Abstract
Background:
While hemodynamics and exercise capacity in patients with chronic thromboembolic pulmonary hypertension (CTEPH) can be improved by invasive therapy such as pulmonary endarterectomy (PEA) and balloon pulmonary angioplasty (BPA), there has been little data on the health-related quality of life (HRQOL) in such patients.
Methods and Results:
This single-center and observational study compared the impact of invasive therapy on HRQOL. We utilized the Medical Outcome Study 36-Item Short Health Survey (SF-36) to measure HRQOL and compared HRQOL changes after PEA and BPA. A total of 48 patients were diagnosed with CTEPH. Of these, 39 patients completed questionnaires before and after invasive therapy. The PEA group (n=15) and the BPA group (n=24) had similar improvements in clinical parameters. With regard to HRQOL score, both groups had fairly low scores in physical functioning (PF), role physical (RP), general health (GH), social functioning (SF), role emotional (RE), and physical component summary (PCS) at baseline. PF, GH, vitality (VT), mental health (MH), and PCS had significant improvements in the PEA group while PCS and all subscales except for bodily pain (BP) had significant improvements in the BPA group. Furthermore, changes between baseline and follow-up were not significantly different between the 2 groups.
Conclusions:
BPA for patients who are ineligible for PEA can recover HRQOL to a similar level to that achieved by PEA.
Chronic thromboembolic pulmonary hypertension (CTEPH) is a rare disease characterized by fibrothrombotic obstructions of the pulmonary arteries (PA), resulting in increased pulmonary vascular resistance (PVR), pulmonary vascular remodeling and eventually leading to right ventricular failure.1
The primary therapy for CTEPH has been pulmonary endarterectomy (PEA),2
which contributes to better hemodynamics, oxygenation, exercise capacity, and prognosis.3,4
CTEPH prognosis had been poor until the development of PEA.5,6
Subsequently, balloon pulmonary angioplasty (BPA) was introduced, which had similarly beneficial effects on hemodynamics, exercise tolerance, and prognosis in patients with inoperable or surgically inaccessible disease.7–12
In spite of the progress in therapeutic options, some patients who have undergone PEA or BPA still had residual symptoms.13,14
Health-related quality of life (HRQOL) measurement is a subjective outcome that represents disease impact on a person’s function and wellbeing, and can be altered by disease treatment.15,16
Beyond objective outcomes such as hemodynamics and exercise tolerance, HRQOL assessment is also important because it is a subjective outcome that reflects the patient’s perspective. Mathai et al reported that HRQOL in patients with pulmonary arterial hypertension (PAH) was associated with World Health Organization functional class (WHO-FC), 6-min walk distance (6MWD), and even transplant-free survival time.17
For CTEPH, there have been several publications on HRQOL. Urushibara et al reported that longer 6MWD and lower PVR were associated with higher HRQOL score in treatment-naïve patients.18
Moreover, in the same study, a surgically treated group had better improvements in HRQOL than a medically treated group.18
Furthermore, Darocha et al reported that HRQOL was improved by BPA.19
We previously identified early introduction of invasive therapies as a factor affecting final HRQOL.20
In spite of these previous studies, there has been no report on the non-inferiority of BPA to PEA in terms of HRQOL improvement. We therefore aimed to clarify the effects of BPA on these patients compared with those who underwent PEA, given that BPA is a fairly new therapy that is generally considered in patients who are not eligible for PEA.21
Based on previous reports, we hypothesized that even patients ineligible for PEA could attain similar HRQOL improvement with BPA. The aim of this study was therefore to compare the impacts of PEA and BPA on HRQOL.
Methods
This study was approved by the Ethics Committee of Kobe University Graduate School of Medicine (approval no. 160171). Written informed consent was given by all patients before catheterization.
Subjects
Between January 2014 and December 2016, a total of 48 patients were diagnosed with CTEPH at Kobe University Hospital. The diagnosis of CTEPH was established on medical history, physical examination, electrocardiogram, chest radiography, echocardiography, computed tomography (CT), lung ventilation-perfusion scintigraphy, right heart catheterization (RHC), and pulmonary angiography (PAG) in accordance with clinical guidelines.21
Treatment approaches were assessed by a multidisciplinary team and patients were re-evaluated after treatment. Patients who could complete questionnaires both before and after invasive therapy were included in this study, while patients who could not complete questionnaires or were treated with a medical therapy were excluded from this study (Figure 1).
Eligibility for Surgery
Eligibility for surgery was discussed by a multidisciplinary team that consisted of cardiologists, interventional cardiologists, and cardiac surgeons, as detailed in our previous report.11
Surgical eligibility was based on preoperative WHO-FC II–IV and surgical accessibility of thrombi identified on PAG. Main lesions in the pulmonary trunk, lobar PA, or segmental PA were defined as the proximal type, while main lesions in the distal segment to sub-segmental PA were defined as the distal type. Patients with the proximal type were considered as candidates for PEA if they had no comorbidities that endangered perioperative safety. BPA was considered if PEA was not indicated. Patients provided written informed consent to undergo PEA or BPA.
