2021 年 3 巻 10 号 p. 615-619
2021 年 3 巻 10 号 p. 615-619
Background: Although many risk factors have been reported to be associated with poor clinical outcomes following transcatheter aortic valve replacement (TAVR), the implications of inadequate cardiac unloading following TAVR remain unknown. We investigated the prognostic impact of inadequate cardiac unloading following TAVR.
Methods and Results: We retrospectively analyzed a cohort of patients with severe aortic stenosis who underwent invasive hemodynamic assessment following TAVR. The impact of inadequate cardiac unloading, defined as an elevated pulmonary capillary wedge pressure (PCWP), on the composite primary endpoint of cardiovascular mortality or heart failure readmission was investigated. Eighty-two patients (median age 86 years; 57 women) were included. Median PCWP following TAVR was 9 mmHg (interquartile range 7–13 mmHg). A higher PCWP tended to be associated with an increased risk of adverse cardiovascular events (adjusted hazard ratio 1.18; 95% confidence interval 0.99–1.41). A cut-off value of PCWP >12 mmHg, calculated by time-dependent receiver operating characteristics analysis, stratified the cumulative incidence of the primary endpoint (2-year incidence of 36% vs. 8%). Uptitration of the diuretic dose was associated with event freedom among those with PCWP >12 mmHg.
Conclusions: Inadequate cardiac unloading following TAVR was associated with an increased risk of adverse clinical events. Uptitration of medical therapies to improve cardiac hemodynamics after TAVR may reduce this risk.
Transcatheter aortic valve replacement (TAVR) is a widely used, evidence-based option for patients with severe aortic stenosis (AS) and high operative risk, and has evolved over the past decade, with procedural complications rare and a shortened length of stay in the vast majority of patients.1–3
Nevertheless, post-TAVR clinical outcomes may not be acceptable as yet in certain subsets of patients, most commonly in those with symptomatic heart failure. Several periprocedural risk factors, including female sex, advanced New York Heart Association class, and left ventricular ejection fraction (LVEF) <30%, are associated with post-TAVR adverse events.4
The prognostic implications specifically of a patient’s hemodynamic profile after TAVR have not been rigorously assessed. Right heart catheterization is currently the gold standard to accurately quantify the intracardiac pressure status. In the present study, we investigated the association between post-TAVR invasive hemodynamics and clinical outcomes.
Patients who underwent TAVR at our institute between October 2017 and October 2019 to treat severe AS, defined as an aortic valve area <1.0 cm2 and peak velocity through the aortic valve >4.0 m/s, were prospectively included in the present study. Low-flow, low-gradient AS was defined by inotrope-loading echocardiography. Patients underwent hemodynamic assessment with right heart catheterization 1 week after TAVR, as detailed below. Following the index discharge, all patients were followed-up until March 2021.
All patients provided informed consent before enrollment in the study. This study was approved by the University of Toyama Ethics Committee (R2015154).
TAVR ProcedureThe indication for and approach of TAVR were determined by the heart team, which consisted of cardiologists, cardiovascular surgeons, and anesthesiologists.2 All patients received balloon-expandable valves (Sapien XT or Sapien 3; Edwards Lifesciences, Irvine, CA, USA) or self-expandable valves (Corevalve or Evolut R; Medtronic, Minneapolis, MN, USA) via a transfemoral or transapical approach under general anesthesia.
Hemodynamic AssessmentRight heart catheterization was performed in the cardiac catheterization laboratory 1 week after TAVR by board-certificated heart failure experts. Standard hemodynamic variables were measured, including cardiac index using the thermodilution method, pulmonary capillary wedge pressure (PCWP), pulmonary artery pressure, and central venous pressure. The position of PCWP was verified by measurement of oxygen saturation before hemodynamic registration of the pressure.
Clinical Variables EvaluatedDemographics, comorbidities, and laboratory, echocardiographic, and medication data were collected 1 week before TAVR and then again after TAVR. The composite primary endpoint was defined as cardiovascular death or heart failure readmission requiring intravenous diuretics with hospitalization during the observational period following the index discharge.
