2021 年 3 巻 4 号 p. 187-193
2021 年 3 巻 4 号 p. 187-193
Background: The impact of preprocedural visit-to-visit blood pressure variability (BPV) on pulmonary vein isolation (PVI) outcome in patients with hypertension (HTN) and atrial fibrillation (AF) remains unclear.
Methods and Results: This study enrolled 138 AF patients with HTN who underwent successful PVI. Patients were classified into 2 groups, those with AF recurrence (AF-Rec; n=42) and those without AF recurrence (No-AF-Rec; n=96). Blood pressure (BP) was measured at least 3 times during sinus rhythm, and systolic and diastolic BPV (Sys-BPV and Dia-BPV, respectively) were defined as the standard deviation of BP. Clinical characteristics were compared between the 2 groups, and the relationship between BPV and AF recurrence was investigated. Sys-BPV and Dia-BPV were significantly higher in the AF-Rec than No-AF-Rec group (Sys-BPV: 10.6±3.7 vs. 6.9±3.5; Dia-BPV: 7.3±3.1 vs. 4.8±3.0; P<0.05 for both). Receiver operating characteristic analysis revealed Sys-BPV 9.1 and Dia-BPV 5.7 as cut-off values for AF recurrence. Kaplan-Meyer analysis demonstrated higher AF recurrence in patients with Sys-BPV >9.1 and Dia-BPV >5.7 (P<0.05 for both). Cox multivariate regression analysis revealed that Sys-BPV >9.1 and Dia-BPV >5.7 were independent predictors of AF recurrence (hazard ratios 3.736 and 2.958, respectively; P<0.05 for both).
Conclusions: Sys-BPV and Dia-BPV were associated with AF recurrence in AF patients with HTN.
Hypertension (HTN) is the most prevalent comorbidity in >50% of patients with atrial fibrillation (AF), and itself is a risk factor for the development of new AF.1–3 In addition, the coexistence of AF and uncontrolled HTN is associated with an increased risk of stroke, intracranial hemorrhage, and major bleeding.4–6 We previously reported that AF recurred significantly more frequently in AF patients with uncontrolled than controlled HTN (52% vs. 26%, respectively; P=0.045), and that left atrial (LA) remodeling progressed in AF patients with uncontrolled HTN, even after catheter ablation (CA), whereas this remodeling was suppressed in patients with controlled HTN.7 Therefore, proper control of HTN is important from the perspective of preventing AF recurrence as well as long-term cardiovascular and cerebrovascular complications.
According to current guidelines, the diagnosis and classification of HTN, as well as subsequent risk management, are performed primarily on the basis of mean blood pressure (BP).8,9 However, the clinical importance of BP variability (BPV), which indicates fluctuations of BP over various time periods (e.g., daily or seasonal) has been recognized. High visit-to-visit BPV (VV-BPV) was proven to be associated with cerebrovascular and cardiovascular events, as well as all-cause deaths, independent of mean BP.10,11 However, there are no specific data indicating whether VV-BPV can affect AF recurrence after CA in AF patients with HTN. Thus, the aims of the present study were to investigate whether VV-BPV is involved in AF recurrence after CA and whether it could be a predictor of AF recurrence independent of mean BP control.
Between February 2014 and May 2018, 239 consecutive patients who had been referred to Fukushima Medical University Hospital for treatment of AF and underwent successful ablation were assessed (Figure 1). Of these 239 patients, 101 were excluded: 91 had no history of HTN and BP during sinus rhythm could not be measured in the remaining 10 because of long-standing persistent AF. Thus, 138 patients were enrolled in the present study. Patients were divided into 2 groups based on AF recurrence after CA, namely those with AF recurrence and/or atrial tachycardia (AT; AF-Rec; n=42) and those without AT/AF recurrence (No-AF-Rec; n=96). All patients included in this study were symptomatic and refractory to at least 1 anti-arrhythmic drug (AAD) before CA.
