2021 年 3 巻 11 号 p. 660-665
2021 年 3 巻 11 号 p. 660-665
Background: The implications of mineralocorticoid receptor antagonists, including the newly introduced esaxerenone, on cardiac reverse remodeling in patients with heart failure with preserved ejection fraction (HFpEF) remain uncertain.
Methods and Results: We included patients with HFpEF who received esaxerenone for hypertension between November 2019 and July 2021 in this retrospective study. Changes in left ventricular mass index (LVMI) were compared between the 6-month pretreatment period (without esaxerenone) and the 6-month treatment period (on esaxerenone). Thirty-three patients (median age 74 years [interquartile range {IQR} 70–81 years]; 33% male, median systolic blood pressure [SBP] 135 mmHg [IQR 123–148 mmHg]) were included in the study and completed 6-month esaxerenone therapy without any adverse events. During the pretreatment period, SBP decreased significantly (P=0.009), whereas LVMI remained unchanged (P=0.30). During the esaxerenone treatment period, both SBP and LVMI decreased significantly (P=0.003 and P=0.001, respectively).
Conclusions: Esaxerenone may have beneficial effects of reverse remodeling in patients with HFpEF when used to treat hypertension. Further studies are needed to understand which patient populations may see greater benefits with esaxerenone.
Hypertension refractory to antihypertensive agents including calcium channel blockers, renin-angiotensin system inhibitors, and diuretics, poses a clinical challenge to the clinician. In these scenarios, mineralocorticoid receptor antagonists (MRAs) are used.1 However, conventional steroidal MRAs, including spironolactone and eplerenone, can cause hormone-related side effects, hyperkalemia, and renal impairment, often necessitating discontinuation.2
Heart failure with preserved ejection fraction (HFpEF) is a heart failure subtype and is often caused by chronic hypertension.3 Mainstays of therapy include diuretics for congestion and antihypertensive agents to relieve afterload on left ventricle, together with the management of underlying modifiable clinical factors associated with the disease.4,5 With the lack of effective therapies proven to affect disease trajectory, patients with HFpEF collectively have a greater risk of cardiovascular death than patients with heart failure with reduced ejection fraction (HFrEF).6
Esaxerenone is a recently introduced promising non-steroidal MRA that specifically inhibits excessive mineralocorticoid receptor activity; thus far, esaxerenone is indicated only for refractory hypertension.7 Given the evidence that other steroidal MRAs (spironolactone and eplerenone) improve prognosis in patients with HFrEF,8–10 esaxerenone may also potentially have beneficial effects on the heart. A recent animal study demonstrated that the administration of esaxerenone reduced cardiac fibrosis, systemic inflammation, and oxidative stress in rats with salt-induced myocardial injury.11 Taking all these findings into consideration, in the present study we investigated the effects of esaxerenone on cardiac reverse remodeling in patients with HFpEF and hypertension.
Consecutive patients who received esaxerenone for the treatment of hypertension refractory to at least 2 antihypertensive agents between November 2019 and July 2021 were considered for inclusion in the present retrospective study. Patients with an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 or those with serum potassium >5.0 mEq/L did not receive esaxerenone and were excluded from the study. Patients with malignancy or secondary hypertension were also excluded.
Of the consecutive patients treated with esaxerenone, those with a left ventricular ejection fraction (LVEF) >40% were included in this study. The diagnosis of heart failure was according to the current guidelines of the Japanese Circulation Society.4 Patients with amyloidosis and hypertrophic cardiomyopathy were excluded. Patients without comprehensive clinical data obtained at 3 time points (6 months before treatment, at baseline, and after 6 months treatment) were also excluded.
The study protocol was approved by the Clinical Research Review Board, University of Toyama (R2015154). The need for written informed consent was waived given the retrospective nature of the study, and an opt-out method was used.
