2020 年 2 巻 3 号 p. 192-202
2020 年 2 巻 3 号 p. 192-202
Background: ETNA-VTE-Japan is a prospective, observational study conducted as part of a postmarketing study regarding the safety and effectiveness of edoxaban in Japanese patients with venous thromboembolism (VTE). The results of the final analysis of data collected at 1 year are presented.
Methods and Results: A total of 1,732 patients were included in this study. The safety and effectiveness were evaluated in 1,702 patients (safety analysis set; SAS) and in 1,698 patients (effectiveness analysis set). In the SAS, 39.4% of patients were aged ≥75 years, 58.2% had body weight ≤60 kg, and 22.2% had creatinine clearance <50 mL/min. Approximately 90% of patients received a dose recommended on the package insert. A total of 46.1% of patients continued treatment for 1 year, with mean and median treatment periods of 235.8 and 263.0 days, respectively. The incidence of bleeding adverse events (AE) was 10.3%; major bleeding, 2.6%; and VTE recurrence, 1.8%. The risk factor commonly associated with bleeding AE and VTE recurrence was cancer. The safety and effectiveness profiles of edoxaban in patients receiving the appropriate low dose (30 mg/day), generally used in patients with high bleeding risk, were similar to those for the appropriate standard dose (60 mg/day).
Conclusions: At 1 year of treatment, there were no major concerns regarding the safety and effectiveness of edoxaban in Japanese patients with VTE.
Venous thromboembolism (VTE) includes deep vein thrombosis (DVT) and pulmonary embolism (PE), and is recognized as a potentially fatal vascular disease following ischemic heart disease and cerebrovascular disease in incidence in Europe and USA.1 In contrast, although the incidence of VTE is lower in Japan than in Europe and the USA,1–3 the frequency of diagnosis of VTE in clinical settings is steadily increasing due to the aging of the population, an increase in the diagnostic rate, and an increase in the number of cancer patients.2,3
Anticoagulation is the mainstay of treatment and of prevention for VTE recurrence. Anticoagulant therapy has long been centered on treatment with the vitamin K antagonist, warfarin, but the efficacy and safety of direct oral anticoagulants (DOAC) were shown to be similar or superior to warfarin in the respective pivotal global clinical trials of each DOAC, and hence they have recently become the first choice of therapy.4–7
Edoxaban, which is a reversible direct factor Xa inhibitor,8–10 is a tablet formulation of DOAC. Edoxaban was initially indicated only for prevention of postoperative VTE after lower extremity orthopedic surgery. It subsequently gained approval in Japan in 2014 for the treatment and prevention of VTE recurrence as well as for the prevention of ischemic stroke and systemic embolism in patients with non-valvular atrial fibrillation (AF).11 Of the DOAC approved in Japan, only edoxaban is approved for all 3 indications. For the treatment of VTE and AF, the daily dose of edoxaban for individual patients is indicated in the package insert (PI): the standard dose for a patient with body weight >60 kg is 60 mg. The dose should be reduced to 30 mg in the presence of one of the following dose adjustment factors: body weight ≤60 kg; creatinine clearance (ClCr) ≤50 mL/min; or concomitant use of a P-glycoprotein inhibitor (cyclosporine, erythromycin, quinidine, or verapamil).
In Japan, the incidence of VTE is increasing.2,3 Under such circumstances, the use of DOAC for VTE treatment and prevention of recurrence is also expected to increase. One of the available formulations of edoxaban is an orally degradable formulation (Lixiana OD tablet), which is suitable for use in elderly patients with dysphagia and is anticipated to become a drug of choice in this group. Efficacy and safety of edoxaban were confirmed in a phase III trial (Hokusai-VTE), but very high-risk patients (i.e., those with ClCr <30 mL/min and concomitant use of dual antiplatelet therapy [DAPT]) were excluded.4 Therefore, it is important to investigate the safety and effectiveness of edoxaban in a real-world clinical setting by conducting large-scale clinical studies in a clinical practice population.
The ETNA-VTE-Japan (Edoxaban Treatment in routiNe clinical prActice in patients with Venous ThromboEmbolism - Japan) study (UMIN000016387) was initiated to collect real-world data for 1 year in Japanese patients with VTE, including those with a high risk of bleeding, who have been excluded from clinical trials and for whom safety information is lacking.
According to a 3-month interim analysis of ETNA-VTE-Japan, there were no major concerns about the safety and effectiveness of edoxaban in Japanese patients with VTE in the first 3 months of treatment in a real-world setting.12 In the present article, we report the final 1-year results regarding the safety and effectiveness of edoxaban. Further, although the current Japanese Circulation Society guidelines (JCS 2017) recommend that the duration of anticoagulation therapy differs according to the diagnosis of VTE,13 the duration of anticoagulation therapy with DOAC has not been sufficiently studied in the real world. For this reason, both the status of treatment continuation of edoxaban according to the diagnosis of VTE and the status of VTE recurrence during the treatment and after the discontinuation of edoxaban were also investigated in patients with acute VTE in this study.
ETNA-VTE-Japan is a prospective, observational real-world study to collect information on the baseline demographic and clinical characteristics of Japanese patients with VTE, as well as on the safety and effectiveness of edoxaban in these patients. The planned enrollment was ≥1,500 patients.
SettingPatients were recruited from medical institutions in Japan. Enrollment began on 1 February 2015 with a central registration system. At completion of the registration period (31 July 2017), 1,732 patients had been enrolled.
PatientsPatients were eligible for inclusion if they had been diagnosed with VTE (DVT and PE) and were intending to receive edoxaban for the first time for the treatment of VTE and to prevent recurrence within the contract period (determined for each participating institution) and within the registration period. Patients were followed up during the observation period even after edoxaban was discontinued.
EthicsThe study was conducted by Daiichi Sankyo Co., Ltd. (Tokyo, Japan) in accordance with the Good Postmarketing Study Practice standards specified by the Ministry of Health, Labour and Welfare in Japan. The study protocol was approved by the In-House Committee of Daiichi Sankyo Co., Ltd., and by the Ministry of Health, Labour and Welfare of Japan, on 8 November 2014. All patients provided written informed consent before registration.