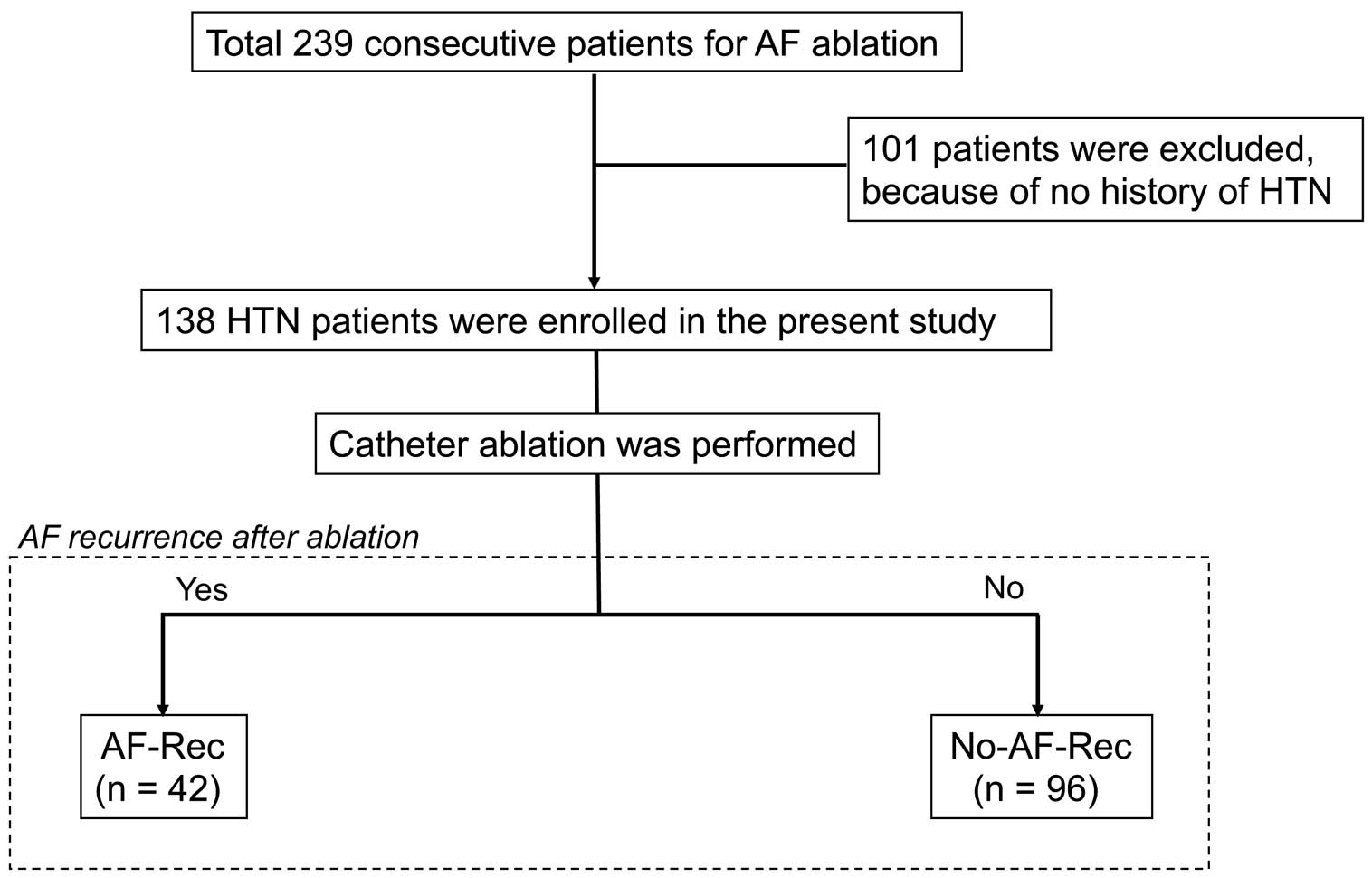
Patient flowchart in the current study. AF, atrial fibrillation; AF-Rec, atrial fibrillation recurrence; HTN, hypertension.
Structural heart disease was excluded by echocardiography and coronary angiography or coronary artery imaging with computed tomography (CT). The definition of HTN was in accordance with the 2017 American College of Cardiology/American Heart Association Blood Pressure Guidelines.8 Before BP measurement, patients rested for >5 min sitting in a chair. Then, at least 2 BP measurements were obtained from seated patients, spaced 1–2 min apart, during sinus rhythm. At the first visit, BP in both arms was measured to detect any possible differences. The arm with the higher value was selected as the reference. An appropriate cuff size (i.e., one in which the bladder encircled 80% of the upper arm) was selected and used for BP measurements. The normal upper limits for systolic and diastolic BP were 140 and 90 mmHg, respectively, based on current guidelines. BP was measured using an automatic sphygmomanometer (Omron HEM-7320-LA; Omron Healthcare, Kyoto, Japan) to minimize BP measurement errors between observers and from visit to visit.12 Mean BP was calculated from measurements recorded on at least 3 different days at the outpatient clinic and on the first day of a patient’s hospital stay prior to the ablation procedure. BPV was calculated as standard deviation (SD) of systolic and diastolic BP. Patients with BP ≥140/90 mmHg were defined as having uncontrolled BP. Treatment with 3 or more antihypertensive drugs and had a history of uncontrolled BP were also considered as uncontrolled BP. Patients with BP <140/90 mmHg without taking antihypertensive medicines were diagnosed as having no HTN. Secondary HTN, such as primary aldosteronism, Cushing syndrome, renovascular HTN, renal diseases, pheochromocytoma, and aortic disease, was excluded prior to study enrollment.8
The study protocol was approved by the Ethics Committee of Fukushima Medical University, and the investigation conformed with the principles outlined in the Declaration of Helsinki. All patients provided written informed consent prior to the procedure.
