2021 年 3 巻 4 号 p. 194-200
2021 年 3 巻 4 号 p. 194-200
Background: We hypothesized that symptom presentation in patients with acute myocardial infarction (AMI) may affect their management and subsequent outcome.
Methods and Results: Using Rural AMI Registry data, 1,337 consecutive patients with AMI who underwent percutaneous coronary intervention were analyzed. Typical symptoms were defined as any symptoms of chest pain or pressure due to myocardial ischemia. We considered the specific symptoms of dyspnea, nausea, or vomiting as atypical symptoms. The primary outcome was 30-day mortality. There were 150 (11.2%) and 1,187 (88.8%) patients who presented with atypical and typical symptoms, respectively. Those who presented with atypical symptoms were significantly older (mean [±SD] age 74±12 vs. 68±13 years; P<0.001) and had a higher Killip class (46.7% vs. 21.8%; P<0.001) than patients presenting with typical symptoms. The prevalence of door-to-balloon time of ≤90 min was significantly lower in patients with atypical than typical symptoms (40.0% vs. 66.3%; P<0.001). At 30 days, there were 55 incidents of all-cause death. Multivariate Cox proportional hazards regression analysis revealed that symptom presentation was associated with 30-day mortality (hazard ratio 2.33; 95% confidence interval 1.20–4.38; P<0.05).
Conclusions: Atypical symptoms in patients with AMI are less likely to lead to timely reperfusion and are associated with increased risk of 30-day mortality.
The diagnosis of acute myocardial infarction (AMI) can be challenging in patients who present with atypical symptoms. Although chest pain is considered a key symptom in the diagnosis of AMI, patients with atypical symptoms may complain of dyspnea, nausea, or vomiting.1 Underdiagnosed AMI may result in treatment delay and patients presenting with serious conditions, such as cardiogenic shock.2 Previous studies have demonstrated that among AMI patients, a higher proportion of those who are elderly, female, or have dementia are likely to present with atypical than typical symptoms,3,4 indicating that symptom presentation is likely to affect the precise diagnosis. Moreover, patients with AMI complaining of atypical symptoms are considered to be in a high-risk subgroup who tend to be managed without beneficial treatment strategies, including percutaneous coronary intervention (PCI). Furthermore, even if these patients were treated with PCI, the door-to-balloon time was significantly longer than ≤90 min, which is the benchmark of timely reperfusion.5 Delays in diagnosis and reperfusion due to atypical symptoms may contribute to adverse outcomes for patients with AMI. However, symptom presentation as a predictor of timely reperfusion and subsequent mortality in patients with AMI who receive emergency PCI is still being debated.6–8 Thus, to investigate this issue further, the present prospective multicenter observational study was conducted among patients with AMI using data from the Rural AMI Registry database.
This study had 2 main objectives: (1) to compare the clinical characteristics and door-to-balloon time in patients with AMI based on symptom presentation; and (2) to assess whether there is an association between symptom presentation and 30-day mortality in patients with AMI who had undergone PCI.
The present analysis was a substudy of the Rural AMI Registry study. The design of the study and its main results have been published previously.9 Briefly, the Rural AMI Registry study prospectively enrolled patients with AMI who were hospitalized within 7 days from onset between January 2013 and March 2014; 41 hospitals in Japan took part in the study. In the present study, the diagnosis of AMI was based on the third universal definition of myocardial infarction (MI).10 The electrocardiogram criteria for ST-segment elevation required a J point elevation ≥0.2 mV in at least 2 contiguous leads, new left bundle branch block, or posterior MI.11 Data were collected via individual chart view by trained data collection personnel at each of the collaborating hospitals, and all data were anonymized and transmitted to the data collection center at the Department of Cardiology and Nephrology, Mie University Graduate School of Medicine (Tsu, Japan) for processing. The Registry contained detailed clinical information, including symptom presentation, history of cardiovascular disease, Killip classification, laboratory data, angiographic data, PCI procedure, time interval from arrival at the emergency department to coronary revascularization, medications, duration of hospitalization, and in-hospital adverse outcomes.