Clinical ManagementAll patients were evaluated once a month and received guideline-directed medical therapy.1,4 Esaxerenone was initiated at a dose of 2.5 mg/day in principal. When patients had an eGFR between 30 and 60 mL/min/1.73 m2, esaxerenone was initiated at a dose of 1.25 mg/day. Systemic blood pressure (SBP) and serum potassium concentrations were carefully followed-up. Blood pressure was measured twice after a 5-min rest in the morning in the outpatient clinic, with mean of the 2 values used. When the serum potassium concentration exceeded 5.0 mEq/L, the dose of esaxerenone was considered for down-titration. When the serum potassium concentration exceeded 5.5 mEq/L, termination of esaxerenone therapy was considered.
Data CollectionDemographics, medications, and laboratory data, including eGFR and serum potassium concentrations at the time of esaxerenone initiation (defined as baseline), were retrieved. Similar clinical data were retrieved 6 months before and 6 months after the initiation of esaxerenone (pretreatment and on-treatment periods, respectively).
At these 3 time points, transthoracic echocardiography was performed in a standard manner by cardiac sonographers blinded to the study protocol. Of note, the left ventricular mass index (LVMI) was serially assessed. The trend in LVMI during the observation period (pretreatment vs. on-treatment period) was defined as the primary outcome to investigate the effect of esaxerenone therapy on changes in myocardial structure.
Statistical AnalysisStatistical analyses were performed using SPSS Statistics 22 (SPSS Inc., Armonk, NY, USA). Two-sided P<0.05 was considered statistically significant. Given small sample size, continuous variables are presented as the median and interquartile range (IQR). Categorical variables are presented as numbers and percentages. Trends were assessed by using the Friedman test and ad hoc Wilcoxon signed-rank test, or the Cochran Q test and ad hoc McNemar test. Logistic regression analyses were used to investigate factors associated with a >30 g/m2 decrease in LVMI during esaxerenone therapy, which was defined as the cut-off for appropriate reverse remodeling according to a previous study.11 Variables with P<0.05 were included in the multivariate analysis.
In all, 36 patients (median age 74 years; 33% male) were included in the study (Table 1). Median left ventricular end-diastolic diameter was 51 mm (IQR 45–54 mm) and the LVEF was 55% (IQR 50–63%). The median LVMI was 148.5 g/m2 (IQR 126.3–186.6 g/m2).
| Demographics | |
| Age (years) | 74 [70–81] |
| Male sex | 11 (33) |
| Ischemic etiology | 8 (24) |
| Body weight (kg) | 55.0 [49.0–66.0] |
| BMI (kg/m2) | 22.4 [19.8–25.7] |
| BSA (m2) | 1.59 [1.42–1.70] |
| NYHA functional class | |
| Class II | 21 (64) |
| Class III | 12 (36) |
| Class IV | 0 |
| Comorbidity | |
| Atrial fibrillation | 16 (49) |
| Diabetes | 9 (27) |
| Hypertension | 33 (100) |
| History of HF hospitalization | 7 (21) |
| Hemodynamics | |
| SBP (mmHg) | 135 [123–148] |
| DBP (mmHg) | 76 [71–84] |
| Heart rate (beats/min) | 75 [65–89] |
| Laboratory data | |
| Serum potassium (mEq/L) | 4.2 [3.9–4.5] |
| eGFR (mL/min/1.73 m2) | 52.9 [42.8–62.1] |
| Plasma BNP (pg/mL) | 164 [100–312] |
| Echocardiography | |
| LVEDd (mm) | 51 [45–54] |
| LVEF (%) | 55 [50–63] |
| Left atrial diameter (mm) | 40 [37–51] |
| LVMI (g/m2) | 148.5 [126.3–186.6] |
| Medication | |
| β-blocker | 28 (85) |
| RAS inhibitor | 27 (82) |
| Calcium channel blocker | 11 (33) |
| Diuretic | 27 (82) |
Continuous variables are presented as the median [interquartile range]; categorical variables are presented as n (%). BMI, body mass index; BNP, B-type natriuretic peptide; BSA, body surface area; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration ratio; HF, heart failure; LVEDd, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; NYHA, New York Heart Association; RAS, renin-angiotensin system; SBP, systolic blood pressure.
All patients had a diagnosis of hypertension. The median SBP at baseline was 135 mmHg (IQR 123–148 mmHg). Twenty-one (64%) patients were receiving ≥3 agents for the treatment of hypertension. No patients had a history of receiving MRAs before the study.
