Abstract
Background:
The prognostic impact of urinary isoxanthopterin (U-IXP), a recently proposed marker of oxidative stress, in patients with heart failure remains unknown.
Methods and Results:
Patients who were admitted to our institute for decompensated heart failure were prospectively included in the study; U-IXP was measured on admission. The association between the U-IXP concentration and a composite primary outcome that included cardiovascular death and heart failure readmissions following the index discharge was investigated. In all, 42 patients (median age 78 years [interquartile range {IQR} 69–85 years]; 25 males) were included in the study. The median U-IXP concentration on admission was 0.58 μmol/g creatinine (Cre; IQR 0.35–0.95 μmol/g Cre). A higher U-IXP concentration was an independent predictor of the primary outcome adjusted for clinical potential confounders and was associated with a significantly higher cumulative incidence of the primary outcome (71% vs. 16%, P=0.001) at a cut-off of 0.93 μmol/g Cre.
Conclusions:
U-IXP on admission was associated with cardiovascular death or heart failure readmission following the index discharge in patients with decompensated heart failure. The clinical implication of aggressive interventions to normalize U-IXP and the detailed prognostic mechanism of U-IXP in heart failure patients remain the next concerns.
Despite guideline-directed medical therapy, mortality and morbidity in patients with heart failure is not satisfactorily low. Thus, further methods to risk stratify the patient cohort using novel markers are warranted. Of note, non-invasive markers are ideal for repeated assessments.
One of the potential risk biomarkers may be associated with reactive oxygen species (ROS), which are key players in vascular endothelial injury and reperfusion injury and are associated with the progression of cardiovascular diseases.1
In humans, xanthine oxidase (XO) generates ROS. Xanthine dehydrogenase in vascular endothelial cells can be converted to XO in response to hypoxic or other stimuli, and the XO is secreted into the systemic circulation and generates ROS in local tissues.2
In patients with heart failure, the uptake of XO in local tissues is enhanced, leading to increased ROS production.3
However, previous studies failed to find oxidative stress-related non-invasive prognostic markers.
XO facilitates the conversion of pterin to isoxanthopterin (IXP), which is secreted into the urine. Urinary IXP (U-IXP) is a useful marker to assess systemic oxidative stress.4
Thus, U-IXP may be a promising novel marker to assess the degree of ROS activity and to predict mortality and morbidity in heart failure patients.5
Although several biomarkers are clinically available to predict prognosis in heart failure patients, U-IXP has the advantages of being a non-invasive marker and being uniquely associated with the ROS system. In this study we investigated the association between U-IXP and clinical outcomes in heart failure patients.
Methods
Patient Selection
Patients who were admitted to our institute for the treatment of decompensated heart failure between July 2019 and September 2020 were prospectively included in the present study. Patients aged <20 years, those admitted at the weekend, those who had undergone cardiac intervention within the past 1 month, and those who declined to take part in the study were excluded. All patients provided written informed consent. This study was approved by the Clinical Research Review Board, University of Toyama (R2015154).
Measurement of U-IXP
Urine samples were obtained on admission from all patients, as well as during a clinically stable phase during the index hospitalization from some patients. U-IXP was measured by HPLC (column, YMC-Triart C18; size 100 mm × 4.6 mm (length × diameter); detector, UV at 2,534 nm; YMC, Kyoto, Japan). The U-IXP/creatinine (Cre) ratio quotient was calculated as an independent variable.
Measurement of Other Clinical Data
Baseline characteristics, including demographics, results of laboratory investigations, echocardiography, and medication data on admission, were obtained. In-hospital length of stay and mortality were recorded. Medication and plasma B-type natriuretic peptide (BNP) concentrations were obtained at index discharge.
Following the index discharge, patients received guideline-directed medical therapy as long as possible until June 2021. The primary outcome of the present study was a composite of cardiovascular death or heart failure readmissions that required intravenous diuretics with careful in-hospital observation. Medication, echocardiography, and plasma BNP concentrations were also obtained at the 6-month follow-up as a secondary outcome.