This study was conducted in accordance with the Declaration of Helsinki. The study protocol was initially approved by the Ethics Committees of Mie University Graduate School of Medicine, Kanazawa University Graduate School of Medicine, Hirosaki University Graduate School of Medicine, and Ehime University Graduate School of Medicine, and subsequently by the local ethics committees at each of the participating centers.
Endpoints and DefinitionsThe primary endpoint was all-cause hospital mortality within 30 days, beginning from the first day of hospitalization. The secondary endpoint was door-to-balloon time. We set the cut-off point of 90 min for door-to-balloon time based on guideline recommendations.12
In the present study, typical symptoms were defined as any symptoms of chest pain or pressure (chest discomfort) due to myocardial ischemia.5 We considered the specific symptoms of dyspnea, nausea/vomiting, abdominal pain, or altered mental status as atypical symptoms. In patients exhibiting multiple symptoms, those patients with chest discomfort were classified into the typical group, even if they had other specific symptoms, such as dyspnea, nausea/vomiting etc. Patients with only atypical symptoms were classified into the atypical group.
Chronic kidney disease (CKD) was defined as the presence of proteinuria, serum creatinine ≥1.3 mg/dL, or estimated glomerular filtration rate ≤60 mL/min/1.73 m2.11 Anemia was defined as an admission hemoglobin <13 g/dL in men or <12 g/dL in women.13 Door-to-balloon time was defined as the interval from the door time, defined as the arrival time at the emergency department where emergency catheterization was performed, to the first device use or balloon inflation.9 Emergency PCI was defined as primary PCI that was performed within 24 h of hospital admission. ‘Night-time’ was defined as the time interval between 18:00 and 08:00 hours.14
Statistical AnalysisContinuous variables are expressed as the mean±SD or median with interquartile range (IQR) and were compared using unpaired t-tests or the Mann-Whitney U-test, as appropriate. Frequency analysis was performed using χ2 tests. Multivariate logistic regression analysis was used to investigate the independent determinants of door-to-balloon time. Multivariate analysis of independent predictors of 30-day mortality was performed using the Cox proportional hazard regression model. After accounting for multicollinearity, potentially clinically relevant variables (atypical symptom, age, sex, diabetes, previous MI, peripheral artery disease, night-time arrival, non-ST-segment elevation MI [NSTEMI], Killip class, CKD, anemia, culprit lesion in the left main coronary artery, the presence of chronic total occlusion, Thrombosis in Myocardial Infarction [TIMI] flow Grade 3 after PCI, and door-to-balloon time) with P<0.05 in univariate models were entered into the multivariate model. Event-free survival analysis was performed using the Kaplan-Meier method with the log-rank test for group comparisons. Two-sided P<0.05 was considered significant. Statistical analyses were performed using JMP Pro Version 12 (SAS institute, Cary, NC, USA).
In all, 1,695 patients with AMI were registered. Of these, 358 patients were excluded from the analysis: 180 patients who did not undergo emergency PCI, 122 patients without assessment of door-to-balloon time, 27 patients with missing information for vital status upon admission, 15 patients with cardiac arrest, 12 patients for whom laboratory data were lacking, and 2 patients for whom MI subtype classification was lacking. Thus, the final sample size was 1,337. As shown in Figure 1, 150 (11.2%) patients presented with atypical symptoms and 1,187 (88.8%) patients presented with typical symptoms. Among the patients with atypical symptoms, 43 presented with dyspnea, 22 presented with nausea/vomiting, 22 presented with altered mental status, 12 presented with back pain, 6 presented with abdominal pain, and 45 presented with other symptoms.