Statistical AnalysisContinuous variables are expressed as the median and interquartile range (IQR) and categorical variables are expressed as numbers and percentages. Continuous and categorical variables were compared between groups with and without the primary endpoint using the Mann-Whitney U test or Fischer’s exact test, respectively. Cox proportional hazard ratio regression analyses were performed to investigate the impact of post-TAVR PCWP on the primary endpoint. The analysis was adjusted for other variables with P<0.02 in the 2-group comparisons (i.e., those with vs. without the primary endpoint), including body surface area and mean right atrial pressure. Time-dependent receiver operating characteristic (ROC) curve analysis was performed to calculate a cut-off value of PCWP to stratify the primary endpoint. The cumulative incidence of the primary endpoint was stratified using a cut-off value of PCWP. Unless specifically stated otherwise, 2-tailed P<0.05 was considered statistically significant. Statistical analyses were conducted using SPSS Statistics 22 (SPSS Inc., Armonk, NY, USA).
Among 115 consecutive patients who underwent TAVR, 82 (median age 86 years [IQR 83–89 years]; 57 women) who received right heart catheterization after TAVR were included in this study. The baseline characteristics of these patients are summarized in the Table. Following TAVR, the median aortic valve area was 1.38 cm2 (IQR 1.21–1.63 cm2) and the peak velocity through the aortic valve was 2.1 m/s (IQR 1.8–2.4 m/s). Plasma B-type natriuretic peptide (BNP) concentrations were 110 pg/mL (IQR 59–230 pg/mL). On right heart catheterization, the median value of mean right atrial pressure was 5 mmHg (IQR 3–6 mmHg), median PCWP was 9 mmHg (IQR 7–13 mmHg), and the median cardiac index was 2.59 L/min/m2 (IQR 2.36–2.98 L/min/m2).
| Total (n=82) |
With events (n=14) |
Without events (n=68) |
P value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 86 [83–89] | 86 [83–88] | 87 [83–89] | 0.67 |
| Male sex | 25 (30) | 6 (43) | 19 (28) | 0.21 |
| Body surface area (m2) | 1.38 [1.29–1.50] | 1.49 [1.38–1.64] | 1.36 [1.27–1.47] | 0.019* |
| Comorbidity | ||||
| Diabetes | 14 (17) | 1 (7) | 13 (19) | 0.26 |
| Coronary artery disease | 22 (27) | 3 (21) | 19 (28) | 0.58 |
| History of stroke | 22 (27) | 6 (43) | 16 (24) | 0.17 |
| History of HF admission | 38 (46) | 6 (43) | 32 (47) | 0.70 |
| Peripheral artery disease | 29 (35) | 6 (43) | 23 (34) | 0.57 |
| Pre-TAVR laboratory data | ||||
| Hemoglobin (g/dL) | 11.4 [10.5–12.4] | 10.8 [10.2–12.0] | 11.4 [10.5–12.3] | 0.66 |
| Serum sodium (mEq/L) | 141 [138–142] | 141 [139–144] | 141 [139–142] | 0.56 |
| Serum total bilirubin (mg/dL) | 0.6 [0.4–0.7] | 0.6 [0.4–0.8] | 0.5 [0.4–0.7] | 0.29 |
| eGFR (mL/min/1.73 m2) | 50.1 [37.9–62.7] | 41.0 [36.8–51.2] | 47.7 [35.4–59.8] | 0.50 |
| Plasma BNP (pg/mL) | 143 [87–287] | 297 [165–387] | 198 [98–298] | 0.048* |
| Pre-TAVR echocardiography | ||||
| Aortic valve area (cm2) | 0.54 [0.44–0.68] | 0.67 [0.49–0.81] | 0.52 [0.43–0.61] | 0.19 |
| Peak velocity through aortic valve (m/s) | 4.4 [4.0–5.0] | 4.1 [3.6–4.4] | 4.7 [4.1–5.2] | 0.058 |
| LV end-diastolic diameter (mm) | 45 [42–50] | 44 [42–46] | 46 [42–50] | 0.39 |
| LVEF (%) | 65 [54–71] | 69 [67–75] | 64 [55–72] | 0.025* |