PEA
PEA was carried out as described in our previous report.11
Briefly, bilateral PEA was performed through a median sternotomy and was conducted with intermittent circulatory arrest for a period limited to 20 min, while the core temperature was maintained at 16℃. Perioperative medical treatment such as catecholamines, nitric oxide, phosphodiesterase III inhibitors, epoprostenol, or diuretics was given until recovery.
BPA
BPA was carried out as described in our previous reports.11,22
Briefly, the femoral vein was selected using a 9-Fr short sheath, and a 6-Fr guiding sheath was inserted into the PA through the femoral vein. A 6-Fr guiding catheter was engaged into the targeting sectional branches and a 0.014-inch guide wire was crossed through the lesion. After assessment on intravascular ultrasound, the lesion was dilated using 2.0–7.0-mm monorail balloons. At each hospitalization, 2 sessions of BPA were performed, and RHC was performed once after the last session to assess efficacy.
HRQOL Assessment
We utilized the Medical Outcome Study 36-Item Short-Form Health Survey (SF-36) version 2, which is a comprehensive measurement of HRQOL.23
Eight subscales are obtained from the questionnaire: physical functioning (PF), role physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role emotional (RE), and mental health (MH). Two summary scores, physical component summary (PCS) and mental component summary (MCS), are calculated from the 8 subscales. All the subscales (raw score) are scaled to have a national average of 50 and a standard deviation of 10 (normalized score). The patients were requested to complete the SF-36 version 2 during hospitalization for baseline RHC and for follow-up RHC after PEA or BPA.
Clinical Parameters
WHO-FC, hemodynamics, oxygenation, exercise capacity, pulmonary function, and brain natriuretic peptide (BNP) were evaluated at diagnosis and after completion of PEA or BPA. Hemodynamics parameters were obtained from RHC, including right atrium pressure, mean pulmonary arterial pressure (mPAP), pulmonary capillary wedge pressure (PCWP), oxygen saturation (SaO2), partial pressure of oxygen (PaO2), and mixed venous oxygen saturation (SvO2). Cardiac output (CO) and cardiac index (CI) were calculated using the Fick method. PVR was calculated using the following formula: (mPAP−PCWP)×80/CO. 6MWD and cardiopulmonary exercise test (CPET) were evaluated for exercise tolerance.24
Pulmonary function was evaluated by measuring vital capacity as a percentage of predicted vital capacity (%VC), the ratio of forced expiratory volume in 1 s to forced vital capacity (FEV1/FVC), and diffusion capacity of carbon monoxide as a percentage of predicted diffusion capacity (%DLCO).
Statistical Analysis
Continuous variables are expressed as mean±SE. We adopted non-parametric statistics due to the small sample size. Differences between the BPA and PEA groups were determined using the Mann-Whitney U-test. Change after invasive therapy was determined using the Wilcoxon signed-rank test. Differences in frequencies were analyzed using the chi-squared test. P<0.05 was considered statistically significant. Spearman correlation coefficient was used to measure the linear relationship between clinical parameters and HRQOL score. Statistical analysis was performed using SPSS statistics 23.0 (SPSS Japan, Tokyo, Japan).
Results
Of the 48 patients, 15 patients underwent PEA, 30 patients underwent BPA, and 3 patients underwent medical therapy. Of the 45 patients treated with PEA or BPA, 3 patients treated with BPA could not complete the HRQOL questionnaire at diagnosis and 3 patients treated with BPA could not complete the follow-up examinations. Therefore, we analyzed the data for 15 PEA-treated patients (PEA group) and 24 BPA-treated patients (BPA group) in this study (Figure 1).
Baseline Characteristics
The baseline characteristics are summarized in
Table 1. Of 5 BPA patients who were categorized as the central group, 2 patients were not eligible for PEA because of advanced age (>75 years old) and 3 patients rejected PEA. The 2 groups had similar age, gender ratio, and therapeutic regimen including pulmonary vasodilators and home oxygen therapy. The patients who underwent PEA had significantly higher mPAP and PVR and significantly lower exercise tolerance than the patients who underwent BPA.
Table 1.
