ie, with echography findings). Finally, there might be individual variability in terms of efficacy of a drug; therefore, grouping drugs by class presents a risk of overgeneralization. Particular attention needs to be paid to atenolol and bisoprolol, given that we could not determine aOR due to the low sample sizes. Similar studies from other centers/countries and multicenter randomized trials are necessary to fully weigh the risks of β-adrenergic blockers and to clearly establish the association with FGR. The present findings are therefore limited to the drugs used in this study.
In spite of certain limitations, this study provides important and significant information on the incidence of FGR with the use of carvedilol, propranolol, metoprolol, atenolol, and bisoprolol. We believe these results will be helpful in clarifying the possible association of β-adrenergic blockers with FGR and will be a stimulus to future clinical trials on the risks and benefits of various drugs in pregnant women with cardiovascular disease.
As a group, β-adrenergic blocker treatment was found to be significantly associated with FGR in pregnant women with cardiovascular disease. Notably, no instances of FGR were observed in the bisoprolol group, although 36% of women in the propranolol group had FGR. Also, the incidence of FGR varied with β-blocker, cautioning against overgeneralization of the results. More extensive investigations are therefore needed to further strengthen such associations, particularly focusing on the multifactorial nature of the FGR and heterogeneity of subjects. Nevertheless, in light of the morbidity and mortality risk associated with cardiovascular disease in pregnancy, the present results will aid in developing a proper framework for clinical management and patient counseling.