Statistical Analysis
Continuous variables are expressed as the median with interquartile range (IQR) regardless of their distribution and were compared between 2 groups using the Mann-Whitney U test. Categorical variables are expressed as numbers and percentages and were compared between 2 groups using Fisher’s exact test. Two-sided P<0.05 was considered statistically significant. Statistical analyses were performed using SPSS Statistics 22 (SPSS Inc., Armonk, NY, USA).
The primary outcome was a composite of cardiovascular death and heart failure readmission. The effect of U-IXP at the time of admission on the primary outcome was the major concern of this study. The cut-off value for U-IXP was calculated by receiver operating characteristic (ROC) curve analysis for the primary endpoint. The patient cohort was the stratified according to the U-IXP cut-off. Kaplan-Meier analyses were performed to assess the cumulative incidence of the primary endpoint. Cox proportional hazard ratio regression analyses were performed to investigate the effect of U-IXP at the time of admission on the primary endpoint. The effect of U-IXP at the time of admission was adjusted for other clinical potential confounders determined at admission, including age, left ventricular ejection fraction (LVEF), plasma BNP, serum sodium, hemoglobin, and uric acid concentrations, and the use of febuxostat.
Results
Baseline Characteristics
Among 57 patients, 12 patients who were admitted on a weekend, 2 who had undergone cardiac intervention within the past 1 month, and 1 who decline to take part in the study were excluded (Figure 1). Thus, 42 patients (median age 78 years [IQR 69–85 years]; 25 males) were included in the study. All patients were hospitalized due to worsening heart failure. Half had previous histories of heart failure admission. Median left ventricular end-diastolic diameter was 51 mm (IQR 44–62 mm) and LVEF was 44% (IQR 29–60%). Plasma BNP at admission was 525 pg/mL (IQR 194–1,173 pg/mL;
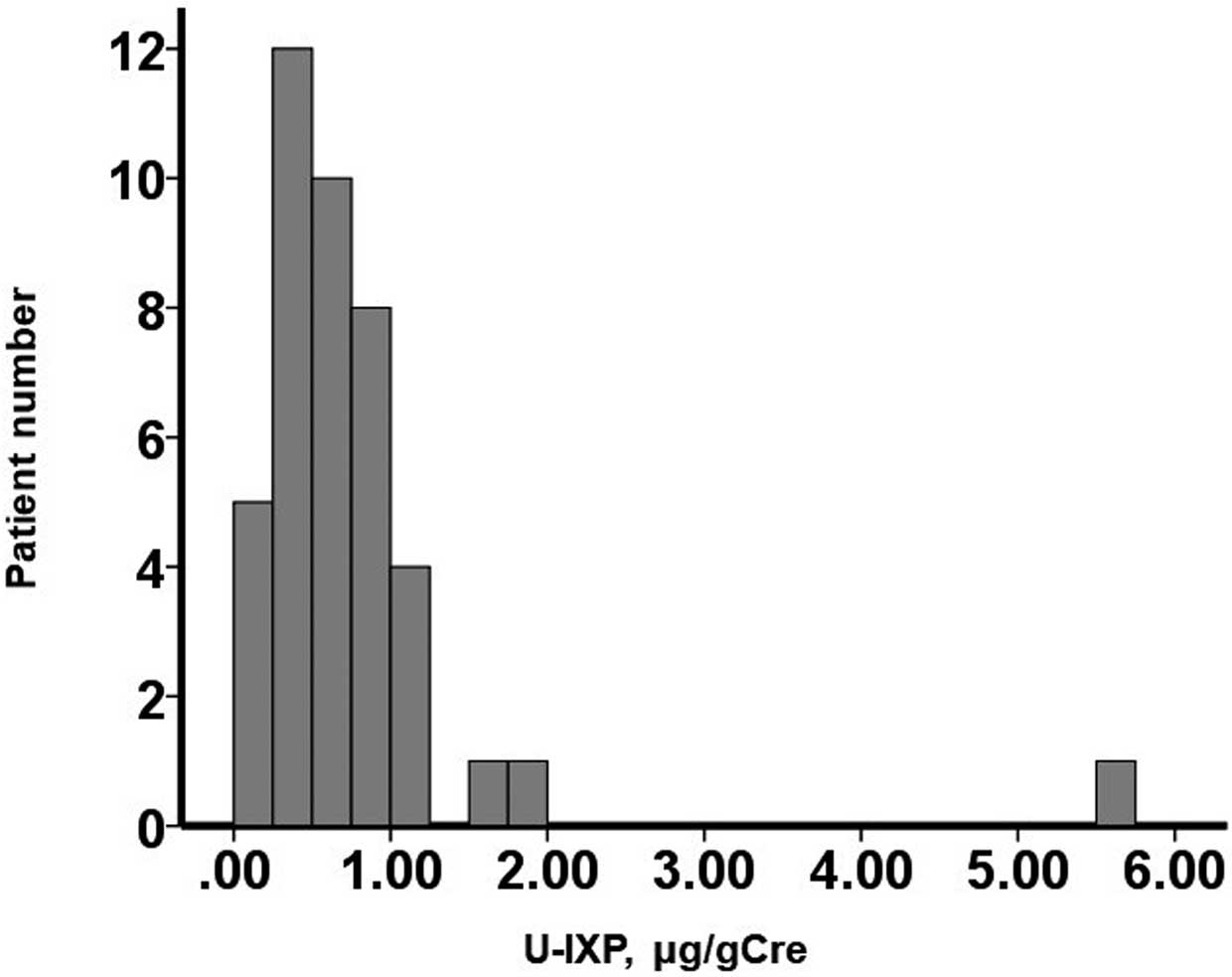
Table 1). The median U-IXP concentration was 0.58 μmol/g Cre (IQR 0.35–0.95 μmol/g Cre). U-IXP was widely distributed, with 11 of 42 patients having high U-IXP (i.e., >0.93 μmol/g Cre, which was the statistically calculated cut-off value [see below];
Figure 2). As a reference for the normal range of U-IXP, 19 patients with benign arrhythmia without any heart diseases had a median U-IXP of 0.44 μmol/g Cre (IQR 0.25–0.65 μmol/g Cre).
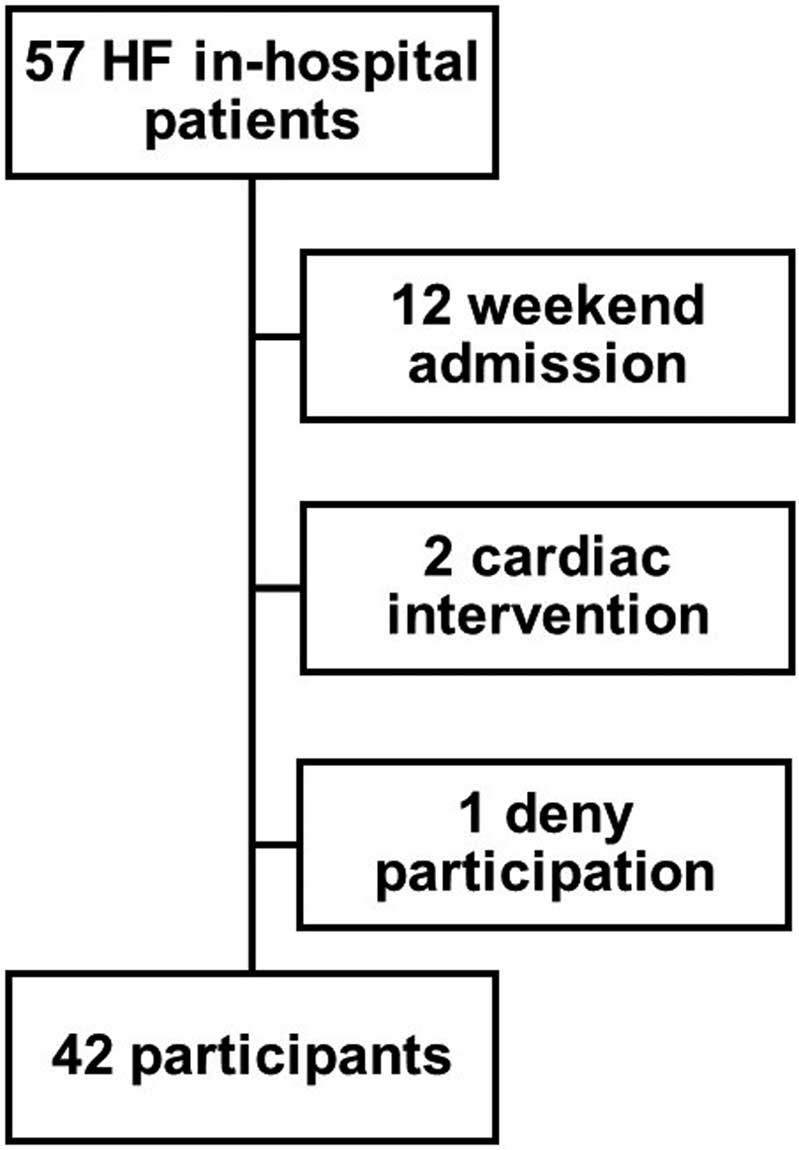
Table 1.
Baseline Characteristics
| |
Total
(n=42) |
High U-IXP
(n=11) |
Low U-IXP
(n=31) |
P value |
| Demographics |
| Age (years) |
78 [69–85] |
84 [78–87] |
77 [69–81] |
0.086 |
| Male sex |
25 (60) |
4 (36) |
21 (68) |
0.072 |
| BMI (kg/m2) |
22.7 [20.0–24.5] |
22.3 [20.3–23.3] |
22.7 [20.3–24.9] |
0.32 |
| Ischemic etiology |
15 (36) |
4 (36) |
11 (35) |
0.96 |
| Comorbidity |
| History of HF admission |
20 (48) |
5 (45) |
15 (48) |
0.57 |
| Dyslipidemia |
19 (45) |
6 (55) |
13 (42) |
0.36 |
| Diabetes |
9 (21) |
2 (18) |
7 (23) |
0.56 |