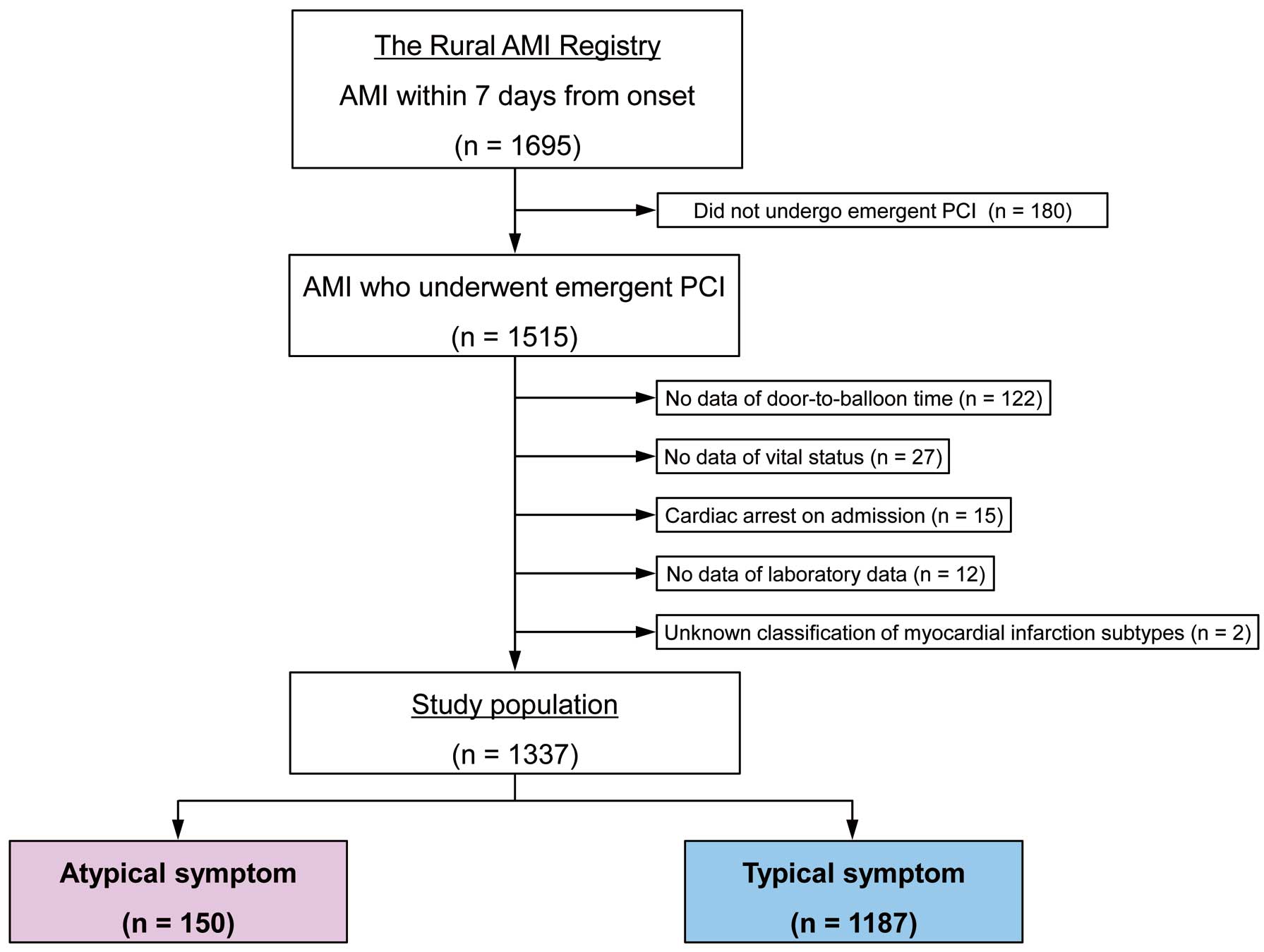
Participant flow chart illustrating the inclusion of patients in the present study. AMI, acute myocardial infarction; PCI, percutaneous coronary intervention.