VariablesThe survey variables were as follows: demographics, risk factors for VTE, clinical characteristics; administration status for edoxaban, prior medication for VTE before starting edoxaban treatment, and concomitant drugs used upon registration; history of non-pharmacological therapy for VTE; history of invasive treatment (including minor surgery other than therapy) for VTE; clinical course; clinical laboratory results; adverse events (AE) including bleeding; and clinical events, including recurrence of VTE (symptomatic and asymptomatic). Bleeding AE were categorized by the attending physicians based on the definitions used in the Hokusai-VTE (which investigated edoxaban vs. warfarin for the treatment of symptomatic venous thromboembolism),4 with slight modifications (Supplementary Table 1). The data for these survey variables were collected using case report forms at 3 months and at 1 year of participation in the study. We analyzed the data up to the 390-day observation period for each patient. Here, we present the results of the final analysis at 1 year.
OutcomesThe data on the survey variables were used in the evaluation of the safety and effectiveness of edoxaban in clinical practice. The safety outcomes collected in this study were AE or adverse drug reactions (ADR), including bleeding events. ADR were defined as AE for which a causal relationship with edoxaban could not be ruled out. Bleeding AE were categorized by the attending physicians based on International Society on Thrombosis and Haemostasis criteria.14 The effectiveness outcome was the incidence of VTE recurrence.
Statistical AnalysisFor categorical variables, proportions were calculated and contingency tables were created. For continuous variables, summary statistics (mean, SD) were calculated. To identify risk factors for all bleeding AE, major bleeding and VTE recurrence, the multivariate Cox proportional hazards model with a step-wise variable selection method (significance level 5%) was used. Variables used in multivariate analysis for all bleeding AE and major bleeding were sex, age, body weight, smoking status, alcohol drinking habit, history of intracranial bleeding, history of gastrointestinal bleeding, baseline comorbidities (hypertension, diabetes mellitus, heart disease, peptic ulcer, cancer [including active cancer], anemia, congenital or acquired disease related to bleeding), pretreatment ClCr, pretreatment aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT), starting daily dose of edoxaban, concomitant use of other medications during the edoxaban treatment period (antiplatelet drugs [including aspirin], non-steroidal anti-inflammatory drugs [NSAIDs] other than low-dose aspirin, p-glycoprotein inhibitors [cyclosporine, erythromycin, quinidine, and verapamil], p-glycoprotein inhibitors [those other than cyclosporine, erythromycin, quinidine, and verapamil], DAPT), and starting daily dose of edoxaban according to the PI (recommended dose, non-recommended under-dose or non-recommended over-dose). Variables used in multivariate analysis for VTE recurrence were sex, age, body weight, smoking status, alcohol drinking habit, VTE risk factors at onset (history of VTE, sitting for ≥4 h, bedrest for ≥7 days, surgery ≤3 months prior to VTE), VTE diagnosis, symptomatic or asymptomatic VTE, days from VTE onset to starting edoxaban treatment, baseline comorbidities (hypertension, diabetes mellitus, dyslipidemia, heart disease, cerebrovascular disorder, peripheral vascular disease, cancer [including active cancer]), pretreatment ClCr, pretreatment AST and/or ALT, starting daily dose of edoxaban, concomitant use of other medications during the edoxaban treatment period (antiplatelet drugs [including aspirin], P-glycoprotein inhibitors [cyclosporine, erythromycin, quinidine, and verapamil], P-glycoprotein inhibitors [those other than cyclosporine, erythromycin, quinidine, and verapamil]), and starting daily dose of edoxaban according to the PI (recommended dose, non-recommended under-dose or non-recommended over-dose). P<0.05 was defined as significant.
Statistical analysis was carried out using SAS System Release 9.2 (SAS Institute Japan, Tokyo, Japan). AE were encoded using the Medical Dictionary for Regulatory Activities Terminology (MedDRA)/J 22.0.
Trial RegistrationETNA-VTE-Japan is registered with the UMIN Clinical Trials Registry (UMIN000016387).
A total of 281 institutions registered 1,732 patients by 31 July 2017, of whom 1,724 patients had data collected and the data set was finalized at 1 year. Data from 22 patients were excluded from the safety evaluation of edoxaban, and safety data were evaluable for 1,702 patients (safety analysis set; Supplementary Figure). The dataset for the effectiveness evaluation consisted of 1,698 patients (effectiveness analysis set; Supplementary Figure).
In the safety analysis set, the mean age was 68.0 years, with 39.4% of patients aged ≥75 years (Table 1). Mean body weight was 59.4 kg, with 58.2% of patients weighing ≤60 kg. Mean ClCr was 77.1 mL/min, and 22.2% of patients (377/1,702) had ClCr ≤50 mL/min. Patients with PE comprised 42.3%, proximal DVT comprised 26.1% and isolated distal DVT only comprised 31.3%. The VTE risk factors were identified in 74.6% of patients, of which the most common one was active cancer (26.9%). The proportion of patients who had received a medication for VTE before starting edoxaban treatment was 56.1%, and unfractionated heparin was most commonly used (38.1%). A history of bleeding was present in 7.6% of patients, including intracranial hemorrhage (2.9%) and gastrointestinal hemorrhage (1.9%; Table 1). A high proportion of patients had baseline comorbidities other than bleeding, such as hypertension (44.1%), diabetes mellitus (14.5%), heart disease (14.1%), cerebrovascular disorder (8.4%) and cancer (including active cancer; 28.1%).