| Pre-TAVR hemodynamics | ||||
| Mean right atrial pressure (mmHg) | 5 [4–7] | 6 [4–7] | 5 [4–7] | 0.54 |
| PCWP (mmHg) | 11 [8–15] | 12 [9–16] | 11 [8–13] | 0.25 |
| Cardiac index (L/min/m2) | 2.66 [2.39–2.98] | 2.45 [2.25–2.77] | 2.72 [2.44–2.98] | 0.12 |
| Pre-TAVR medication | ||||
| β-blocker | 21 (26) | 3 (21) | 18 (26) | 0.69 |
| RAS inhibitor | 50 (61) | 8 (57) | 42 (62) | 0.75 |
| Post-TAVR laboratory data | ||||
| Hemoglobin (g/dL) | 10.4 [9.7–11.0] | 10.0 [9.5–10.3] | 10.6 [9.9–11.1] | 0.024* |
| Serum sodium (mEq/L) | 140 [138–141] | 141 [138–143] | 140 [138–141] | 0.32 |
| Serum total bilirubin (mg/dL) | 0.5 [0.4–0.6] | 0.6 [0.4–0.7] | 0.5 [0.4–0.6] | 0.11 |
| eGFR (mL/min/1.73 m2) | 48.6 [36.2–64.3] | 45.3 [39.1–64.6] | 51.1 [33.9–63.6] | 0.83 |
| Plasma BNP (pg/mL) | 110 [59–230] | 264 [112–348] | 101 [55–187] | 0.026* |
| Post-TAVR echocardiography | ||||
| Aortic valve area (cm2) | 1.38 [1.21–1.63] | 1.48 [1.33–1.65] | 1.37 [1.19–1.61] | 0.16 |
| Peak velocity through aortic valve (m/s) | 2.1 [1.8–2.4] | 1.8 [1.7–2.3] | 2.1 [1.9–2.4] | 0.18 |
| Para-valvular leak | 20 (24) | 5 (36) | 15 (22) | 0.28 |
| LV end-diastolic diameter (mm) | 45 [41–50] | 44 [41–45] | 47 [41–50] | 0.17 |
| LVEF (%) | 64 [56–71] | 70 [63–74] | 63 [54–70] | 0.031* |
| Post-TAVR hemodynamics | ||||
| SBP (mmHg) | 117 [105–128] | 115 [101–127] | 118 [106–128] | 0.58 |
| Heart rate (beats/min) | 70 [63–78] | 66 [63–71] | 72 [63–78] | 0.45 |
| Mean right atrial pressure (mmHg) | 5 [3–6] | 7 [4–9] | 4 [3–6] | 0.012* |
| PCWP (mmHg) | 9 [7–13] | 13 [11–18] | 9 [7–12] | 0.002* |
| Cardiac index (L/min/m2) | 2.64 [2.43–2.94] | 2.41 [2.26–2.73] | 2.58 [2.37–2.93] | 0.097 |
| Post-TAVR medication | ||||
| β-blocker | 27 (33) | 5 (36) | 22 (32) | 0.81 |
| RAS inhibitor | 57 (70) | 11 (79) | 46 (68) | 0.42 |
Continuous variables are expressed as the median [interquartile range] and were compared using the Mann-Whitney U test. Categorical variables are expressed as n (%) and were compared using Fischer’s exact test. *P<0.05. BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; HF, heart failure; LV, left ventricular; LVEF, left ventricular ejection fraction; PCWP, pulmonary capillary wedge pressure; RAS, renin-angiotensin system; SBP, systolic blood pressure; TAVR, transcatheter aortic valve replacement.
During the observation period (median 811 days; IQR 627–952 days) after the index discharge, 14 patients experienced the primary endpoint (7 cardiovascular deaths, 11 heart failure readmissions, and 4 patients with both). Several baseline characteristics differed between those with and without the composite primary endpoint (Table). Of note, post-TAVR PCWP (median [IQR] 13 [11–18] vs. 9 [7–12] mmHg; P=0.002) and BNP (median [IQR] 264 [112–348] vs. 101 [55–187] pg/mL; P=0.026) were higher in patients with than without the primary endpoint, although the clinical implication of such differences may be modest.
Post-TAVR PCWP was associated with the occurrence of the composite primary endpoint, with an unadjusted hazard ratio (HR) of 1.26 (95% confidence interval [CI] 1.11–1.42; P<0.001). After adjustment for body surface area and mean right atrial pressure, both of which differed significantly between the 2 groups, the adjusted HR was 1.18 (95% CI 0.99–1.41; P=0.062).