Table 1 presents the baseline clinical characteristics of the patient population. Patients with atypical symptoms were significantly older than those exhibiting typical symptoms (74±12 vs. 68±13 years; P<0.001). Of note, there were significant differences in the prevalence of dyslipidemia (38.0% vs. 46.9%; P<0.05) and previous MI (2.0% vs. 7.0%; P<0.05) according to symptom presentation. In addition, patients with atypical symptoms more often exhibited Killip class >I (46.7% vs. 21.8%; P<0.001), CKD (26.7% vs. 9.6%; P<0.001), and anemia (41.3% vs. 20.5%; P<0.001) than those with typical symptoms. With regard to discharge medication, patients with atypical symptoms were less frequently prescribed evidence-based medical therapy, including statins (78.7% vs. 85.7%; P<0.05) and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (76.0% vs. 83.4%; P<0.05). The Supplementary Figure shows B-type natriuretic peptide concentrations according to symptom presentation type.
| All patients (n=1,337) |
Atypical symptoms (n=150) |
Typical symptoms (n=1,187) |
P value | |
|---|---|---|---|---|
| Age (years) | 68±13 | 74±12 | 68±13 | <0.001 |
| Male sex | 1,020 (76.3) | 109 (72.7) | 911 (76.7) | 0.28 |
| Hypertension | 882 (66.0) | 104 (69.3) | 778 (65.5) | 0.35 |
| Diabetes | 429 (32.1) | 58 (38.7) | 371 (31.3) | 0.07 |
| Dyslipidemia | 614 (45.9) | 57 (38.0) | 557 (46.9) | <0.05 |
| Current smoker | 473 (35.4) | 46 (30.7) | 427 (36.0) | 0.20 |
| Previous MI | 86 (6.4) | 3 (2.0) | 83 (7.0) | <0.05 |
| Previous PCI | 105 (7.9) | 8 (5.3) | 97 (8.2) | 0.20 |
| Previous stroke | 71 (5.3) | 11 (7.3) | 60 (5.1) | 0.26 |
| Peripheral artery disease | 33 (2.5) | 6 (4.0) | 27 (2.3) | 0.23 |
| Night-time arrival | 525 (39.3) | 66 (44.0) | 459 (38.7) | 0.21 |
| STEMI | 1,140 (85.3) | 127 (84.7) | 1,013 (85.3) | 0.83 |
| Killip class >I | 329 (24.6) | 70 (46.7) | 259 (21.8) | <0.001 |
| SBP (mmHg) | 134±31 | 127±36 | 135±31 | <0.05 |
| DBP (mmHg) | 78±20 | 72±23 | 79±20 | <0.001 |
| Heart rate (beats/min) | 77±20 | 82±25 | 76±19 | <0.05 |
| Serum creatinine (mg/dL) | 0.81 [0.69–1.02] | 0.97 [0.78–1.32] | 0.81 [0.68–0.99] | <0.001 |
| CKD | 154 (11.5) | 40 (26.7) | 114 (9.6) | <0.001 |
| Maintenance dialysis | 12 (0.9) | 0 (0) | 12 (1.0) | 0.09 |
| Hemoglobin (g/dL) | 14.0±2.1 | 13.0±2.2 | 14.1±2.1 | <0.001 |
| Anemia | 305 (22.8) | 62 (41.3) | 243 (20.5) | <0.001 |
| Baseline creatine phosphokinase (IU/L) | 162 [98–402] | 200 [116–563] | 157 [98–379] | <0.05 |
| Peak creatine phosphokinase (IU/L) | 2,144 [1,016–4,096] | 2,197 [1,049–4,426] | 2,143 [999–4,087] | 0.31 |
| Type of MI | 0.07 | |||
| Type 1 | 1,292 (96.6) | 148 (98.7) | 1,144 (96.4) | |
| Type 2 | 22 (1.6) | 0 (0.0) | 22 (1.9) | |
| Type 4b | 23 (1.7) | 2 (1.3) | 21 (1.8) | |
| Discharge medications | ||||
| Statin | 1,135 (84.9) | 118 (78.7) | 1,017 (85.7) | <0.05 |
| β-blocker | 918 (68.7) | 98 (65.3) | 820 (69.1) | 0.35 |
| ACEI or ARB | 1,104 (82.6) | 114 (76.0) | 990 (83.4) | <0.05 |
| Aspirin | 1,307 (97.8) | 149 (99.3) | 1,158 (97.6) | 0.11 |
| Ticlopidine or clopidogrel | 1,257 (94.0) | 138 (92.0) | 1,119 (94.3) | 0.29 |
| Warfarin | 114 (8.5) | 10 (6.7) | 104 (8.8) | 0.37 |
Categorical variables are presented as n (%) and continuous variables are presented as the mean±SD or median [interquartile range]. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; DBP, diastolic blood pressure; MI, myocardial infarction; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; STEMI, ST-elevation myocardial infarction.