Electrophysiological Study and Ablation ProcedureThe ablation procedure was performed as described previously.7,13 Briefly, AADs were withheld for at least 5 half-lives and the existence of LA thrombi were excluded by transesophageal echocardiography. Ablation was performed under sedation with intravenous dexmedetomidine and fentanyl. Twelve-lead surface electrocardiograms (ECGs) and intracardiac electrograms were recorded simultaneously by a digital multichannel system (RMC-5000; Nihon-Kohden, Tokyo, Japan), filtered at 30–400 Hz for bipolar electrograms and at 0.05–400 Hz for unipolar electrograms. An electrode was positioned in the bundle of His area, and another electrode was positioned in the distal coronary sinus (CS). Following transseptal puncture, 3-dimensional electroanatomical mapping of the left atrium was reconstructed using a contact force-sensing catheter (Thermocool SmartTouch® SF; Biosense Webster, Irvine, CA, USA).
Circumferential pulmonary vein isolation (PVI) was performed in the power-controlled mode. Radiofrequency energy was delivered at 30 W along the posterior wall, roof and bottom, and at 40 W in the remaining areas using a dragging technique with a temperature limit of 45℃. During the procedure, unfractionated heparin was administered to maintain an activated clotting time >300 s. After successful PVI, intravenous isoproterenol was administered for the provocation of recovered pulmonary vein (PV) conduction and non-PV firing (non-PV foci). If the PV was still isolated and non-PV focal firing was not detected, a bolus of adenosine was administered to check for dormant conduction and to provoke the firing of non-PV foci. If no AF induction was confirmed by CS burst pacing of up to 200 ms, the procedure was deemed completed. In case of AF induction, substrate modification, such as linear ablation or complex fractionated atrial electrogram (CFAE) ablation, was considered. If a low-voltage area (bipolar voltage <0.5 mV) and/or a dense scar (the absence of local voltage or a bipolar voltage <0.1 mV without capture) were detected, linear ablation and/or box isolation of these areas was performed. If there were no low-voltage areas and/or dense scars, CFAE ablation was performed.
Follow-upAll patients were followed up at 1, 3, 6 and 12 months and every 3 months thereafter. Anticoagulation therapy was continued for at least 2 months after the procedure. Patients with a CHA2DS2-VASc score ≥2 were recommended to continue anticoagulation for 2 months after PVI, regardless of AF recurrence. AADs were continued for 3 months after the procedure, and then all AADs were discontinued. At each visit, a 12-lead ECG and 24-h Holter ECG were performed. The study endpoint was the recurrence of AT/AF, defined as documentation lasting >30 s recorded in a 12-lead ECG or 24-h Holter ECG after the blanking period of 3 months.
Statistical AnalysisData were compared between the 2 groups using a 2-tailed paired Student’s t-test. Results are presented as the mean±SD. Dichotomous data are presented as numbers and percentages and were compared using a Chi-squared test. Receiver operating characteristic (ROC) analysis was used to calculate sensitivity, specificity, area under the ROC curve (AUC), and the optimal cut-off with 95% confidence intervals (CIs). Freedom from recurrence was evaluated using the Kaplan-Meier method. A Cox proportional hazard model was used to determine independent predictors of recurrence, with results presented as hazard ratios (HRs) and 95% CIs. Multivariate Cox regression analysis with stepwise forward selection was performed using covariates that had P<0.05 in the univariate analysis to determine the predictors of AF recurrence. Binominal logistic regression analysis was performed to elucidate the predictors of systolic BPV (Sys-BPV) or diastolic-BPV (Dia-BPV) higher than the cut-off value.
All analyses were performed in SPSS for Windows, version 26.0 (SPSS Inc., Chicago, IL, USA). All statistical tests were 2-sided and P<0.05 was considered statistically significant.