A cut-off value of PCWP to stratify the primary endpoint was calculated to be 12 mmHg (Figure 1). The cumulative incidence of the primary endpoint differed significantly when stratified according to the PCWP cut-off value (P=0.001; Figure 2). The 2-year cumulative incidence of either cardiovascular death or heart failure hospitalization was significantly greater in those with a post-TAVR PCWP >12 mmHg (36% vs. 8%). This cut-off value also stratified patients on the cumulative incidence of heart failure readmissions alone (P=0.003).
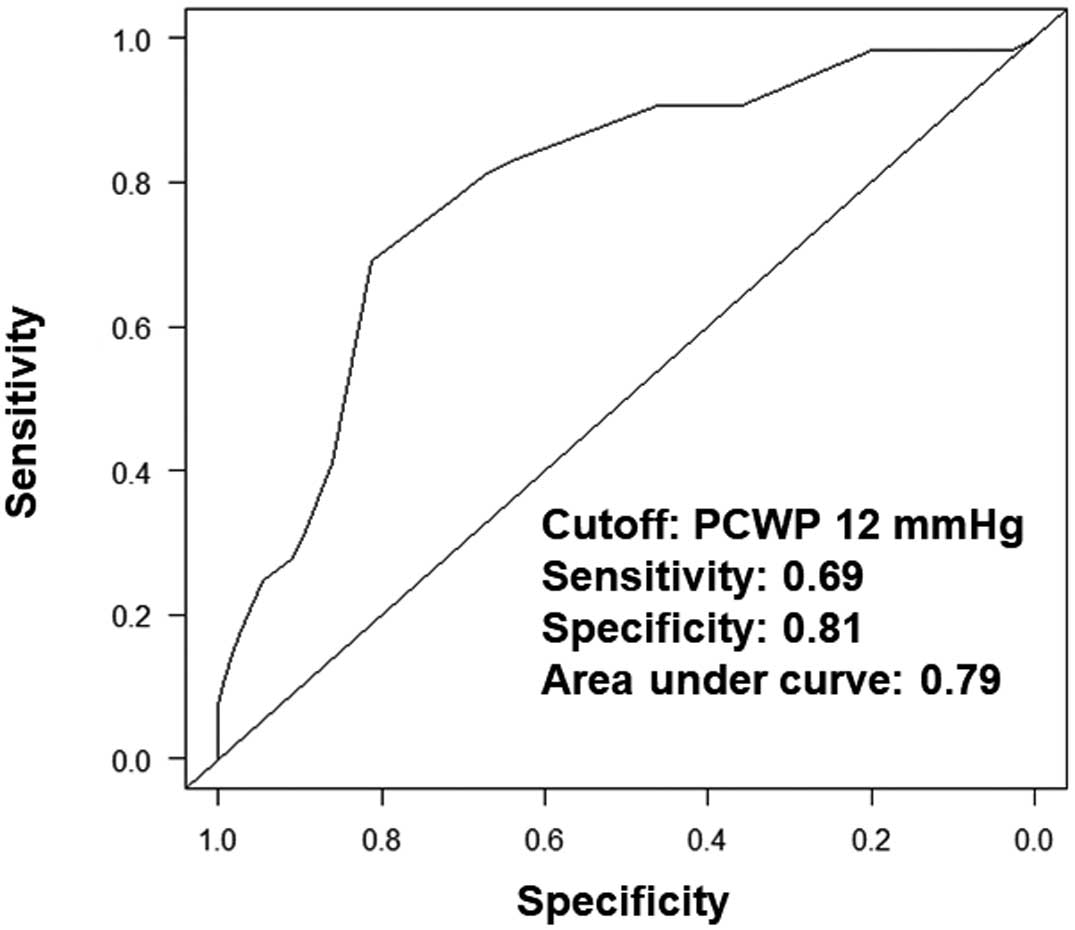
Time-dependent receiver operating characteristic curve analysis to calculate a cut-off value for pulmonary capillary wedge pressure (PCWP) to predict the primary endpoint.

Cumulative incidence of the primary endpoint stratified by the cut-off value for pulmonary capillary wedge pressure (PCWP). *P<0.05 (log-rank test).
The median change in PCWP from before to after TAVR was −6 mmHg (IQR −10, −2 mmHg). The effect of the change in PCWP on the primary endpoint did not reach statistical significance (P=0.069; HR 1.07, 95% CI 0.99–1.16).