Esaxerenone was initiated at a dose of 2.5, 1.25, and 0.625 mg/day in 17, 15, and 1 of 33 patients, respectively. All patients continued esaxerenone therapy during the 6-month observation period without any reported adverse events.
Trends in Major Parameters, Including LVMIDuring the pretreatment period (without esaxerenone), SBP decreased significantly (P=0.009; Figure 1A), whereas LVMI and the plasma B-type natriuretic peptide (BNP) concentration were unchanged (P=0.30 and P=0.84, respectively; Figure 1B,C).
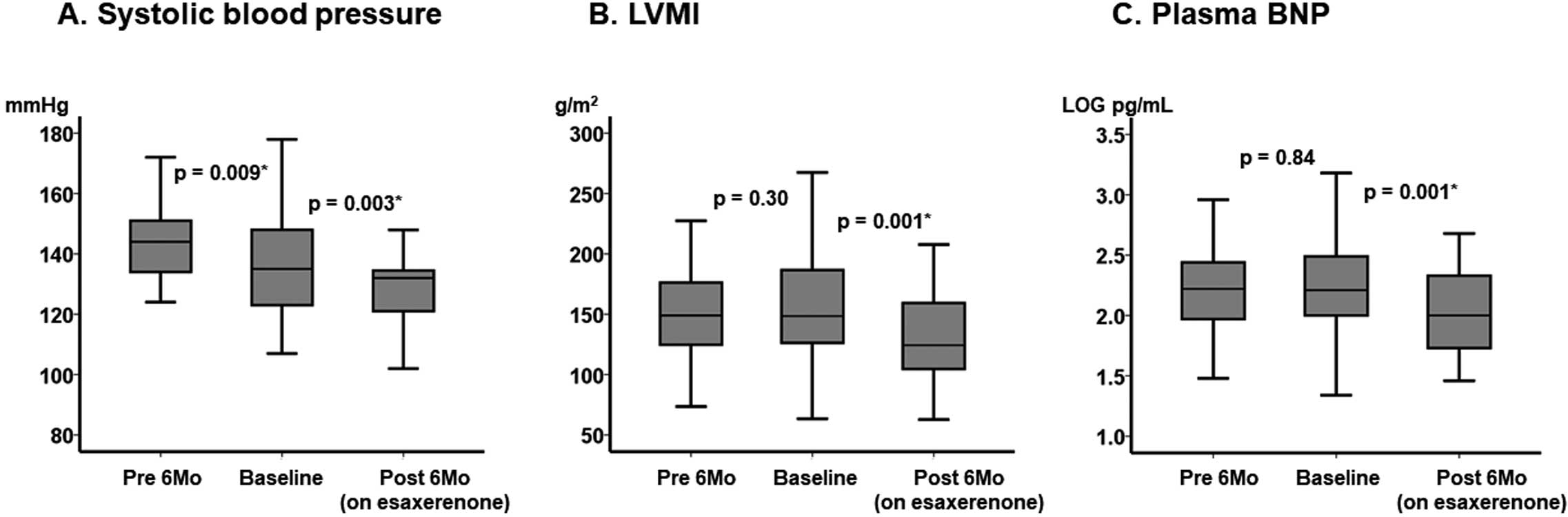
Trends in major clinical parameters from 6 months before treatment (pretreatment) to baseline and 6 months after the initiation of esaxerenone: (A) systolic blood pressure (SBP), (B) left ventricular mass index (LVMI), and (C) plasma B-type natriuretic peptide (BNP) concentrations. The boxes show the interquartile range, with the median value indicated by the horizontal line; whiskers show the range. P values were determined using the Wilcoxon signed-rank test.
Following the initiation of esaxerenone, SBP decreased further (P=0.003; Figure 1A), accompanied by significant decreases in LVMI and plasma BNP concentrations (P=0.001 for both; Figure 1B,C).