| Total (n=1,702) |
Edoxaban | |||||
|---|---|---|---|---|---|---|
| Over-dose 60 mg (n=60)† |
Appropriate standard dose 60 mg (n=516)† |
Appropriate low dose 30 mg (n=986)† |
Under-dose 30 mg (n=106)† |
Under-dose 15 mg (n=19)† |
||
| Sex | ||||||
| Female | 1,026 (60.3) | 35 (58.3) | 179 (34.7) | 748 (75.9) | 41 (38.7) | 15 (78.9) |
| Age (years) | ||||||
| Mean±SD | 68.0±14.3 | 69.4±13.2 | 61.2±14.0 | 71.4±13.5 | 66.7±12.3 | 78.9±13.1 |
| ≥75 | 671 (39.4) | 29 (48.3) | 105 (20.3) | 486 (49.3) | 35 (33.0) | 13 (68.4) |
| Body weight (kg) | ||||||
| Mean±SD | 59.4±13.9 | 58.1±7.6 | 73.2±12.0 | 51.2±7.6 | 70.2±9.3 | 48.2±9.9 |
| ≤60 | 990 (58.2) | 46 (76.7) | 0 (0.0) | 926 (93.9) | 0 (0.0) | 18 (94.7) |
| Pretreatment ClCr (mL/min)‡ | ||||||
| <15 | 2 (0.1) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 0 (0.0) | 1 (5.3) |
| ≥15–<30 | 48 (2.8) | 0 (0.0) | 0 (0.0) | 45 (4.6) | 0 (0.0) | 3 (15.8) |
| ≥30–≤50 | 327 (19.2) | 18 (30.0) | 0 (0.0) | 299 (30.3) | 0 (0.0) | 10 (52.6) |
| >50–<80 | 642 (37.7) | 22 (36.7) | 153 (29.7) | 413 (41.9) | 51 (48.1) | 3 (15.8) |
| ≥80 | 667 (39.2) | 20 (33.3) | 363 (70.3) | 227 (23.0) | 55 (51.9) | 2 (10.5) |
| Not calculable | 16 (0.9) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 0 (0.0) | 0 (0.0) |
| Mean±SD | 77.1±34.7 | 71.0±28.8 | 101.7±35.5 | 64.0±26.6 | 87.4±32.8 | 47.8±29.4 |
| Patient status | ||||||
| Inpatient | 1,021 (60.0) | 43 (71.7) | 319 (61.8) | 599 (60.8) | 53 (50.0) | 4 (21.1) |
| VTE diagnosis | ||||||
| PE with or without DVT | 720 (42.3) | 33 (55.0) | 261 (50.6) | 373 (37.8) | 45 (42.5) | 5 (26.3) |
| Symptomatic | 555 (32.6) | 31 (51.7) | 217 (42.1) | 266 (27.0) | 34 (32.1) | 4 (21.1) |
| Proximal DVT | 444 (26.1) | 14 (23.3) | 131 (25.4) | 263 (26.7) | 23 (21.7) | 7 (36.8) |
| Symptomatic | 315 (18.5) | 12 (20.0) | 98 (19.0) | 178 (18.1) | 17 (16.0) | 5 (26.3) |
| Isolated distal DVT only | 532 (31.3) | 13 (21.7) | 123 (23.8) | 345 (35.0) | 38 (35.8) | 7 (36.8) |
| Symptomatic | 304 (17.9) | 9 (15.0) | 82 (15.9) | 182 (18.5) | 22 (20.8) | 4 (21.1) |
| Other (e.g., DVT at unknown site) |
2 (0.1) | 0 (0.0) | 0 (0.0) | 2 (0.2) | 0 (0.0) | 0 (0.0) |
| Risk factors at VTE onset | ||||||
| No | 395 (23.2) | 13 (21.7) | 140 (27.1) | 203 (20.6) | 27 (25.5) | 7 (36.8) |
| Yes | 1,269 (74.6) | 47 (78.3) | 364 (70.5) | 758 (76.9) | 79 (74.5) | 12 (63.2) |
| Cancer (active) | 458 (26.9) | 16 (26.7) | 123 (23.8) | 284 (28.8) | 31 (29.2) | 4 (21.1) |
| History of VTE | 161 (9.5) | 6 (10.0) | 57 (11.0) | 80 (8.1) | 14 (13.2) | 0 (0.0) |
| Drug treatment for VTE before starting edoxaban | ||||||
| Yes | 955 (56.1) | 34 (56.7) | 293 (56.8) | 552 (56.0) | 62 (58.5) | 7 (36.8) |
| Unfractionated heparin | 649 (38.1) | 30 (50.0) | 212 (41.1) | 367 (37.2) | 33 (31.1) | 4 (21.1) |
| Warfarin | 262 (15.4) | 4 (6.7) | 67 (13.0) | 156 (15.8) | 27 (25.5) | 4 (21.1) |
| History of bleeding | ||||||
| Present | 130 (7.6) | 3 (5.0) | 34 (6.6) | 77 (7.8) | 13 (12.3) | 2 (10.5) |
| Intracranial bleeding | 50 (2.9) | 1 (1.7) | 15 (2.9) | 27 (2.7) | 6 (5.7) | 0 (0.0) |
| Gastrointestinal bleeding | 33 (1.9) | 2 (3.3) | 4 (0.8) | 24 (2.4) | 3 (2.8) | 0 (0.0) |
| Baseline comorbidities other than bleeding | ||||||
| Hypertension | 750 (44.1) | 29 (48.3) | 222 (43.0) | 418 (42.4) | 66 (62.3) | 8 (42.1) |
| Diabetes mellitus | 247 (14.5) | 12 (20.0) | 81 (15.7) | 126 (12.8) | 23 (21.7) | 5 (26.3) |
| Dyslipidemia | 482 (28.3) | 19 (31.7) | 140 (27.1) | 275 (27.9) | 41 (38.7) | 5 (26.3) |
| Heart disease | 240 (14.1) | 7 (11.7) | 43 (8.3) | 162 (16.4) | 23 (21.7) | 5 (26.3) |
| Cerebrovascular disorder | 143 (8.4) | 8 (13.3) | 27 (5.2) | 93 (9.4) | 9 (8.5) | 4 (21.1) |
| Peptic ulcer | 105 (6.2) | 5 (8.3) | 36 (7.0) | 53 (5.4) | 9 (8.5) | 1 (5.3) |
| Cancer (including active cancer) | 479 (28.1) | 16 (26.7) | 124 (24.0) | 301 (30.5) | 33 (31.1) | 4 (21.1) |
| Anemia | 157 (9.2) | 2 (3.3) | 33 (6.4) | 113 (11.5) | 6 (5.7) | 3 (15.8) |
| Congenital or acquired disease related to bleeding |
20 (1.2) | 0 (0.0) | 6 (1.2) | 11 (1.1) | 3 (2.8) | 0 (0.0) |
Data given as n (%) unless otherwise specified. †15 patients for whom dose adjustment factors were unknown were excluded. ‡Calculated with the Cockcroft-Gault formula. ClCr, creatinine clearance; DVT, deep vein thrombosis; PE, pulmonary embolism; VTE, venous thromboembolism.
Compared with the appropriate standard-dose 60-mg group, patients receiving the appropriate low dose (30 mg) were older, had lower body weight and ClCr, and greater baseline comorbidity of cancer. A similar trend was confirmed in the non-recommended under-dose 30-mg group and, in addition, the proportion of patients with a history of bleeding was also greater compared with the appropriate standard-dose 60-mg group.