Baseline Subject Characteristics
| Variables |
PEA
(n=15) |
BPA
(n=24) |
P-value |
| Age (years) |
61.7±3.3 |
64.1±2.6 |
0.449 |
| Time from baseline to follow-up (months) |
6.8±1.0 |
13.3±1.9 |
0.022 |
| Time from symptom onset to invasive therapy (months) |
55.3±13.5 |
32.8±6.7 |
0.146 |
| Time from last invasive therapy to follow-up (months) |
4.4±0.7 |
7.9±1.5 |
0.097 |
| No. BPA sessions |
NA |
3.25±0.3 |
NA |
| Female |
10 (67) |
20 (83) |
0.208* |
| Central disease |
15 (100) |
5 (21) |
<0.001* |
| WHO-FC (I/II/III/IV) |
0/2/10/3 |
0/3/21/0 |
0.399 |
| Treatment |
| PAH-specific monotherapy |
3 (20) |
4 (17) |
0.556* |
| PAH-specific combination therapy |
2 (13) |
5 (21) |
0.444* |
| Warfarin |
15 (100) |
24 (100) |
NA |
| HOT |
6 (40) |
11 (46) |
0.721* |
| Right heart catheterization |
| mPAP (mmHg) |
42.7±1.6 |
33.8±2.2 |
0.004 |
| mRAP (mmHg) |
5.2±0.7 |
4.8±0.6 |
0.679 |
| PCWP (mmHg) |
9.1±1.1 |
8.0±0.7 |
0.539 |
| CI (L/min/m2) |
1.82±0.10 |
2.04±0.13 |
0.432 |
| PVR (Wood units) |
12.2±1.2 |
9.0±1.0 |
0.015 |
| SaO2 (%) |
90.1±1.2 |
90.3±0.9 |
0.915 |
| SvO2 (%) |
59.2±1.6 |
63.3±1.4 |
0.080 |
| Exercise capacity |
| 6MWD (m) |
281±40 |
352±20 |
0.038 |
| Peak V̇O2 (mL/min/kg) |
12.3±1.2 |
14.1±1.4 |
0.525 |
| V̇E/V̇CO2 slope |
47.3±4.6 |
43.0±3.7 |
0.365 |
| Pulmonary function |
| %VC |
86.0±4.8 |
90.7±3.6 |
0.415 |
| FEV1/FVC |
0.745±0.022 |
0.750±0.017 |
0.253 |
| %DLCO |
60.6±3.1 |
60.7±2.6 |
0.853 |
Data given as mean±SE, n, or n (%). *Mann-Whitney U-test (chi-squared test). 6MWD, 6-min walk distance; %DLCO, diffusion capacity of carbon monoxide as the percent of predicted; %VC, vital capacity as the percent of predicted; BPA, balloon pulmonary angioplasty; CI, cardiac index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; HOT, home oxygen therapy; NA, not available; mPAP, mean pulmonary arterial pressure; mRAP, mean right atrial pressure; PAH, pulmonary arterial hypertension; PaO2, partial pressure of oxygen; PCWP, pulmonary capillary wedge pressure; PEA, pulmonary endarterectomy; PVR, pulmonary vascular resistance; SaO2, arterial oxygen saturation; SvO2, mixed venous oxygen saturation; V̇E/V̇CO2, minute ventilation/carbon dioxide production; V̇O2, oxygen uptake; WHO-FC, World Health Organization functional class.
Table 2
lists the effects of PEA and BPA on clinical parameters. WHO-FC, hemodynamics (mPAP, PVR, CI), SaO2, SvO2, 6MWD, and BNP had significant improvements following both invasive therapies. While the BPA group had significant improvements in CPET parameters (peak V̇O2, V̇E/V̇CO2
slope), the PEA group showed a tendency toward improvement in those parameters that did not reach significance. These results were in accordance with previous reports,3,8–11
showing that the present patients were treated appropriately.
Table 2.