The angiographic and procedural characteristics are summarized in Table 2. There were significant differences in the infarct-related coronary vessels based on symptom presentation. Patients with atypical symptoms more frequently exhibited multivessel disease than those with typical symptoms (60.0% vs. 46.8%; P<0.05). Of note, drug-eluting stents were less likely to be used in patients presenting with atypical than typical symptoms (55.0% vs. 63.4%; P<0.05). In addition, there were significant differences in the use of intra-aortic balloon pumps (24.0% vs. 14.8%; P<0.05) and mechanical ventilation (23.3% vs. 6.7%; P<0.001) between patients presenting with atypical and typical symptoms. Importantly, the prevalence of door-to-balloon time of ≤90 min was significantly lower in patients with atypical than typical symptoms (40.0% vs. 66.3%; P<0.001).
| All patients (n=1,337) |
Atypical symptoms (n=150) |
Typical symptoms (n=1,187) |
P value | |
|---|---|---|---|---|
| Infarct-related coronary vessel | ||||
| Left main artery | 31 (2.3) | 10 (6.7) | 21 (1.8) | <0.05 |
| Left anterior descending artery | 610 (45.6) | 49 (32.7) | 561 (47.3) | <0.001 |
| Left circumflex artery | 152 (11.4) | 12 (8.0) | 140 (11.8) | 0.15 |
| Right coronary artery | 535 (40.0) | 79 (52.7) | 456 (38.4) | <0.001 |
| Multivessel disease | 646 (48.3) | 90 (60.0) | 556 (46.8) | <0.05 |
| Presence of chronic total occlusion | 116 (8.7) | 11 (7.3) | 105 (8.8) | 0.53 |
| TIMI flow Grade 0 before PCI of the culprit lesion | 881 (65.9) | 94 (62.7) | 787 (66.3) | 0.38 |
| TIMI flow Grade 3 after PCI of the culprit lesion | 1,201 (89.8) | 129 (86.0) | 1,072 (90.3) | 0.11 |
| Drug-eluting stent implantation | 826 (62.4) | 82 (55.0) | 744 (63.4) | <0.05 |
| Use of intra-aortic balloon pump | 212 (15.9) | 36 (24.0) | 176 (14.8) | <0.05 |
| Use of mechanical ventilation | 115 (8.6) | 35 (23.3) | 80 (6.7) | <0.001 |
| Use of temporary pacing | 214 (16.0) | 41 (27.3) | 173 (14.6) | <0.001 |
| Renal replacement therapy | 33 (2.5) | 11 (7.3) | 22 (1.9) | <0.001 |
| Catecholamine therapy | 272 (20.3) | 63 (42.0) | 209 (17.6) | <0.001 |
| Door-to-balloon time (min) | 76 [55–109] | 101 [66–158] | 74 [54–103] | <0.001 |
| Door-to-balloon time ≤90 min | 847 (63.4) | 60 (40.0) | 787 (66.3) | <0.001 |
Categorical variables are presented as n (%) and continuous variables are expressed as the median [interquartile range]. PCI, percutaneous coronary intervention; TIMI, Thrombosis in Myocardial Infarction.