We also analyzed data from patients with acute VTE diagnosed in the 14 days before starting edoxaban treatment. The baseline characteristics of this group of patients (n=1,024) in the safety analysis set are summarized in Supplementary Table 2 (by VTE diagnosis). Most of the baseline characteristics were similar to those of all patients in the safety analysis set (n=1,702).
Dose Level and Dose Adjustment FactorsThe starting dose of edoxaban in the safety analysis set was 60 mg/day in 34.1%, 30 mg/day in 64.6%, and 15 mg/day in 1.3% (Figure 1). Of the total 1,702 patients, 1,687 patients had information available on dose adjustment factors. Of these, 89.0% (1,502/1,687) received edoxaban at the appropriate 60-mg and 30-mg dose (92.6%, 986/1,065 with dose adjustment factors; and 83.0%, 516/622 without dose adjustment factors). Of the 1,065 patients with dose adjustment factors, body weight ≤60 kg was a factor in 93.0% (990/1,065). The remaining patients (185/1,687, 11.0%) received a non-recommended dose: 125 (7.4%) received an under-dose (30 mg: 103, 6.1%; 15 mg: 22, 1.3%) and 60 (3.6%) received an over-dose (60 mg). The main reasons for under-dose were older age and renal dysfunction (which did not fulfill the criteria for decreasing the dose of edoxaban as written in the PI).

Medication status in Japanese patients with venous thromboembolism. *Data from 15 patients for whom the presence of dose adjustment factors was undetermined, were excluded. P-gp, P-glycoprotein.
Of the 1,702 patients in the safety analysis set, 784 (46.1%) continued and 918 (53.9%) discontinued treatment at 1 year. The main reasons for discontinuation were as follows: completion of treatment as planned, 23.2%; did not return for treatment or transferred to a different hospital, 14.3%; clinical events or AE, 12.5%; and switched to other drugs for VTE, 5.2%. Mean±SD and median duration of edoxaban treatment during the 1-year observation period were 235.8±141.5 days and 263.0 days, respectively. Of the 1,024 patients with acute VTE, 411 (40.1%) continued and 613 (59.9%) discontinued treatment, and the main reasons for discontinuation were: completion of treatment as planned, 27.0%; did not return for treatment or transferred to a different hospital, 16.2%; clinical events or AE, 14.2%; and switched to other drugs for VTE, 4.4%. Mean±SD and median duration of edoxaban treatment was 218.8±142.0 days and 210.5 days, respectively. When patients with acute VTE were stratified by VTE diagnosis, the continuation rate was lower and the mean and median duration of edoxaban treatment were shorter in patients with isolated distal DVT only (31.0%, 196.5±139.4 days and 175.0 days, respectively), compared with those with PE (44.7%, 225.4±142.5 days and 232.0 days, respectively) and proximal DVT (43.9%, 234.7±141.8 days and 274.0 days, respectively).
Clinical Outcomes (Safety and Effectiveness)The incidence of bleeding AE during the edoxaban treatment period in the safety analysis set (n=1,702) and VTE recurrence in the on-treatment population (the on-treatment period being the time during which patients were receiving edoxaban, as opposed to the treatment period, which may have included periods during which edoxaban treatment was interrupted) and in the effectiveness analysis set (n=1,698) are listed in Table 2 (details of bleeding AE are shown in Supplementary Table 3). The incidence and number of patients with bleeding AE and VTE recurrence were as follows: bleeding AE, 10.3% (n=176); major bleeding, 2.6% (n=45); VTE recurrence, 1.8% (n=30); and symptomatic VTE recurrence, 0.8% (n=14). The most common type of major bleeding was gastrointestinal bleeding (0.9%, n=16). The cumulative incidence of bleeding AE and VTE recurrence are shown in Figure 2. Approximately half of bleeding AE occurred in the first 3 months (90 days) of edoxaban treatment and there was no specific trend in the time of onset of VTE recurrence. The incidence of bleeding AE was 10.4% in patients who received the appropriate low-dose edoxaban (30 mg/day) and 10.5% in patients who received the appropriate standard dose (60 mg/day). The incidence of major bleeding was 2.8% in the appropriate low-dose 30-mg group and 2.7% in the appropriate standard-dose 60-mg group. The incidence of VTE recurrence was 1.7% in the appropriate low-dose 30-mg group and 2.1% in the appropriate standard-dose 60-mg group. The incidence of bleeding AE was 11.7% (7/60) in patients receiving over-dose 60 mg and 10.4% (11/106) in patients receiving under-dose 30 mg. Further, the incidence of VTE recurrence in patients receiving under-dose was 0.0% (0/105) for the 30-mg/day dose and 10.5% (2/19) for the 15-mg/day dose.