Parameter Change From Baseline to Follow-up
| Variables |
PEA (n=15) |
BPA (n=24) |
| n |
Baseline |
Follow-up |
P-value† |
n |
Baseline |
Follow-up |
P-value† |
| WHO-FC (I/II/III/IV) |
15 |
0/2/10/3 |
2/2/11/0 |
0.020 |
24 |
0/3/21/0 |
4/5/15/0 |
0.015 |
| mPAP (mmHg) |
15 |
42.7±1.6 |
24.6±1.7 |
0.001 |
24 |
33.8±2.2 |
21.9±1.7 |
<0.001 |
| mRAP (mmHg) |
15 |
5.2±0.7 |
5.1±0.7 |
0.925 |
24 |
4.8±0.6 |
2.9±0.5 |
0.024 |
| PCWP (mmHg) |
15 |
9.1±1.1 |
9.3±1.1 |
0.826 |
24 |
8.0±0.7 |
6.9±0.6 |
0.384 |
| CI (L/min/m2) |
15 |
1.82±0.10 |
2.34±0.16 |
0.003 |
24 |
2.04±0.13 |
2.35±0.10 |
0.014 |
| PVR (Wood units) |
15 |
12.2±1.2 |
4.6±0.7 |
0.001 |
24 |
9.0±1.0 |
4.1±0.3 |
<0.001 |
| SaO2 (%) |
15 |
90.1±1.2 |
94.2±0.6 |
0.006 |
22 |
90.3±0.9 |
94.4±0.9 |
0.002 |
| SvO2 (%) |
15 |
59.2±1.6 |
64.3±1.5 |
0.029 |
22 |
63.3±1.4 |
69.6±1.0 |
0.001 |
| BNP (pg/mL) |
15 |
416±77 |
161±40 |
0.001 |
23 |
200±67 |
43±14 |
0.008 |
| 6MWD (m) |
15 |
281±40 |
372±33 |
0.031 |
23 |
352±20 |
386±21 |
0.024 |
| Peak V̇O2 (mL/min/kg) |
8 |
12.3±1.2 |
14.2±1.1 |
0.362 |
14 |
14.1±1.4 |
17.5±1.3 |
0.009 |
| V̇E/V̇CO2 slope |
8 |
47.3±4.6 |
39.4±2.5 |
0.161 |
14 |
43.0±3.7 |
31.1±1.7 |
0.003 |
Data given as mean±SE or n. †Paired Wilcoxon signed-rank test. BNP, brain natriuretic peptide. Other abbreviations as in Table 1.
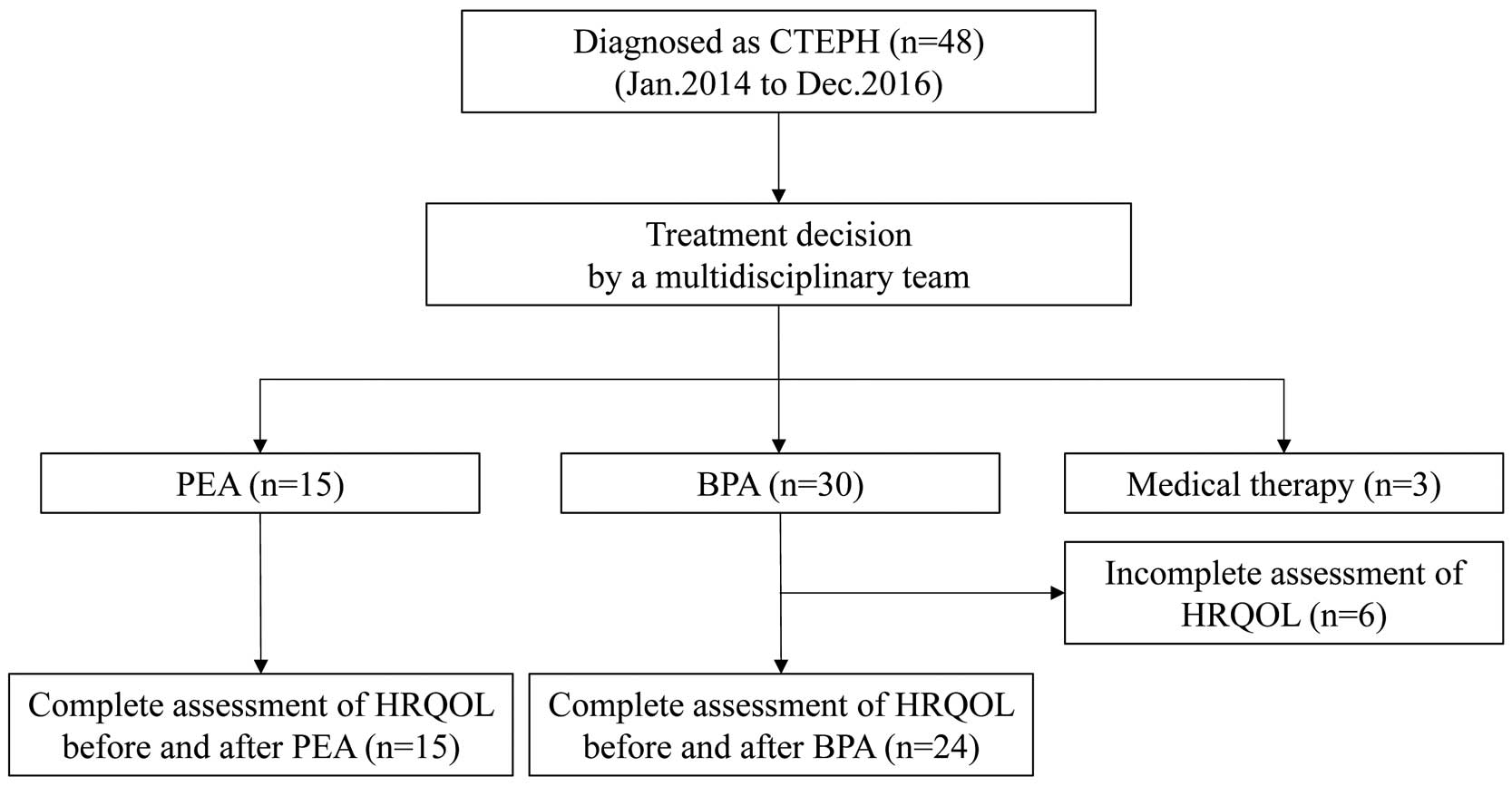
Table 3, Figure 2
present the effects of PEA and BPA on HRQOL. Both groups had low HRQOL at baseline. In particular, 5 of 8 subscales (PF, RP, GH, SF, and RE) and PCS had low scores <1 SD below the mean. The other subscales (BP, VT, and MH) had low scores >1 SD below the mean while MCS was normal. PEA significantly improved PF, GH, VT, and MH with a tendency toward improvement in RP, SF, and RE. In contrast, BPA significantly improved all the aforementioned parameters. BP did not improve in either group. Both PEA and BPA contributed to significantly improved PCS. We compared the changes in HRQOL scores affected by the 2 invasive therapies to determine if these 2 invasive therapies had different effects on HRQOL improvement. There were no significant differences in HRQOL score between the 2 groups (Table 3;
Figure 2C). Although PEA and BPA contributed to better HRQOL scores, almost all of them in both groups were still low compared with the national average. In particular, PF, RP, and PCS were still <40 even after the invasive therapies.