Comparison of the Prognostic Impact of BNP vs. PCWPThe unadjusted HR for PCWP >12 mmHg for the primary endpoint was 5.86 (95% CI 1.96–17.5; P=0.002). For patients with BNP >178 pg/mL, with this cut-off value also calculated using ROC curve analysis, the unadjusted HR was 4.00 (95% CI 1.34–12.0; P=0.013).
Implication of Diuretic Dose Adjustment Among Those With PCWP >12 mmHgOf the 22 patients with PCWP >12 mmHg, the furosemide equivalent dose was increased from a median of 20 to 30 mg in 13 patients without any future events). The furosemide equivalent dose (median 20 mg) was unchanged in the remaining 9 patients, who experienced future events (Figure 3).
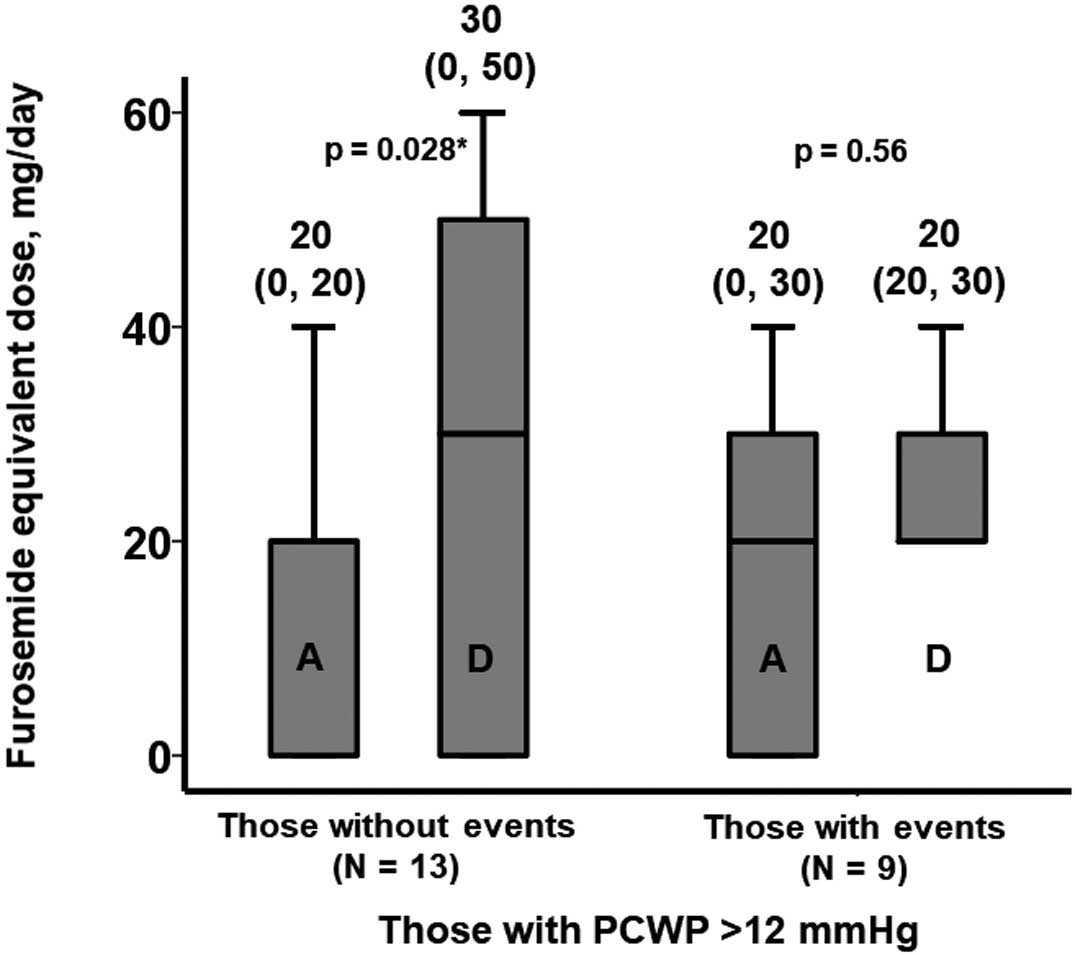
Changes in the furosemide equivalent dose during the index hospitalization among patients with a pulmonary capillary wedge pressure (PCWP) >12 mmHg. A, admission; D, discharge. *P<0.05 (Wilcoxon signed-rank test). Values above columns are the median (interquartile range) furosemide equivalent dose.