| History of VT |
6 (14) |
1 (9) |
5 (16) |
0.50 |
| Atrial fibrillation |
13 (31) |
5 (45) |
8 (26) |
0.20 |
| Laboratory data |
| Hemoglobin (g/dL) |
11.2 [9.7–13.0] |
10.5 [9.8–11.6] |
11.9 [9.9–13.4] |
0.37 |
| Serum sodium (mEq/L) |
140 [137–142] |
140 [137–143] |
140 [139–142] |
0.84 |
| eGFR (mL/min/1.73 m2) |
50.1 [35.0–70.3] |
50.5 [30.2–55.3] |
49.6 [35.5–70.7] |
0.69 |
| Plasma BNP (pg/mL) |
525 [194–1,173] |
577 [393, 939] |
507 [163–1,185] |
0.43 |
| Troponin I (pg/mL) |
51.3 [26.9–269.4] |
62.9 [39.9–245.3] |
49.8 [21.6–293.5] |
0.68 |
| Uric acid (mg/dL) |
6.5 [5.3–8.3] |
6.2 [5.2–8.1] |
6.6 [5.4–8.3] |
0.78 |
| Echocardiography |
| LVDd (mm) |
51 [44–62] |
47 [41–57] |
52 [46–63] |
0.27 |
| LVEF (%) |
44 [29–60] |
40 [31–62] |
46 [29–60] |
0.98 |
| Moderate or greater MR |
18 (43) |
3 (27) |
15 (48) |
0.22 |
| Moderate or greater TR |
14 (33) |
7 (64) |
7 (23) |
0.013* |
| Medication |
| β-blocker |
23 (55) |
6 (55) |
17 (55) |
0.63 |
| ACEI |
22 (52) |
8 (73) |
14 (45) |
0.11 |
| MRA |
15 (36) |
5 (45) |
10 (32) |
0.33 |
| Febuxostat |
11 (26) |
2 (18) |
9 (29) |
0.39 |
Unless indicated otherwise, data are given as the median [interquartile range] or n (%). *P<0.05. ACEI, angiotensin-converting enzyme inhibitor; BMI, body mass index; BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; HF, heart failure; LVDd, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; MRA, mineralocorticoid receptor antagonist; TR, tricuspid regurgitation; U-IXP, urinary isoxanthopterin; VT, ventricular tachyarrhythmia.