Table 3.
Effect of Invasive Therapy on HRQOL
| Variables |
PEA (n=15) |
BPA (n=24) |
Change between baseline
and follow-up |
| Baseline |
Follow-up |
P-value† |
Baseline |
Follow-up |
P-value† |
PEA |
BPA |
P-value‡ |
| PF |
12.4±5.2 |
31.7±4.3 |
0.001 |
25.4±2.9 |
38.6±2.7 |
0.001 |
+19.2±4.0 |
+13.2±3.3 |
0.191 |
| (34.3±7.4) |
(61.7±6.1) |
|
(52.7±4.1) |
(71.5±3.9) |
|
(+27.3±5.6) |
(+18.8±4.7) |
|
| RP |
27.6±4.9 |
38.7±3.8 |
0.124 |
28.3±3.3 |
38.5±2.7 |
0.002 |
+11.1±6.7 |
+10.2±3.3 |
0.638 |
| (47.5±8.9) |
(67.9±6.9) |
|
(48.7±6.0) |
(67.4±5.0) |
|
(+20.4±12.2) |
(+18.8±6.1) |
|
| BP |
48.9±3.9 |
49.2±2.4 |
0.916 |
47.6±2.5 |
46.1±2.3 |
0.647 |
+0.3±3.4 |
−1.5±2.7 |
0.618 |
| (71.8±8.8) |
(72.4±5.4) |
|
(68.7±5.7) |
(65.3±5.1) |
|
(+0.6±7.6) |
(−3.4±6.0) |
|
| GH |
37.7±2.7 |
47.8±2.2 |
0.001 |
36.9±2.1 |
44.0±2.0 |
0.005 |
+10.1±1.4 |
+7.2±1.9 |
0.212 |
| (41.3±5.1) |
(59.9±4.1) |
|
(39.8±3.9) |
(53.0±3.7) |
|
(+18.7±2.6) |
(+13.3±3.6) |
|
| VT |
42.9±4.0 |
52.1±2.2 |
0.044 |
43.6±1.9 |
48.7±1.9 |
0.007 |
+9.2±3.7 |
+5.1±1.5 |
0.449 |
| (47.5±8.2) |
(66.3±4.4) |
|
(49.0±4.0) |
(59.4±3.8) |
|
(+18.7±7.4) |
(+10.4±3.1) |
|
| SF |
36.1±4.2 |
45.7±3.0 |
0.113 |
35.4±2.9 |
42.6±2.8 |
0.044 |
+9.6±5.0 |
+7.1±3.3 |
1.000 |
| (60.0±8.0) |
(78.3±5.7) |
|
(58.9±5.6) |
(72.4±5.4) |
|
(+18.3±9.6) |
(+13.5±6.3) |
|
| RE |
39.3±4.2 |
43.2±4.1 |
0.327 |
34.4±3.6 |
42.2±3.4 |
0.007 |
+4.0±5.1 |
+7.8±2.9 |
0.352 |
| (66.1±8.3) |
(73.9±8.0) |
|
(56.6±7.0) |
(71.9±6.7) |
|
(+7.8±10.0) |
(+15.3±5.6) |
|
| MH |
47.7±3.2 |
52.8±2.4 |
0.049 |
44.9±2.1 |
51.4±1.7 |
0.002 |
+5.1±2.4 |
+6.5±1.6 |
0.786 |
| (67.3±6.0) |
(77.0±4.4) |
|
(62.1±3.9) |
(74.4±3.2) |
|
(+9.7±4.5) |
(+12.3±3.1) |
|
| PCS |
19.6±4.7 |
32.2±4.1 |
0.023 |
24.3±3.0 |
35.1±2.9 |
0.002 |
+12.6±4.7 |
+10.8±2.9 |
0.786 |
| MCS |
53.2±2.7 |
57.5±2.1 |
0.100 |
49.6±1.7 |
52.4±1.3 |
0.189 |
+4.3±2.1 |
+2.8±1.8 |
0.743 |
Data given as mean±SE. HRQOL scores are presented as normalized scores and raw scores are given in parentheses. †Wilcoxon signed-rank test or ‡Mann-Whitney U-test. BP, bodily pain; GH, general health; HRQOL, health-related quality of life; MCS, mental component summary; MH, mental health; PCS, physical component summary; PF, physical functioning; RE, role emotional; RP, role physical; SF, social functioning; VT, vitality.