In this study we observed a higher risk of the combined endpoint of cardiovascular death or heart failure hospitalization in patients with inadequate cardiac unloading as defined by a PCWP >12 mmHg following TAVR. Among those with PCWP >12 mmHg, the dose of loop diuretics was uptitrated in patients without any events.
Inadequate Cardiac Unloading Following TAVRBecause we observed a higher risk of adverse cardiovascular events in patients with high post-procedure filling pressures, early attention to clinical volume status should be a focus of the care team to potentially mitigate risk. Right heart catheterization may not always be available in all hospitals due to its invasive nature and the resources needed for continuous hemodynamic monitoring. Plasma BNP concentrations are a well-known prognostic biomarker for increased morbidity and mortality in both heart failure patients and those undergoing TAVR,5,6 and should be considered as part of the routine clinical assessment to better estimate filling pressures when invasive assessment is not possible.7 However, discordant interpretations can occur in the presence of obesity, advanced age, atrial fibrillation, and chronic kidney disease.8 Of note, given Laplace’s law, the BNP concentration may not accurately indicate intracardiac pressure in the AS cohort (i.e., in patients with a thick left ventricular wall, then BNP concentration is underestimated).9 Furthermore, the prognostic predictability of BNP in the present study, assessed by the HR, was inferior to that of the invasively measured PCWP.
Interestingly, the cut-off of PCWP was 12 mmHg, which is relatively lower than the conventional values in contemporary heart failure studies.10 The remodeled left ventricle may be unstable following TAVR and even a slight increase in the filling pressures in this postoperative state may increase the risk for incident heart failure. Until a method to accurately and non-invasively quantify cardiac unloading is developed, we would like to recommend that right heart catheterization is performed after TAVR.
Implication of Aggressive Cardiac Unloading Following TAVRData on effective therapeutic strategies for patients with inadequate cardiac unloading following TAVR are lacking. A recent study demonstrated that the use of β-blockers was associated with lower heart failure recurrence in patients with plasma BNP ≥400 pg/mL and LVEF <50% following TAVR.11 A meta-analysis demonstrated that renin-angiotensin system inhibitors were associated with greater survival following TAVR.12 We recently reported a case where medical optimization with non-invasive positive pressure ventilation therapy was used to treat persistent inadequate cardiac unloading following TAVR.13
In the present study, the uptitration of diuretics for those with PCWP >12 mmHg was associated with a lower incidence of heart failure events. Appropriate intervention for mild cardiac congestion may improve clinical outcomes. Of note, we should be careful of imprudent uptitration of loop diuretics, particularly for those with low cardiac output and impaired renal function. For the appropriate intervention tools, the concomitant use of recently introduced agents, including tolvaptan, angiotensin receptor-neprilysin inhibitor, and sodium-glucose cotransporter 2 inhibitors, could be considered.
Study LimitationsThe cohort size and number of events were moderate in size; we also included a restricted number of potential confounders in the multivariate analyses. Other uninvestigated confounders may have affected the outcomes. For example, a history and the severity of heart failure may have affected clinical outcomes. We performed right heart catheterization 1 week after TAVR rather than periprocedurally to avoid the effect of procedure-related invasion, but the appropriate timing of right heart catheterization and alternative methodologies to estimate the degree of cardiac loading remain to be determined. We used PCWP as alternative for left ventricular end-diastolic pressure and cannot exclude the effects of other factors, including pulmonary diseases. We did not include patients with severe pulmonary diseases in this study, and our findings may not be applicable to such patients. Multivariate analysis did not reach statistical significance with P=0.062. Further studies with larger cohorts are warranted. We did not assess all-cause mortality as an endpoint given the age and comorbidities of the study cohort. We did not repeat right heart catheterization, and the implications of interventions for elevated PCWP need to be evaluated in future studies.
Inadequate cardiac unloading, as defined by mildly elevated PCWP, was associated with an increased risk of cardiovascular mortality and heart failure recurrence following TAVR. Post-TAVR care protocols in vulnerable patient subsets, especially those with a history of systolic or diastolic heart failure, are needed to reduce future adverse events.
None.
This study did not receive any specific funding.
K.K. is a member of Circulation Reports’ Editorial Team. The remaining authors have no conflicts of interest to disclose.
This study was approved by the University of Toyama Ethics Committee (R2015154).
Original data are available from the corresponding author upon reasonable request.