The risk factors associated with a delay in door-to-balloon time (>90 min) are analyzed in Table 3. On multivariable logistic regression analysis, atypical symptoms (odds ratio [OR] 2.76; 95% confidence interval [CI] 1.91–4.02; P<0.001), age >75 years (OR 1.47; 95% CI 1.13–1.91; P<0.05), night-time arrival (OR 1.68; 95% CI 1.32–2.13; P<0.001), NSTEMI (OR 3.34; 95% CI 2.43–4.62; P<0.001), and the presence of chronic total occlusion (OR 1.59; 95% CI 1.05–2.39; P<0.05) were significantly associated with the door-to-balloon time. The results of a similar analysis excluding patients with NSTEMI are provided in the Supplementary Table.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Atypical symptoms | 2.95 | 2.09–4.20 | <0.001 | 2.76 | 1.91–4.02 | <0.001 |
| Age >75 years | 1.64 | 1.30–2.07 | <0.001 | 1.47 | 1.13–1.91 | <0.05 |
| Night-time arrival | 1.55 | 1.23–1.94 | <0.001 | 1.68 | 1.32–2.13 | <0.001 |
| NSTEMI | 3.16 | 2.32–4.33 | <0.001 | 3.34 | 2.43–4.62 | <0.001 |
| Killip class >I | 1.35 | 1.04–1.74 | <0.05 | 0.95 | 0.71–1.26 | 0.71 |
| CKD | 1.47 | 1.04–2.06 | <0.05 | 1.14 | 0.78–1.65 | 0.50 |
| Anemia | 1.57 | 1.21–2.03 | <0.001 | 1.21 | 0.90–1.63 | 0.21 |
| Presence of CTO | 1.63 | 1.11–2.39 | <0.05 | 1.59 | 1.05–2.39 | <0.05 |
CI, confidence interval; CKD, chronic kidney disease; CTO, chronic total occlusion; NSTEMI, non-ST segment elevation myocardial infarction; OR, odds ratio.
Under these conditions, the median duration of the hospital stay was 14 days (IQR 10–19 days). At Day 30, there were 55 recorded all-cause deaths. The 30-day mortality was significantly higher in patients with atypical than typical symptoms (20 [13.3%] vs. 35 [2.9%]; P<0.001). On multivariate Cox proportional hazard regression analysis, atypical symptoms (hazard ratio [HR] 2.33; 95% CI 1.20–4.38; P<0.05), age >75 years (HR 3.46; 95% CI 1.72–7.45; P<0.001), Killip class >I (HR 3.27; 95% CI 1.62–6.98; P<0.001), culprit lesion in the left main coronary artery (HR 2.63; 95% CI 1.02–5.98; P<0.05), the presence of chronic total occlusion (HR 2.84; 95% CI 1.35–5.64; P<0.05), and TIMI flow Grade 3 after PCI (HR 0.37; 95% CI 0.20–0.72; P<0.05) were associated with 30-day all-cause mortality (Table 4).
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Atypical symptoms | 4.03 | 2.17–7.23 | <0.001 | 2.33 | 1.20–4.38 | <0.05 |
| Age >75 years | 5.45 | 2.86–11.27 | <0.001 | 3.46 | 1.72–7.45 | <0.001 |
| Killip class >I | 7.14 | 3.77–14.47 | <0.001 | 3.27 | 1.62–6.98 | <0.001 |
| CKD | 3.47 | 1.84–6.28 | <0.001 | 1.64 | 0.85–3.04 | 0.14 |
| Anemia | 2.07 | 1.14–3.69 | <0.05 | 0.98 | 0.52–1.82 | 0.96 |
| Culprit lesion in the LMCA | 5.23 | 2.11–11.18 | <0.05 | 2.63 | 1.02–5.98 | <0.05 |
| Presence of CTO | 3.15 | 1.56–5.93 | <0.05 | 2.84 | 1.35–5.64 | <0.05 |
| TIMI flow Grade 3 after PCI | 0.31 | 0.17–0.60 | <0.001 | 0.37 | 0.20–0.72 | <0.05 |
| Door-to-balloon time >90 min | 1.83 | 1.03–3.27 | <0.05 | 1.14 | 0.61–2.11 | 0.68 |
HR, hazard ratio; LMCA, left main coronary artery. Other abbreviations as in Tables 2,3.