The magnitude of changes in SBP did not differ between the pretreatment and the treatment periods (median [IQR] −4 [−8, 1] vs. −7 [−16, −2] mmHg, respectively; P=0.28; Figure 2A), whereas there were significant changes in LVMI and plasma BNP concentrations during the treatment period (on esaxerenone) compared with the pretreatment period (P=0.001 and P=0.003, respectively; Figure 2B,C).
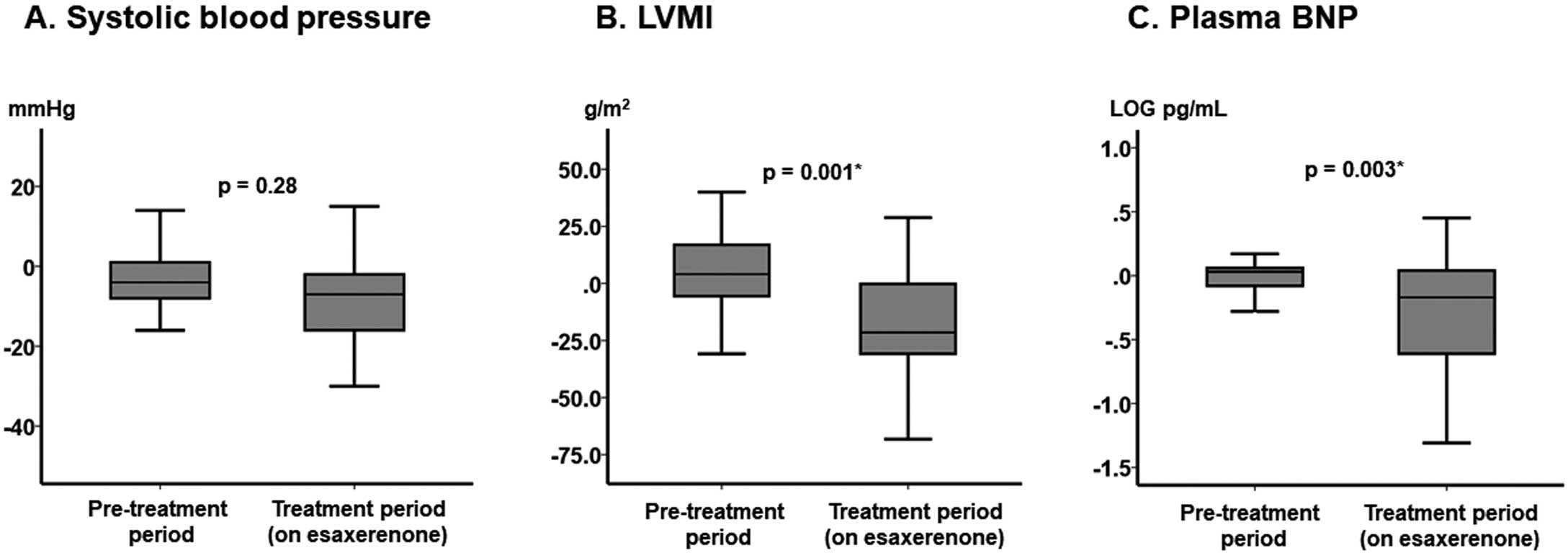
Changes in major clinical parameters between the pretreatment period and the on-treatment period: (A) systolic blood pressure (SBP), (B) left ventricular mass index (LVMI), and (C) plasma B-type natriuretic peptide (BNP) concentrations. The boxes show the interquartile range, with the median value indicated by the horizontal line; whiskers show the range. P values were determined using the Mann-Whitney U test.
The use of other antihypertensive agents remained unchanged during the study period (P>0.05 for all; Table 2). Serum potassium concentrations did not increase significantly following the initiation of esaxerenone. eGFR remained unchanged during the study period (P=0.56). Left ventricular end-diastolic diameter, left atrial diameter, and the E/e’ ratio decreased significantly, whereas LVEF increased significantly following the administration of esaxerenone (P<0.05 for all).