We analyzed the correlation between HRQOL score and clinical parameters before and after invasive therapies in order to investigate whether the association between HRQOL score and clinical parameters was altered by invasive therapies. Before invasive therapies, 3 of 8 subscales and PCS had significant correlations with clinical parameters: PF with BNP (r=−0.34) and 6MWD (r=0.44), RP with SaO2
(r=0.43), SF with mPAP (r=−0.40), and PCS with 6MWD (r=0.33;
Supplementary Table 1). After invasive therapies, 4 of 8 subscales and PCS had significant correlations with clinical parameters: PF with time from symptom onset to invasive therapy (r=−0.42) and 6MWD (r=0.43); RP with age (r=−0.33), PVR (r=−0.32), SvO2
(r=0.45), and 6MWD (r=0.39); SF with PVR (r=−0.40); RE with SvO2
(r=0.42), and 6MWD (r=0.40); and PCS with time from symptom onset to invasive therapy (r=−0.33), PVR (r=−0.33), SvO2
(r=0.50), and 6MWD (r=0.49;
Supplementary Table 2).
Discussion
The present study evaluated HRQOL scores in patients to whom therapeutic approaches were assigned in accordance with current guidelines and by consensus.
This study has shown that both treatments could significantly improve hemodynamics, oxygenation, 6MWD, and BNP. The PEA group, however, did not show significant improvement in CPET parameters (Table 2). We believe that there are 2 reasons for this. First, the sample size in the PEA group was small. Second, the time from invasive therapy to follow-up was shorter in the PEA group compared with the BPA group (4.4 months vs. 7.9 months). This short follow-up period might have resulted in the apparent insufficient improvements in parameters from CPET, because these parameters in PEA-treated patients had been shown to improve with time.25
For HRQOL scores, PF, GH, VT, and MH were significantly improved by both PEA and BPA, leading to significantly better PCS in both groups. Even though significant improvements in RP, SF, and RE were seen only in the BPA group, the parameters showed a similar tendency toward improvement in both groups. We think that this non-significance was due to small sample size in the PEA group. Furthermore, the improvement in HRQOL score by invasive therapies was not significantly different between the PEA group and the BPA group. Patients who have undergone PEA or BPA have been shown to have better hemodynamics, exercise tolerance, and longitudinal clinical prognosis.3,4,8–12
There have been only a few studies, however, in which HRQOL score was utilized as a clinical outcome. Although PEA and BPA contribute to better HRQOL in patients with CTEPH,18,19
the question of whether patients who have undergone BPA could have improved HRQOL to the same extent as that in PEA has not been addressed. In this study, we show for the first time that HRQOL improvement in BPA-treated patients was not inferior to that of PEA-treated patients, suggesting that patients ineligible for PEA can receive HRQOL improvement by BPA similar to that of patients with operable disease.
Based on the aforementioned findings, patients with CTEPH can improve HRQOL in addition to hemodynamics and exercise tolerance by invasive therapy. But even after attaining mPAP ≤25 mmHg, almost all of the HRQOL scores in both groups were still low compared with the national average. In particular, PF, RP, and PCS were still <1 SD below the mean. This indicates that even after PEA or BPA, patients still have impaired HRQOL. We believe that 2 factors can contribute to better HRQOL in patients with CTEPH. First, in our previous report, we showed that early introduction of PEA or BPA could contribute to better HRQOL after invasive therapy in CTEPH.20
From the present results, early diagnosis and introduction of invasive therapy are likely essential for better HRQOL after invasive therapy. Second, we might reconsider the goal of invasive therapy in CTEPH. In our previous reports, both extensive revascularization by BPA and sequential hybrid therapy with PEA and additional BPA contributed to amelioration of symptoms and exercise capacity in patients with CTEPH.13,14
It can therefore be hypothesized that additional BPA in addition to PEA or standard BPA might lead to better HRQOL. Further investigation is required to elucidate whether additional BPA can contribute to better HRQOL in patients previously treated with PEA or BPA.