Kaplan-Meier curves demonstrated that cumulative mortality within 30 days was significantly greater among patients with atypical than typical symptoms (Figure 2).
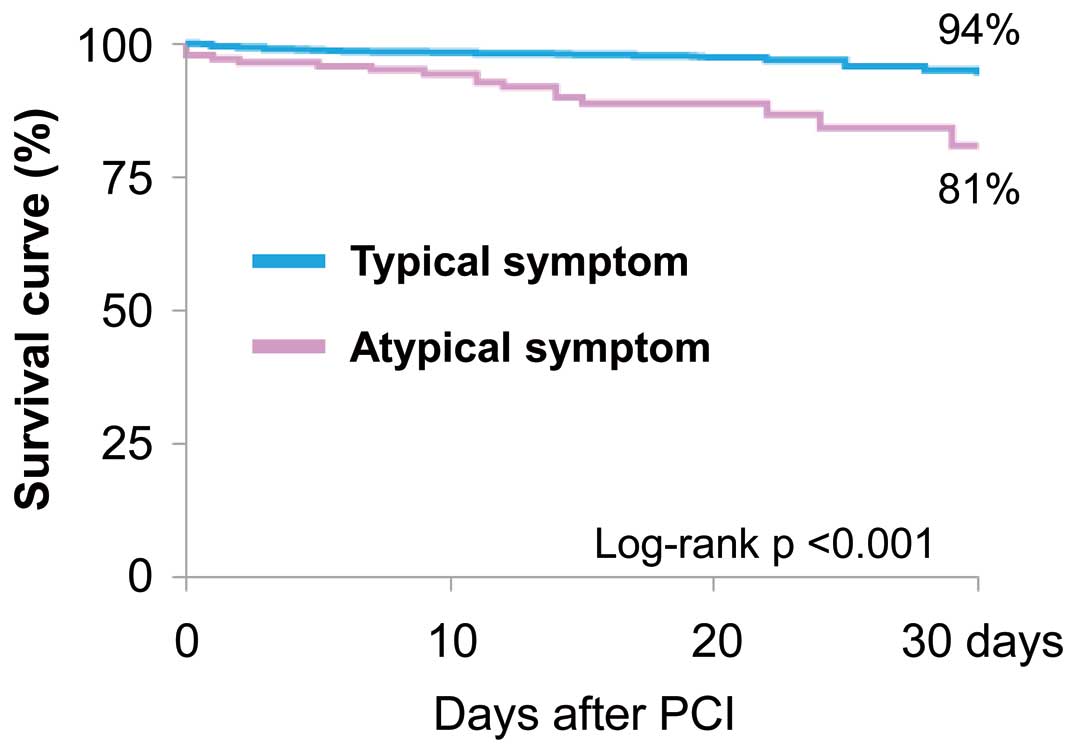
Kaplan-Meier time-to-event curves for the freedom from all-cause death within 30 days after percutaneous coronary intervention (PCI) for patients stratified according to symptom presentation.
The important findings of the present study are that: (1) patients with AMI exhibiting atypical symptoms were more likely to have multiple comorbidities than those exhibiting typical symptoms; (2) a delay in door-to-balloon time was more common in patients with atypical than typical symptoms; and (3) symptom presentation was associated with 30-day mortality, even in patients who underwent emergency PCI.
The incidence of atypical symptoms in the present study was 11.2%, which is consistent with previous data from AMI patients who underwent emergency PCI.6 Previous studies have reported an association between atypical symptoms and advanced age, female sex, and a history of diabetes.3,4 However, this study showed no significant differences in the frequency of symptom presentation according to sex and history of diabetes, which is also consistent with other studies.15,16 Such inconclusive results may be explained by heterogeneity among the study population. Meanwhile, in the present study, we also found significant differences in the prevalence of heart failure and renal dysfunction according to symptom presentation. Indeed, all these factors have been associated with a higher risk of death. Although the mechanisms underlying the interplay between atypical symptoms and higher risk factor profile are not completely understood, it is possible to speculate that the patients with AMI who exhibited atypical symptoms may be too severely ill to complain of chest pain.