| 6 months before |
Baseline | 6 months after (on esaxerenone) |
P value | |
|---|---|---|---|---|
| Hemodynamics | ||||
| DBP (mmHg) | 81 [75–88] | 76 [71–84] | 74 [67–82] | <0.001* |
| Heart rate (beats/min) | 82 [70–89] | 75 [65–89] | 76 [69–82] | <0.001* |
| Body weight (kg) | 54.8 [48.7–65.5] | 55.0 [49.0–66.0] | 54.4 [48.7–65.6] | 0.076 |
| Medications | ||||
| β-blocker | 25 (76) | 28 (85) | 28 (85) | 1.0 |
| ACEI/ARB | 27 (82) | 27 (82) | 27 (82) | 1.0 |
| Calcium channel blocker | 10 (30) | 11 (33) | 11 (33) | 0.72 |
| Diuretics | 26 (79) | 27 (82) | 27 (82) | 0.86 |
| Laboratory data | ||||
| Hemoglobin (g/dL) | 11.2 [10.2–12.4] | 11.3 [10.1–12.7] | 11.6 [10.3–12.9] | 0.076 |
| Serum potassium (mEq/L) | 4.1 [4.0–4.5] | 4.2 [3.9–4.5] | 4.3 [4.0–4.6] | 0.80 |
| eGFR (mL/min/1.73 m2) | 53.6 [44.9–64.6] | 52.9 [42.8–62.1] | 54.8 [43.4–59.9] | 0.56 |
| Echocardiography | ||||
| LVEDd (mm) | 49 [45–53] | 51 [45–54] | 49 [43–52] | 0.001* |
| LVEF (%) | 58 [49–63] | 55 [50–63] | 61 [51–67] | 0.017* |
| E/e’ ratio | 17.2 [14.3–18.8] | 16.9 [14.1–18.4] | 16.1 [13.4–17.4] | 0.028* |
| Left atrial diameter (mm) | 42 [39–50] | 40 [37–51] | 39 [35–48] | 0.005* |
Continuous variables are presented as the median [interquartile range]; categorical variables are presented as n (%). *P<0.05. Trends were assessed using the Friedman test for the continuous variables and the Cochran Q test for the categorical variables. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; DBP, diastolic blood pressure. Other abbreviations as in Table 1.
Eleven (33%) patients achieved a >30 g/m2 decrease in LVMI following 6 months of esaxerenone therapy. Higher LVMI at baseline was significantly associated with this endpoint in both univariate and multivariate analyses (odds ratio 1.03; 95% confidence interval 1.00–1.05; P=0.037; Table 3).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 0.91 (0.83–0.99) | 0.021* | 0.91 (0.83–1.00) | 0.059 |
| Ischemic etiology | 1.28 (0.24–6.70) | 0.77 | ||
| BSA | 0.87 (0.03–25.8) | 0.94 | ||
| Atrial fibrillation | 0.26 (0.05–1.26) | 0.093 | ||
| Diabetes | 1.94 (0.40–9.45) | 0.41 | ||
| SBP | 0.98 (0.94–1.02) | 0.23 | ||
| Heart rate | 1.04 (0.99–1.09) | 0.10 | ||
| Plasma BNP | 1.00 (0.99–1.01) | 0.29 | ||
| Left atrial diameter | 0.97 (0.90–1.05) | 0.45 | ||
| LVMI | 1.03 (1.01–1.05) | 0.017* | 1.03 (1.00–1.05) | 0.037* |
| β-blocker | 0.71 (0.10–5.03) | 0.73 | ||
| RAS inhibitor | 1.00 (0.15–6.53) | 1.0 | ||
| Esaxerenone dose | 0.67 (0.22–2.04) | 0.48 | ||
*P<0.05 by logistic regression analysis. CI, confidence interval; OR, odds ratio. Other abbreviations as in Table 1.
There was no significant correlation between changes in SBP and changes in LVMI during the 6 months of esaxerenone therapy (r=−0.11, P=0.54).
In this study we investigated the impact of 6 months of esaxerenone therapy on changes in LVMI in patients with hypertension and HFpEF. The major findings are that: (1) during the pretreatment period, 6 months conventional antihypertension treatment significantly decreased SBP, whereas LVMI and BNP concentrations remained unchanged; (2) esaxerenone decreased SBP without any significant adverse events; and (3) LVMI and BNP concentrations both decreased in the 6-month period following the initiation of esaxerenone.