There were no significant differences in baseline characteristics between the high and low U-IXP groups, except for a higher prevalence of moderate or greater tricuspid regurgitation in the high U-IXP group (Table 1).
In-Hospital Course
During the index hospitalization, the median in-hospital length of stay was 15 days (IQR 10–27 days). No patients required intravenous inotropes or mechanical circulatory support. The high U-IXP group tended to have a longer in-hospital stay (25 vs. 14 days; P=0.14) and had significantly higher mortality (2/11 vs. 0/31; P=0.014) than the low U-IXP group (Table 2).
Table 2.
In-Hospital Clinical Course
| |
High U-IXP
(n=11) |
Low U-IXP
(n=31) |
P value |
| In-hospital stay (days) |
25 [12–39] |
14 [10–24] |
0.14 |
| In-hospital death |
2 (18) |
0 (0) |
0.014* |
Unless indicated otherwise, data are given as the median [interquartile range] or n (%). *P<0.05. U-IXP, urinary isoxanthopterin.
During the observation period following the index discharge, 3 patients died (cardiovascular deaths) and 9 patients had heart failure readmissions (composite primary endpoint).
In the univariate analysis, U-IXP was a significant predictor of the primary endpoint (hazard ratio 1.60; 95% confidence interval 1.14–2.26; P=0.007;
Table 3). U-IXP remained a significant predictor after adjusting for other clinically significant potential confounders (P<0.05 for each;
Table 3).
Table 3.
Impact of Urinary Isoxanthopterin on the Primary Endpoint
| |
HR
(95% CI) |
P value |
| Univariate analysis |
| U-IXP alone |
1.60 (1.14–2.26) |
0.007* |
| Multivariate analysis |
| U-IXP adjusted for age |
1.63 (1.13–2.36) |
0.010* |
| U-IXP adjusted for LVEF on admission |
1.57 (1.11–2.23) |
0.011* |
| U-IXP adjusted for log[BNP] on admission |
1.62 (1.11–2.31) |
0.008* |
| U-IXP adjusted for sodium on admission |
1.62 (1.15–2.29) |
0.006* |
| U-IXP adjusted for hemoglobin on admission |
1.60 (1.13–2.27) |
0.008* |
| U-IXP adjusted for uric acid on admission |
1.62 (1.14–2.30) |
0.007* |
| U-IXP adjusted for the use of febuxostat |
1.65 (1.16–2.36) |
0.005* |
*P<0.05. CI, confidence interval; HR, hazard ratio. Other abbreviations as in Table 1.
The cut-off value for U-IXP on admission to predict the primary endpoint was 0.93 μmol/g Cre, with a sensitivity of 0.58, a specificity of 0.87, and an area under the curve of 0.75 (Figure 3). The patient cohort was stratified into 2 groups using this cut-off value throughout the study.
The high U-IXP group had a significantly higher incidence of the primary endpoint than the low U-IXP group (71% vs. 16%; P=0.001;
Figure 4). A similar trend was observed among patients being administered antihyperuricemic agents (n=11; P=0.018).
Other Clinical Outcomes Following Index Discharge
The prevalence of anti-heart failure medications did not differ significantly between the 2 groups at index discharge and the 6-month follow-up (Table 4). Plasma BNP concentrations were not significantly different between the 2 groups during the observational period. The prevalence of moderate or greater tricuspid regurgitation was higher in the high U-IXP group at the 6-month follow-up.
Table 4.