The present study demonstrated that the use of intra-aortic balloon pumps and mechanical ventilation was higher among patients presenting with atypical symptoms, likely due to a higher incidence of cardiogenic shock at presentation among patients with AMI who exhibit atypical symptoms. Because patients with atypical symptoms are at risk of a delay in timely reperfusion because of a need for time-consuming therapy, such as securing airways and mechanical support before PCI, continuing efforts to minimize door-to-balloon time are desirable in these patients. Under these conditions, this study demonstrated that there were significant differences in the infarct-related coronary vessels based on symptom presentation. Specifically, a culprit lesion in the left main coronary artery was significantly more likely in patients with AMI exhibiting atypical symptoms compared with those exhibiting typical symptoms. In general, hemodynamic instability, cardiogenic shock, and resuscitated cardiac arrest are often complications in patients with AMI due to left main trunk coronary artery disease.17,18 Therefore, differences in the culprit coronary vessels may explain, in part, the differences in symptom presentation in patients with AMI.
In this study, symptom presentation was associated with 30-day mortality in patients with AMI who underwent PCI, even after adjustment for various clinical factors. Several previous studies have investigated the association between the presence of chest pain and prognosis among patients with coronary artery disease. In patients with stable angina or previous MI, inducible myocardial ischemia that becomes evident during a stress test is associated with unfavorable outcomes, regardless of whether symptoms are present.19,20 Consequently, revascularization appears to offer a prognostic advantage compared with medical therapy, even in patients with silent myocardial ischemia.21 Although there were robust associations between complete revascularization and favorable outcomes in patients with AMI,22,23 revascularization for non-culprit lesions may be less likely in patients with atypical symptoms because of the absence of chest pain. Because patients with atypical symptoms are more likely to have multivessel coronary artery disease, future investigations should examine whether achieving complete revascularization improves their prognosis.
This study has several limitations. First, the analysis of symptom presentation in patients with AMI was not prespecified in the original trial design; thus, these results should be considered hypothesis generating. In addition, the number of patients with endpoints was limited, and therefore the present findings should be interpreted with caution. However, the present study clearly demonstrated the prognostic importance of the assessment of symptom presentation, which was easily evaluated at first contact with AMI patients in the emergency department. Second, the effects of confounding factors that we did not analyze should be considered. A recent study showed the importance of minimizing the door-to-balloon time as well as the onset to balloon time in patients with AMI undergoing PCI.24 If subjects with atypical symptoms experience a longer delay before hospital admission, this may be a relevant factor. However, to determine accurate symptom onset would be difficult in patients complaining of atypical symptoms. Therefore, the use of a more objective clinical tool to estimate the duration from symptom onset to presentation is desirable.25,26 Third, although we were not able to assess symptom presentation using an alternative definition,27 the definition of atypical symptoms varies between studies and there is no general consensus as to their assessment in patients with suspected AMI. Thus, more data are needed to examine this issue further. Fourth, we were unable to adjust for differences in population density, economic status, traffic networks, and number of physicians in the participating hospitals, which may have affected our results. Finally, pathophysiological assessment with nuclear cardiac imaging, which has established links between silent myocardial ischemia, autonomic neuropathy, and subsequent future cardiovascular events,28,29 was not available in the present study. Therefore, this study may provide the foundation for large-scale prospective clinical studies with imaging analysis to confirm the study results.
This multicenter study of AMI demonstrated that patients exhibiting atypical symptoms represent a high-risk subgroup that experiences a delay in timely reperfusion and is associated with an increased risk of 30-day mortality. Future investigations with objective diagnostic criteria may support the findings of the present study.
The authors acknowledge the efforts of the investigators at the 41 participating centers in helping with this study. A list of participating centers is provided in the Supplementary Appendix.
This study did not receive any specific funding.
The authors declare that there are no conflicts of interest.
This study was approved by the Ethics Committees of Mie University Graduate School of Medicine (Reference no. 2478).
Please find supplementary file(s);
http://dx.doi.org/10.1253/circrep.CR-21-0006