MCS and BP did not show significant improvement in either the PEA or BPA groups. For MCS, previous studies have shown a significant improvement by PEA18
and BPA.19
MCS improvement in the present study, however, was not significant. MCS might have been preserved from baseline or the small improvement might have been due to the small sample size. In patients with chronic heart failure, BP was not lower than the national average.26
BP score in the present study was similarly in accordance with this result. Furthermore, not many patients with CTEPH have a history of chest pain in clinical practice. Therefore, we do not believe that BP was affected by CTEPH, and was appropriate for the evaluation of CTEPH.
There are several studies in which 6MWD and PVR were associated with HRQOL scores in patients with PAH or CTEPH.17,18
In this study, we analyzed the correlation between HRQOL scores and clinical parameters before and after invasive therapy in order to investigate whether association between HRQOL score and clinical parameters was altered by invasive therapy. Before invasive therapy, BNP, mPAP, SaO2, and 6MWD had correlations with HRQOL scores. After invasive therapy, age, time from symptom onset to invasive therapy, PVR, SvO2, and 6MWD had correlations with HRQOL scores. This indicates that 6MWD would be a useful marker reflecting HRQOL in patients with CTEPH both before and after therapy. This would also suggest that patients with low 6MWD have low HRQOL.
Study Limitations
This study had several limitations. First, the present study was a retrospective, single-center, and small-sample study. Larger prospective studies are needed to further investigate HRQOL in CTEPH patients. Second, baseline characteristics between the PEA group and the BPA group, such as mPAP, PVR, and 6MWD, were significantly different. Although HRQOL improvement between the 2 groups was similar, these differences might reflect selection bias. Third, there may be gender-related differences. In this study, a higher percentage of women were included. We were unable, however, to analyze gender-related differences because of the small sample size. A fourth limitation is the measurement of HRQOL itself. SF-36 is not a disease-specific HRQOL measurement, but a comprehensive measurement of HRQOL. We adopted this measurement because its scores can be compared with the healthy population.23,27
It may, however, be less sensitive to changes in HRQOL in CTEPH patients.16
Therefore, it might be better to utilize a disease-specific measurement to assess the effect of invasive therapy.
Conclusions
Both BPA and PEA contribute to better HRQOL in patients with CTEPH. This suggests that patients ineligible for PEA can achieve equivalent clinical outcomes to those patients eligible for PEA. Almost all CTEPH patients can achieve improved HRQOL as well as hemodynamics and exercise tolerance by PEA or BPA.
Acknowledgments
We would like to thank our clinical data managers, Yoko Suzuki and Mayumi Hasegawa, for their contribution to this study. This work was supported by JSPS KAKENHI Grant Number JP18K17669.
Disclosures
Sources of Funding:
There is no grant support for this manuscript.
Conflict of Interest Statement:
K.N. has received research grants from Actelion Pharmaceuticals Japan, Bayer Holding, and GlaxoSmithKline. K.H. received research grants from Actelion Pharmaceuticals Japan, and Bayer Holding. The other authors declare no conflicts of interest.
Supplementary Files
Please find supplementary file(s);
http://dx.doi.org/10.1253/circrep.CR-19-0016
References
- 1.
Simonneau G, Torbicki A, Dorfmuller P, Kim N. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: e160112.
- 2.
Kim NH, Delcroix M, Jenkins DP, Channick R, Dartevelle P, Jansa P, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol 2013; 62: D92–D99.
- 3.
Jenkins D, Madani M, Fadel E, D’Armini AM, Mayer E. Pulmonary endarterectomy in the management of chronic thromboembolic pulmonary hypertension. Eur Respir Rev 2017; 26: e160111.
- 4.
Delcroix M, Lang I, Pepke-Zaba J, Jansa P, D’Armini AM, Snijder R, et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: Results from an international prospective registry. Circulation 2016; 133: 859–871.
- 5.
Riedel M, Stanek V, Widimsky J, Prerovsky I. Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest 1982; 81: 151–158.
- 6.
Lewczuk J, Piszko P, Jagas J, Porada A, Wojciak S, Sobkowicz B, et al. Prognostic factors in medically treated patients with chronic pulmonary embolism. Chest 2001; 119: 818–823.
- 7.
Feinstein JA, Goldhaber SZ, Lock JE, Ferndandes SM, Landzberg MJ. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 2001; 103: 10–13.
- 8.
Mizoguchi H, Ogawa A, Munemasa M, Mikouchi H, Ito H, Matsubara H. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 748–755.