| Bleeding AE | Edoxaban treatment period | |||||
|---|---|---|---|---|---|---|
| Total (n=1,702) |
Over-dose 60 mg (n=60)† |
Appropriate standard dose 60 mg (n=516)† |
Appropriate low dose 30 mg (n=986)† |
Under-dose 30 mg (n=106)† |
Under-dose 15 mg (n=19)† |
|
| All bleeding | 176 (10.3) | 7 (11.7) | 54 (10.5) | 103 (10.4) | 11 (10.4) | 0 (0.0) |
| Major bleeding | 45 (2.6) | 1 (1.7) | 14 (2.7) | 28 (2.8) | 2 (1.9) | 0 (0.0) |
| Intracranial bleeding‡ | 9 (0.5) | 1 (1.7) | 2 (0.4) | 6 (0.6) | 0 (0.0) | 0 (0.0) |
| Gastrointestinal bleeding§ | 16 (0.9) | 0 (0.0) | 2 (0.4) | 13 (1.3) | 1 (0.9) | 0 (0.0) |
| Clinically relevant non-major bleeding |
50 (2.9) | 3 (5.0) | 8 (1.6) | 34 (3.4) | 5 (4.7) | 0 (0.0) |
| Nuisance bleeding | 88 (5.2) | 4 (6.7) | 33 (6.4) | 46 (4.7) | 4 (3.8) | 0 (0.0) |
| VTE recurrence | On-treatment population | |||||
| Total (n=1,698) |
Over-dose 60 mg (n=60)† |
Appropriate standard dose 60 mg (n=516)† |
Appropriate low dose 30 mg (n=983)† |
Under-dose 30 mg (n=105)† |
Under-dose 15 mg (n=19)† |
|
| All | 30 (1.8) | 0 (0.0) | 11 (2.1) | 17 (1.7) | 0 (0.0) | 2 (10.5) |
| Symptomatic | 14 (0.8) | 0 (0.0) | 5 (1.0) | 8 (0.8) | 0 (0.0) | 1 (5.3) |
| PE | 6 (0.4) | 0 (0.0) | 3 (0.6) | 3 (0.3) | 0 (0.0) | 0 (0.0) |
| Symptomatic | 4 (0.2) | 0 (0.0) | 1 (0.2) | 3 (0.3) | 0 (0.0) | 0 (0.0) |
| DVT alone | 24 (1.4) | 0 (0.0) | 8 (1.6) | 14 (1.4) | 0 (0.0) | 2 (10.5) |
| Symptomatic | 10 (0.6) | 0 (0.0) | 4 (0.8) | 5 (0.5) | 0 (0.0) | 1 (5.3) |
Data given as n (%). †15 patients for whom dose adjustment factors were unknown were excluded. One case of nuisance bleeding was seen in a patient with 60 mg, for whom dose adjustment factors were unknown. ‡Intracranial tumor hemorrhage, brainstem hemorrhage, cerebellar hemorrhage, cerebral hemorrhage, hemorrhagic cerebral infarction, thalamus hemorrhage, and subdural hematoma. §Ischemic colitis, intestinal hemorrhagic diverticulum, gastric hemorrhage, gastric ulcer hemorrhage, gastrointestinal hemorrhage, esophageal hemorrhage, rectal ulcer hemorrhage, upper gastrointestinal hemorrhage, lower gastrointestinal hemorrhage, large intestinal hemorrhage, small intestinal hemorrhage, and acute hemorrhagic ulcerative colitis. AE, adverse event. Other abbreviations as in Table 1.

Kaplan-Meier curves of cumulative incidence of (A) bleeding adverse events during the edoxaban treatment period in the safety analysis set (n=1,702), and (B) venous thromboembolism (VTE) recurrence in the on-treatment population in the effectiveness analysis set (n=1,698).
Significant factors associated with bleeding AE (all bleeding) during the edoxaban treatment period, with major bleeding during the edoxaban treatment period and with VTE recurrence in the on-treatment population, identified on multivariate analysis, are listed in Table 3 (all P<0.05). On multivariate analysis the factors associated with significantly increased risk for all bleeding were cancer, anemia, congenital or acquired disease related to bleeding, and concomitant use of antiplatelet drugs and NSAIDs other than low-dose aspirin. On multivariate analysis, cancer and hypertension were identified as being associated with significantly increased risk of major bleeding. Finally, cancer was identified as being associated with significantly increased risk of VTE recurrence.
| HR | 95% CI | P-value | |
|---|---|---|---|
| Bleeding AE | |||
| Baseline comorbidity: cancer | 2.240 | 1.653–3.036 | <0.0001 |
| Baseline comorbidity: anemia | 1.734 | 1.154–2.605 | 0.0081 |
| Baseline comorbidity: congenital or acquired disease related to bleeding | 3.024 | 1.406–6.502 | 0.0046 |
| Concomitant use of antiplatelet drugs (including aspirin) (during the edoxaban treatment period) |
1.788 | 1.183–2.704 | 0.0059 |
| Concomitant use of NSAIDs other than low-dose aspirin (during the edoxaban treatment period) |
1.636 | 1.117–2.396 | 0.0115 |
| Major bleeding | |||
| Baseline comorbidity: hypertension | 2.109 | 1.154–3.854 | 0.0152 |
| Baseline comorbidity: cancer | 3.324 | 1.839–6.006 | <0.0001 |
| VTE recurrence | |||
| Baseline comorbidity: cancer | 3.783 | 1.836–7.796 | 0.0003 |
NSAID, non-steroidal anti-inflammatory drug. Other abbreviations as in Table 1.
The risks/benefits in high-risk patients (older age, low body weight, renal dysfunction, cancer, concomitant antiplatelet drugs or concomitant use of DAPT) treated with the recommended doses of edoxaban are listed in Table 4. The incidence of all bleeding, major bleeding, and of recurrent VTE in cancer patients, who had significantly increased risk of bleeding and VTE recurrence on multivariate analysis, was 16.2%, 5.2%, and 3.8%, respectively. The incidence of all bleeding and of major bleeding in patients with concomitant antiplatelet drugs, who had significantly increased bleeding risk on multivariate analysis, was 16.9% and 5.4%, respectively. Furthermore, although the number of patients was limited to 18, the incidence of all bleeding and of major bleeding in patients with concomitant DAPT was 27.8% and 5.6%, respectively, the highest incidences in all the risk categories examined.
| Bleeding AE | VTE recurrence (On-treatment) % (n/N) (95% CI) |
||
|---|---|---|---|
| All bleeding % (n/N) (95% CI) |
Major bleeding % (n/N) (95% CI) |
||
| All patients | 10.5 (157/1,502) | 2.8 (42/1,502) | 1.9 (28/1,499) |
| (9.0–12.1) | (2.0–3.8) | (1.2–2.7) | |
| Elderly (≥75 years) | 10.8 (64/591) | 2.2 (13/591) | 1.2 (7/590) |
| (8.4–13.6) | (1.2–3.7) | (0.5–2.4) | |
| Low body weight (≤60 kg) | 10.5 (97/926) | 2.8 (26/926) | 1.8 (17/924) |
| (8.6–12.6) | (1.8–4.1) | (1.1–2.9) | |
| Low body weight (<40 kg) | 13.5 (10/74) | 4.1 (3/74) | 1.4 (1/74) |
| (6.7–23.5) | (0.8–11.4) | (0.0–7.3) | |
| Renal dysfunction (pretreatment ClCr ≤50 mL/min) | 11.3 (39/345) | 2.9 (10/345) | 1.7 (6/343) |
| (8.2–15.1) | (1.4–5.3) | (0.6–3.8) | |
| Baseline comorbidity: cancer | 16.2 (69/425) | 5.2 (22/425) | 3.8 (16/423) |
| (12.9–20.1) | (3.3–7.7) | (2.2–6.1) | |
| Concomitant use of antiplatelet drugs (including aspirin) (during the edoxaban treatment period) |
16.9 (25/148) | 5.4 (8/148) | 0.0 (0/148) |
| (11.2–23.9) | (2.4–10.4) | (0.0–2.5) | |
| Concomitant use of DAPT (during the edoxaban treatment period) |
27.8 (5/18) | 5.6 (1/18) | 0.0 (0/18) |
| (9.7–53.5) | (0.1–27.3) | (0.0–18.5) | |
DAPT, dual antiplatelet therapy. Other abbreviations as in Table 1.