Effects of Esaxerenone on Blood Pressure and Renal FunctionAs expected, SBP decreased significantly while patients were on esaxerenone therapy. We observed a median decrease of approximately 7 mmHg, which was overall lower compared with previous studies.12,13 These findings may stem from a relatively lower baseline SBP (median 135 mmHg) and the lower dose of esaxerenone, due to a higher prevalence of chronic kidney disease, in the present study.
Unlike other steroidal MRAs,14 esaxerenone may have a more immediate renoprotective effect.15 A Phase III study demonstrated that albuminuria improved during esaxerenone therapy used in addition to angiotensin-converting enzyme II inhibitors in patients with diabetes and microalbuminuria.13 Aside from improved odds of long-term stabilization of the clinical status of heart failure with MRA, which, in turn, may improve renal function, we observed stabilization in eGFR without any adverse events, including hyperkalemia, in this study.
Effects of Esaxerenone on LVMI in HFpEF PatientsThere are scarce data regarding the efficacy of esaxerenone in heart failure populations, including HFpEF, because the only current indication for esaxerenone is for hypertension. One prior correspondence analysis reported a decrease in blood pressure and plasma BNP concentrations in patients with hypertension and heart failure, including both HFpEF and HFrEF, with esaxerenone therapy.16 There are no established medications to treat HFpEF,4 although esaxerenone therapy is effective in resistant hypertension, which often predisposes many to an increased risk of poor ventricular compliance and clinical volume overload. This is the reason why we chose to study the effects of esaxerenone in patients with hypertension and HFpEF.
A decrease in blood pressure may ultimately be the predominant mechanism in reducing LVMI,17 instead of the class effect of esaxerenone. However, a comparable decrease in blood pressure by conventional antihypertensive agents during the pretreatment period did not result in a significant change in LVMI. Instead, the pleiotropic effects of neurohormonal modulation from esaxerenone may have a direct effect on cardiac structure and function.
Steroidal MRAs, including spironolactone and eplerenone, reduce mortality and morbidity in patients with HFrEF, as seen in large-scale clinical trials.8–10 Comprehensive data from randomized trials on HFpEF thus far have not shown a clear clinical benefit.4 The direct myocardial effects of esaxerenone also remain unknown. An experimental animal study recently found that esaxerenone reduced inflammation and oxidative stress and suppressed the progression of cardiac remodeling and fibrosis in rats with salt-induced myocardial injury.11 More detailed, larger mechanistic studies, perhaps using cardiac magnetic resonance imaging in patients on esaxerenone therapy, may offer better insights into potential changes in cardiac structure and function on therapy.
Further PerspectivesThere are no established medications to treat HFpEF thus far,4 although several agents, including sodium-glucose cotransporter 2 inhibitors and sacubitril/valsartan, appear promising.18 MRAs are recommended for patients with refractory hypertension who are receiving several antihypertensive agents.1 However, given our findings, esaxerenone may be beneficial at an earlier stage as part of hypertension therapies, particularly when clinical HFpEF is present. We observed morphological changes only in the left ventricle during esaxerenone therapy. The effects of esaxerenone on mortality and morbidity in the HFpEF cohort are the next concerns.
Study LimitationsThis study had a small sample size. This was a proof-of-concept study that investigated the effect of esaxerenone on HFpEF. We performed intragroup comparisons (pre- vs. post-treatment), but lacked a control group. The medications remained unchanged during both periods, although uninvestigated confounders may have existed nevertheless. We could not assess optimal dosing, appropriate patient selection, or long-term mortality and morbidity, all of which remain to be evaluated in future studies.
Esaxerenone may facilitate cardiac remodeling in patients with HFpEF when administered for the treatment of hypertension. Further studies are needed to understand which patient populations may benefit clinically from esaxerenone.
None.
T.I. receives grant support from JSPS KAKENHI (JP20K17143). K.K. is a member of Circulation Reports’ Editorial Board. The remaining authors have no conflicts of interest to disclose.
This study was approved by the Clinical Research Review Board, University of Toyama (R2015154).
Data are available from the corresponding author upon reasonable requests.