- 9.
Kataoka M, Inami T, Hayashida K, Shimura N, Ishiguro H, Abe T, et al. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 756–762.
- 10.
Sugimura K, Fukumoto Y, Satoh K, Nochioka K, Miura Y, Aoki T, et al. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J 2012; 76: 485–488.
- 11.
Taniguchi Y, Miyagawa K, Nakayama K, Kinutani H, Shinke T, Okada K, et al. Balloon pulmonary angioplasty: An additional treatment option to improve the prognosis of patients with chronic thromboembolic pulmonary hypertension. EuroIntervention 2014; 10: 518–525.
- 12.
Aoki T, Sugimura K, Tatebe S, Miura M, Yamamoto S, Yaoita N, et al. Comprehensive evaluation of the effectiveness and safety of balloon pulmonary angioplasty for inoperable chronic thrombo-embolic pulmonary hypertension: Long-term effects and procedure-related complications. Eur Heart J 2017; 38: 3152–3159.
- 13.
Yanaka K, Nakayama K, Shinke T, Shinkura Y, Taniguchi Y, Kinutani H, et al. Sequential hybrid therapy with pulmonary endarterectomy and additional balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. J Am Heart Assoc 2018; 7: e008838.
- 14.
Shinkura Y, Nakayama K, Yanaka K, Kinutani H, Tamada N, Tsuboi Y, et al. Extensive revascularisation by balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension beyond haemodynamic normalisation. EuroIntervention 2018; 13: 2060–2068.
- 15.
Revicki DA, Osoba D, Fairclough D, Barofsky I, Berzon R, Leidy NK, et al. Recommendations on health-related quality of life research to support labeling and promotional claims in the United States. Qual Life Res 2000; 9: 887–900.
- 16.
Mathai SC, Ghofrani HA, Mayer E, Pepke-Zaba J, Nikkho S, Simonneau G. Quality of life in patients with chronic thromboembolic pulmonary hypertension. Eur Respir J 2016; 48: 526–537.
- 17.
Mathai SC, Suber T, Khair RM, Kolb TM, Damico RL, Hassoun PM. Health-related quality of life and survival in pulmonary arterial hypertension. Ann Am Thorac Soc 2016; 13: 31–39.
- 18.
Urushibara T, Tanabe N, Suda R, Kato F, Kasai H, Takeuchi T, et al. Effects of surgical and medical treatment on quality of life for patients with chronic thromboembolic pulmonary hypertension. Circ J 2015; 79: 2696–2702.
- 19.
Darocha S, Pietura R, Pietrasik A, Norwa J, Dobosiewicz A, Pilka M, et al. Improvement in quality of life and hemodynamics in chronic thromboembolic pulmonary hypertension treated with balloon pulmonary angioplasty. Circ J 2017; 81: 552–557.
- 20.
Tamada N, Nakayama K, Yanaka K, Onishi H, Shinkura Y, Tsuboi Y, et al. Early introduction of pulmonary endarterectomy or balloon pulmonary angioplasty contributes to better health-related quality of life in patients with chronic thromboembolic pulmonary hypertension. JACC Cardiovasc Interv 2018; 11: 1114–1116.
- 21.
Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119.
- 22.
Kinutani H, Shinke T, Nakayama K, Taniguchi Y, Otake H, Takaya T, et al. High perfusion pressure as a predictor of reperfusion pulmonary injury after balloon pulmonary angioplasty for chronic thromboembolic pulmonary hypertension. Int J Cardiol Heart Vasc 2016; 11: 1–6.
- 23.
Fukuhara S, Ware JE Jr, Kosinski M, Wada S, Gandek B. Psychometric and clinical tests of validity of the Japanese SF-36 Health Survey. J Clin Epidemiol 1998; 51: 1045–1053.
- 24.
Tsuboi Y, Tanaka H, Nishio R, Sawa T, Terashita D, Nakayama K, et al. Associations of exercise tolerance with hemodynamic parameters for pulmonary arterial hypertension and for chronic thromboembolic pulmonary hypertension. J Cardiopulm Rehabil Prev 2017; 37: 341–346.
- 25.
Leung Wai Sang S, Morin JF, Hirsch A. Operative and functional outcome after pulmonary endarterectomy for advanced thromboembolic pulmonary hypertension. J Card Surg 2016; 31: 3–8.
- 26.
Izawa KP, Watanabe S, Omiya K, Yamada S, Oka K, Tamura M, et al. Health-related quality of life in relation to different levels of disease severity in patients with chronic heart failure. J Jpn Phys Ther Assoc 2005; 8: 39–45.
- 27.
Fukuhara S, Bito S, Green J, Hsiao A, Kurokawa K. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J Clin Epidemiol 1998; 51: 1037–1044.