ADR were reported in 13.6% of patients, while serious ADR were reported in 3.8% (Supplementary Table 4).
VTE Recurrence According to Edoxaban StatusThe incidence of VTE recurrence in patients with acute VTE (n=1,023) is shown in Table 5. The incidence of VTE recurrence was 1.6% (n=16) in the on-treatment population and 3.7% (n=38) in the intention-to-treat (ITT) population (during the whole 1-year observation period; Table 5A,B). The cumulative incidence of VTE recurrence in patients with acute VTE in the on-treatment population and in the ITT population is shown in Figure 3. There was no specific trend in the time of onset of VTE recurrence. When patients were stratified by acute VTE diagnosis, the incidence of VTE recurrence in the on-treatment population was 1.7% (7/420), 1.4% (4/280) and 1.5% (5/323) in patients with PE, proximal DVT and isolated distal DVT only, respectively (Table 5A,B). In contrast, in patients who were observed following discontinuation or interruption of edoxaban treatment (n=670), the incidence of VTE recurrence during the period of discontinuation or interruption was 3.4% (n=23; Table 5C). When patients were stratified by acute VTE diagnosis, the incidence of VTE recurrence during the period of edoxaban discontinuation or interruption was 2.3% (6/258), 4.1% (7/172) and 4.2% (10/240), respectively (Table 5C).
| A | On-treatment population | |||
|---|---|---|---|---|
| Total (n=1,023) |
PE (n=420) |
Proximal DVT (n=280) |
Isolated distal DVT only (n=323) |
|
| All | 16 (1.6) | 7 (1.7) | 4 (1.4) | 5 (1.5) |
| Symptomatic | 7 (0.7) | 4 (1.0) | 2 (0.7) | 1 (0.3) |
| PE | 4 (0.4) | 3 (0.7) | 1 (0.4) | 0 (0.0) |
| Symptomatic | 3 (0.3) | 2 (0.5) | 1 (0.4) | 0 (0.0) |
| DVT alone | 12 (1.2) | 4 (1.0) | 3 (1.1) | 5 (1.5) |
| Symptomatic | 4 (0.4) | 2 (0.5) | 1 (0.4) | 1 (0.3) |
| B | ITT population (during the entire 1-year observation period) | |||
| Total (n=1,023) |
PE (n=420) |
Proximal DVT (n=280) |
Isolated distal DVT only (n=323) |
|
| All | 38 (3.7) | 12 (2.9) | 11 (3.9) | 15 (4.6) |
| Symptomatic | 20 (2.0) | 7 (1.7) | 7 (2.5) | 6 (1.9) |
| PE | 11 (1.1) | 8 (1.9) | 2 (0.7) | 1 (0.3) |
| Symptomatic | 8 (0.8) | 5 (1.2) | 2 (0.7) | 1 (0.3) |
| DVT alone | 27 (2.6) | 4 (1.0) | 9 (3.2) | 14 (4.3) |
| Symptomatic | 12 (1.2) | 2 (0.5) | 5 (1.8) | 5 (1.5) |
| C | During the period of edoxaban discontinuation or interruption | |||
| Total (n=670) |
PE (n=258) |
Proximal DVT (n=172) |
Isolated distal DVT only (n=240) |
|
| All | 23 (3.4) | 6 (2.3) | 7 (4.1) | 10 (4.2) |
| Symptomatic | 14 (2.1) | 4 (1.6) | 5 (2.9) | 5 (2.1) |
| PE | 7 (1.0) | 5 (1.9) | 1 (0.6) | 1 (0.4) |
| Symptomatic | 5 (0.7) | 3 (1.2) | 1 (0.6) | 1 (0.4) |
| DVT alone | 16 (2.4) | 1 (0.4) | 6 (3.5) | 9 (3.8) |
| Symptomatic | 9 (1.3) | 1 (0.4) | 4 (2.3) | 4 (1.7) |
Data given as n (%). ITT, intention to treat. Other abbreviations as in Table 1.