Clinical Course Following the Index Discharge
| |
High U-IXP
(n=11) |
Low U-IXP
(n=31) |
P value |
| At index discharge |
| Plasma BNP (pg/mL) |
248 [142–391] |
160 [149–261] |
0.84 |
| β-blocker |
6/11 |
25/31 |
0.091 |
| ACEI |
8/11 |
29/31 |
0.067 |
| MRA |
6/11 |
23/31 |
0.20 |
| At 6-month follow-up |
| Plasma BNP (pg/mL) |
257 [126–838] |
162 [66–217] |
0.57 |
| β-blocker |
4/8 |
23/28 |
0.064 |
| ACEI |
7/8 |
22/28 |
0.57 |
| MRA |
4/8 |
19/28 |
0.35 |
| LVDd (mm) |
57 [48–69] |
59 [47–63] |
1.0 |
| LVEF (%) |
38 [23–55] |
43 [38–59] |
0.53 |
| Moderate or greater MR |
2/4 |
2/15 |
0.11 |
| Moderate or greater TR |
3/4 |
1/15 |
0.003* |
Unless indicated otherwise, data are given as the median [interquartile range] or as the number of patients/total number of patients in whom data are available. *P<0.05. Aabbreviations as in Table 1.
Among 11 patients who had high U-IXP on admission, U-IXP remained high during the index hospitalization in 2. Of these 2 patients, 1 was readmitted for heart failure 373 days after the index discharge and the other died due to worsening heart failure and multiorgan dysfunction during the index hospitalization.
Discussion
In this study we investigated the association between U-IXP on admission and the composite primary endpoint, consisting of cardiovascular death and heart failure readmission, in heart failure patients. The major findings of this study are that: (1) there was a wide distribution of U-IXP concentrations, and approximately one-quarter of patients had higher U-IXP; (2) most baseline characteristics did not differ significantly between the high and low U-IXP groups; (3) the high U-IXP group tended to have higher in-hospital mortality and a longer in-hospital stay; and (4) higher U-IXP was associated with cardiovascular death or heart failure readmission.
U-IXP and Cardiovascular Diseases
Baseline characteristics did not differ significantly after stratification of the patient cohort by U-IXP levels, except for the degree of tricuspid regurgitation. The prevalence of moderate or greater tricuspid regurgitation was higher in the high U-IXP group. XO, which is one of the producers of oxidative stress, is mainly formed in the liver.6
Congestive liver provoked by greater tricuspid regurgitation may lead to the greater production of XO, which, in turn, leads to high U-IXP. Older age and female sex were also associated with higher U-IXP levels, although these associations did not reach statistical significance. Men tend to develop cardiovascular diseases, such as acute coronary syndrome earlier than females, and the reason for this considered to be the sex difference in oxidative stress.7
In addition, this difference could be explained, in part, by known triggers of oxidative stress, namely age, smoking, diabetes, dyslipidemia, air pollution, and noise.8
In contrast, conventional markers of cardiac function, including LVEF and BNP levels, were not associated with the U-IXP level. Instead of cardiac function alone, U-IXP may indicate the severity of systemic congestion, systemic inflammation, and systemic hypoxia.9
In the present study, U-IXP consistently was an independent predictor of the primary endpoint in addition to these conventional prognostic markers, as discussed below.
Prognostic Implication of U-IXP
No studies have investigated the prognostic impact of the non-invasive biomarker U-IXP in heart failure cohorts. In a previous study, Lindsay et al. focused on the U-IXP level, which they reported to be correlated with the severity of Duchenne muscular dystrophy.4
Incremental oxidative stress negatively affects myocardial calcium handling and leads to the development of arrhythmia and cardiac remodeling, all of which cause worsening heart failure. Furthermore, the activated oxidative stress signal impairs vascular function by negatively affecting endothelial and smooth muscle cell growth, proliferation, and migration, facilitating cellular apoptosis and increasing vascular tone. Taking all of this into consideration, it is plausible that U-IXP levels are associated with the future recurrence of heart failure and cardiovascular mortality.