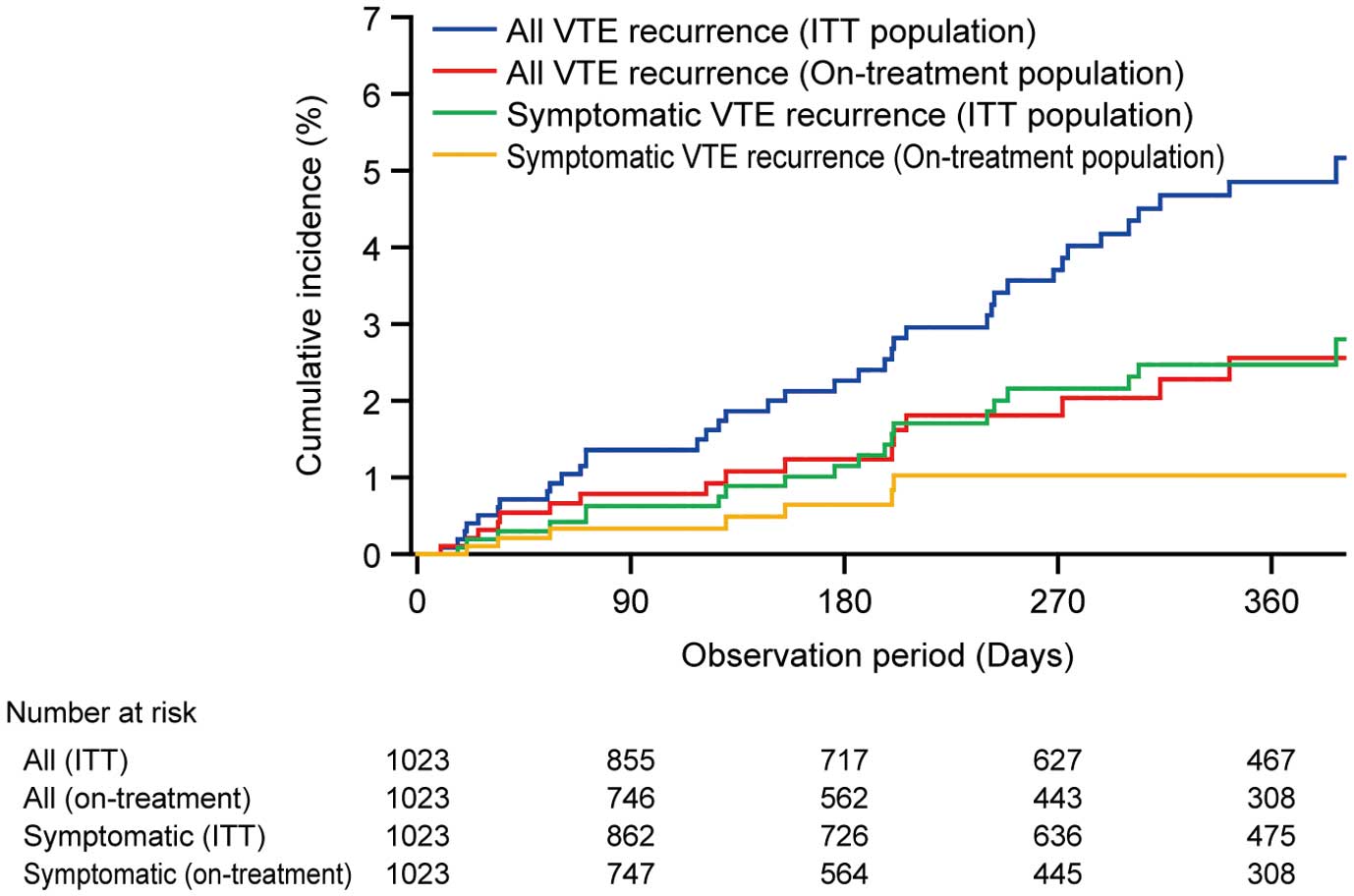
Kaplan-Meier curves of cumulative incidence of venous thromboembolism (VTE) recurrence, in the effectiveness analysis set for patients with acute VTE (n=1,023). ITT, intention to treat.
We report here the 1-year safety and effectiveness of edoxaban in Japanese patients with VTE from a postmarketing observational study. This study is characterized by the inclusion of patients with very high risk of bleeding (i.e., ClCr <30 mL/min and concomitant use of DAPT), who had been excluded from the phase III study, Hokusai-VTE.4 According to the 3-month interim analysis from this observational study, there were no major concerns regarding the safety and effectiveness of edoxaban during the early phase of treatment.12 The present analysis of the 1-year data also confirmed that there were no major concerns about the safety and effectiveness of edoxaban in Japanese patients with VTE during a long-term period of treatment in a real-world setting.
The proportions of VTE patients with high bleeding risk (e.g., the elderly and those with low body weight or renal dysfunction) are higher in Japan than in Western populations.4–7,15–17 Given that most patients with high bleeding risk receive the low dose (30 mg) of edoxaban according to the PI, information regarding the safety and effectiveness of edoxaban in such patients is of particular importance. As in the 3-month interim analysis, in the present study we also found that the safety and effectiveness of edoxaban in patients who received the appropriate low dose (30 mg/day) were similar to those in patients who received the appropriate standard dose (60 mg/day) during a 1-year long-term period of treatment.
The present study included a greater proportion of patients with high bleeding risk compared with the Hokusai-VTE4 (age ≥75 years, 39.4% vs. 13.6%; body weight ≤60 kg, 58.2% vs. 12.7%; renal function ≤50 mL/min, 22.2% vs. 6.5%; cancer present, 26.9% vs. 9.2%). According to the starting dose of edoxaban, the proportion of patients at high risk of bleeding was greater at the appropriate low dose of 30 mg than at the appropriate standard dose of 60 mg in the present study. Similarly, the proportion of patients with high bleeding risk was also greater in the under-dose 30-mg group than in the appropriate standard-dose 60-mg group. We assume that the reason for this was that even patients without dose adjustment factors were more likely to receive under-dose of 30 mg if they had advanced age, cancer, or a history of bleeding, which are considered to be high risks for bleeding.
With regard to the status of edoxaban, approximately two-thirds of the initial daily dose was the low dose of 30 mg, due to the high proportion of Japanese patients with high bleeding risk. Approximately 90% of patients received the dosage specified in the PI. In contrast, the under-dose rate was 7.4%, mainly due to older age and renal dysfunction (which did not meet the criteria for decreasing the dose of edoxaban in the PI). A certain number of patients in real-world clinical settings, such as elderly patients, will receive an under-dose in consideration of the risk of bleeding, even in the absence of dose adjustment factors. The present under-dose rates, however, were lower than those in the RIETE registry, which enrolled patients with VTE receiving DOAC mainly such as rivaroxaban or apixaban.18 This may have been due to the appropriate dose reduction criteria, which have been established only for edoxaban of the DOAC available for VTE treatment. Approximately half of the patients discontinued edoxaban in 1 year, and the main reason for discontinuation was “completion of treatment as planned”.
Regarding clinical outcomes (safety and effectiveness), the present incidence of bleeding AE was lower than in the Hokusai-VTE (21.7%), although the incidence of VTE recurrence was similar (Hokusai-VTE: 1.6% in the on-treatment population).4 Regarding the time of onset of bleeding AE, approximately half occurred in the first 3 months of edoxaban treatment, and a similar trend was confirmed in Hokusai-VTE.4 This suggests that more attention should be paid to bleeding AE in the early phase of anticoagulation therapy for VTE. The incidence of major bleeding in the present study was slightly greater than that in the Hokusai-VTE (1.4%), which was considered to be due to the higher rate of baseline comorbidity of cancer in the present study compared with the Hokusai-VTE (9.2%),4 as well as the higher incidence of major bleeding in patients with cancer in the present study. The incidence of major bleeding in the present study, however, was not higher than the COMMAND VTE Registry (approximately 6.0% during 1 year), despite the same proportion of patients with cancer (approximately 23%) in both studies.19 This suggests that edoxaban is well tolerated in a real-world setting in Japanese VTE patients during 1-year long-term treatment.