Of note, the prognostic impact of U-IXP levels remained after adjusting for other prognostic markers, including LVEF and BNT. In addition to the severity of cardiac function alone, U-IXP represents systemic inflammation and ischemia, both of which would have a negative prognostic impact on worsening cardiovascular diseases. The prognostic impact of U-IXP was independent of serum uric acid level, probably due to their different signaling pathways.
Future Concerns
One of the advantages of measuring U-IXP rather than conventional blood biomarkers is the non-invasiveness of the measurement. Samples can be obtained from patients even at home. The prognostic impact of the trend in U-IXP is the next concern. In a subgroup of the present study, 2 patients with persistently elevated U-IXP had worse in-hospital outcomes. Given the small sample size, we could not analyze the additive value of U-IXP to conventional prognostic biomarkers, including BNP, in predicting clinical outcomes. Therapeutic intervention to decrease the U-IXP level and its prognostic implication remain uncertain. Given the findings of the present study, febuxostat may not affect the U-IXP level.
Study Limitations
This was a single-center study with a small sample size. This is a proof-of-concept study, and further studies with larger sample sizes are warranted to validate our findings. The period for which samples were stored frozen varied for each patient. However, in general, U-IXP levels are not affected by the period of frozen storage if appropriately preserved. We did not collect U-IXP data at index discharge for all patients. The prognostic impact of U-IXP at the time of admission may be affected by in-hospital management. The prognostic impact of U-IXP on other uninvestigated outcomes, including exercise capacity, remains to be evaluated in future studies. The detailed mechanism as to why elevated U-IXP is associated with poor clinical outcomes remains uncertain given the nature of this retrospective study, and further studies are warranted to clarify the physiology.
Conclusions
U-IXP on admission was associated with cardiovascular death or heart failure readmission following index discharge in patients with decompensated heart failure. Further studies are warranted to clarify the underlying physiology.
Disclosure
K.K. is a member of
Circulation Reports’ Editorial Board. The remaining authors have no conflicts of interest to declare.
IRB Information
This study was approved by the Clinical Research Review Board, University of Toyama (R2015154).
Data Availability
Data are available from the corresponding authors upon reasonable request.
References
- 1.
Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 2011; 301: H2181–H2190.
- 2.
Adachi T, Fukushima T, Usami Y, Hirano K. Binding of human xanthine oxidase to sulphated glycosaminoglycans on the endothelial-cell surface. Biochem J 1993; 289: 523–527.
- 3.
Landmesser U, Spiekermann S, Dikalov S, Tatge H, Wilke R, Kohler C, et al. Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: Role of xanthine-oxidase and extracellular superoxide dismutase. Circulation 2002; 106: 3073–3078.
- 4.
Lindsay A, McCourt PM, Karachunski P, Lowe DA, Ervasti JM. Xanthine oxidase is hyper-active in Duchenne muscular dystrophy. Free Radic Biol Med 2018; 129: 364–371.
- 5.
Wakabayashi I, Nakanishi M, Ohki M, Suehiro A, Uchida K. A simple and useful method for evaluation of oxidative stress in vivo by spectrofluorometric estimation of urinary pteridines. Sci Rep 2020; 10: 11223.
- 6.
Battelli MG, Bolognesi A, Polito L. Pathophysiology of circulating xanthine oxidoreductase: New emerging roles for a multi-tasking enzyme. Biochim Biophys Acta 2014; 1842: 1502–1517.
- 7.
Kander MC, Cui Y, Liu Z. Gender difference in oxidative stress: A new look at the mechanisms for cardiovascular diseases. J Cell Mol Med 2017; 21: 1024–1032.
- 8.
Hulsmans M, Holvoet P. The vicious circle between oxidative stress and inflammation in atherosclerosis. J Cell Mol Med 2010; 14: 70–78.
- 9.
Cantu-Medellin N, Kelley EE. Xanthine oxidoreductase-catalyzed reactive species generation: A process in critical need of reevaluation. Redox Biol 2013; 1: 353–358.