As previously mentioned, a high proportion of Japanese patients are at high risk for bleeding, and such patients are given a low dose of edoxaban (30 mg). In contrast, physicians may be concerned about a possible lack of effectiveness with the low dose of 30 mg. Thus, the safety and effectiveness results of the 30 mg group in this study are particularly important. In the present study, the safety and effectiveness of the appropriate low-dose 30-mg group were similar to those of the appropriate standard-dose 60-mg group. Therefore, there were no major concerns about the safety and effectiveness of edoxaban at a low dose.
In the present study, the incidence of bleeding AE was not remarkably increased in the over-dose 60-mg group. Furthermore, in the under-dose 30-mg group, in which the proportion of patients at high risk for bleeding was high, a greater incidence of bleeding was not seen, nor was a greater incidence of VTE recurrence, as noted in the RIETE registry,18 seen either. However, due to the limited number of patients in the over-dose and under-dose groups, ranging from a few dozen to a hundred, it is difficult to evaluate these groups in a multifaceted manner. Additional studies with a greater number of patients are needed to evaluate the safety and effectiveness of over-dose and under-dose edoxaban.
Although the incidence of bleeding AE was not high, the factors associated with bleeding AE identified on multivariate analysis were as follows: cancer, anemia, and bleeding-related congenital or acquired disease, and the concomitant use of antiplatelet drugs and NSAIDs other than low-dose aspirin. In contrast, hypertension and cancer were identified as being associated with major bleeding. Most bleeding risk factors identified in the present study are the same as those reported in previous studies.20–24 Cancer was identified as being associated with VTE recurrence on multivariate analysis. This risk factor has also been reported in previous studies.20 Furthermore, in large-scale observational studies conducted in the real world, many patients with high risk (e.g., low body weight, renal impairment or cancer), who are not included sufficiently in pivotal clinical trials, are likely to be accumulated. Because such high-risk patients are often treated in clinical practice, it is important to assess the risk/benefit balance of these patients in real-world studies, which may be helpful for physicians in clinical practice. Therefore, the current study assessed the risk (as rate of bleeding AE) and benefit (as rate of VTE recurrence) for high-risk patients, and investigated the safety and effectiveness on multivariate analysis. With reference to the multivariate analysis and risk/benefit in the high-risk patients, anticoagulation requires more careful monitoring for bleeding in patients considered to have a particularly high risk of bleeding. This study suggests that patients with cancer should be treated with anticoagulant therapy in consideration of the risk of embolization as well as the risk of bleeding.
With regard to anticoagulation therapy for VTE and prevention of recurrence, treatment of patients with acute VTE is particularly important. The incidence of VTE recurrence in these patients in the present analysis was similar to that in the Hokusai-VTE. In addition, the recommended duration of anticoagulation for treatment or suppression of VTE recurrence varies according to the diagnosis of VTE and the related risk of recurrence for each. In the present patients with acute VTE, edoxaban treatment periods were short and continuation rates were low in patients with isolated distal DVT only, who are at low risk for VTE recurrence according to the guidelines. Furthermore, the incidence of VTE recurrence in the on-treatment population was slightly higher in patients with PE, and the results were similar to those reported previously.12 In contrast, the incidence of VTE recurrence after discontinuation or interruption of edoxaban was high in all acute VTE patients, and also according to VTE diagnosis: it was high not only in patients with PE and proximal DVT (for whom the JCS 2017 guidelines state that long-term continuation is recommended for at least 3 months), but also in patients with isolated distal DVT only, for whom the guideline states that no standardized anticoagulation should be used.13 When details of patients with isolated distal DVT only, who had VTE recurrence after discontinuation or interruption of edoxaban, were examined (Supplementary Table 5), many patients had VTE risk factors such as cancer, varicose veins in the lower extremities, myasthenia gravis or ulcerative colitis. When risk factors other than cancer or a transient risk were defined as “unprovoked” according to JCS 2017, 9 out of 10 patients had VTE risk factors that were included in the “cancer” category or in the “unprovoked” category,13 and most of the patients with risk factors in the “unprovoked” category had VTE recurrence a few months after “completed the edoxaban treatment as planned”. For the duration of anticoagulation therapy for patients with the risk factor in the “cancer” category, longer anticoagulation is recommended in JCS 2017.13 Whereas a treatment duration ≥3 months is recommended for patients with risk factors in the “unprovoked” category, the recently reported COMMAND-VTE study suggested the usefulness of continued anticoagulation therapy for ≥1 year compared with a treatment duration <1 year.19 Similarly, in the present study, the usefulness of continuing anticoagulation therapy for patients with risk factors in the “unprovoked” category was also suggested. The timing of discontinuation should be carefully determined, and anticoagulation therapy may be considered for as long as possible if individual conditions permit, even in patients with isolated distal DVT only, whose risk factors for VTE are “cancer” and “unprovoked”.
Study LimitationsThe study has the following limitations. It was an observational study, and there were no control groups. Only Japanese patients were included, which may limit the generalizability of the results. Second, comparison between the present results and those of clinical trials is difficult due to the different patient background characteristics (e.g., patients with very high bleeding risk were included in ETNA-VTE-Japan but excluded from the Hokusai-VTE).4
There were no major concerns about the safety and effectiveness of edoxaban in Japanese patients with VTE in 1 year of treatment in a real-world setting. Furthermore, there were no major concerns about the safety and effectiveness of the appropriate low-dose edoxaban (30 mg/day), which is likely to be prescribed for patients with high bleeding risk.
Editorial assistance for the preparation of this manuscript was provided by Kokoro Koyama, PhD, of inScience Communications, Springer Healthcare, and Rie Ishibashi. This assistance was funded by Daiichi Sankyo Co., Ltd.
The individual identified participant data (including data dictionaries) will not be shared.
Daiichi Sankyo Co., Ltd. (Tokyo, Japan).
M.N. has received lecture fees from Daiichi Sankyo Co., Ltd. N.Y. has received lecture fees from Daiichi Sankyo Co., Ltd., Bayer Yakuhin, Ltd., Bristol-Myers Squibb K.K., and Pfizer Japan Inc. T.A., K.S., and K.U. are employees of Daiichi Sankyo Co., Ltd.
The study protocol was approved by the In-House Committee of Daiichi Sankyo Co., Ltd., and by the Ministry of Health, Labour and Welfare of Japan, on 8 November 2014.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circrep.CR-19-0127