The characteristics of patients in the 2 groups are presented in Table 1. There were no significant differences in age, sex, body mass index (BMI), the proportion of persistent AF, and the duration of AF between the 2 groups. Mean systolic BP was significantly higher in the AF-Rec than No-AF-Rec group (132±13 mmHg vs. 127±11 mmHg; P=0.005). In addition, there were significant differences in Sys-BPV and Dia-BPV between the AF-Rec and No-AF-Rec groups (Sys-BPV, 10.6±3.7 vs. 6.9±3.5, respectively [P<0.001]; Dia-BPV, 7.3±3.1 vs. 4.8±3.0, respectively [P<0.001]). Patients in the AF-Rec group had a higher prevalence of uncontrolled BP than those in the No-AF-Rec group (55% vs. 23%; P<0.001). With regard to echocardiographic parameters, LA volume index (LAVI) tended to be higher in the AF-Rec than No-AF-Rec group, but the difference did not reach statistical significance. There were no significant differences between the 2 groups in estimated glomerular filtration rate (eGFR), in the type and mean number of antihypertensive drugs used, or in the CHA2DS2-VASc score.
| AF-Rec (n=42) |
No-AF-Rec (n=96) |
P value | |
|---|---|---|---|
| Age (years) | 63±8 | 65±8 | 0.154 |
| Male sex | 37 (88) | 80 (83) | 0.477 |
| BMI (kg/m2) | 25.3±3.6 | 25.0±2.9 | 0.621 |
| Paroxysmal AF | 24 (57) | 60 (62) | 0.556 |
| Persistent AF | 18 (43) | 36 (38) | 0.556 |
| Duration of AF (months) | 15.8±12.0 | 16.3±18.0 | 0.888 |
| Mean systolic BP (mmHg) | 133±13 | 127±11 | 0.005* |
| Mean diastolic BP (mmHg) | 77±8 | 77±9 | 0.966 |
| Uncontrolled BP | 23 (55) | 22 (23) | 0.001* |
| Sys-BPV | 10.6±3.7 | 6.9±3.5 | <0.001* |
| Dia-BPV | 7.3±3.1 | 4.8±3.0 | <0.001* |
| Comorbidity | |||
| Diabetes | 12 (29) | 21 (22) | 0.400 |
| Dyslipidemia | 13 (31) | 31 (32) | 0.878 |
| Echocardiography | |||
| LVEF (%) | 59.8±8.0 | 60.9±9.9 | 0.442 |
| E/e’ | 8.7±3.3 | 8.3±3.3 | 0.721 |
| LAVI (mL/m2) | 43.6±13.9 | 39.2±14.4 | 0.098 |
| eGFR (mL/min/1.73 m2) | 60.2±17.4 | 64.9±14.6 | 0.104 |
| Antihypertensive drugs | |||
| CCBs | 26 (62) | 56 (58) | 0.697 |
| ARBs | 30 (71) | 63 (66) | 0.507 |
| ACEI | 4 (10) | 17 (18) | 0.221 |
| β-blocker | 24 (57) | 51 (54) | 0.749 |
| Diuretics | 12 (29) | 30 (31) | 0.755 |
| α-blocker | 9 (21) | 20 (21) | 0.938 |
| No. drugs per patient | 2.5±0.8 | 2.4±1.0 | 0.908 |
| CHA2D2-VASc score | 2.0±0.9 | 2.0±0.9 | 0.712 |
| Additional ablation plus PVI | |||
| SVCI | 0 (0) | 1 (1) | 0.510 |
| Box isolation | 3 (7) | 4 (4) | 0.467 |
| CFAE | 2 (5) | 2 (2) | 0.392 |
Unless indicated otherwise, data are given as the mean±SD or as n (%). ACEI, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; AF-Rec, atrial fibrillation recurrence; ARBs, angiotensin II receptor blockers; BMI, body mass index; BP, blood pressure; CCBs, calcium channel blockers; CFAE, complex fractionated atrial electrogram; Dia-BPV, diastolic BP variability; eGFR, estimated glomerular filtration rate; HTN, hypertension; LAVI, left atrium volume index; LVEF, left ventricular ejection fraction; PVI, pulmonary vein isolation; SVCI, superior vena cava isolation; Sys-BPV, systolic BP variability. *P values are statistically significant.
The entire study population underwent successful PVI. One patient in the No-AF-Rec group had superior vena cava (SVC) firing as a non-PV focus, so SVC isolation was performed. Three patients in the AF-Rec group and 4 in the No-AF-Rec group underwent box isolation because they had a low-voltage area in the LA posterior wall. CFAE ablation was performed for 2 patients in each group. During the 13±7 months of follow-up, recurrence was documented in 42 of all 138 patients (30.4%).
ROC curve analysis demonstrated that the cut-off values of Sys-BPV and Dia-BPV for AF recurrence were 9.1 (AUC 0.791; 95% CI 0.708−0.874; P<0.001) and 5.7 (AUC 0.767; 95% CI 0.682−0.852; P<0.001), respectively, as shown in Figure 2.
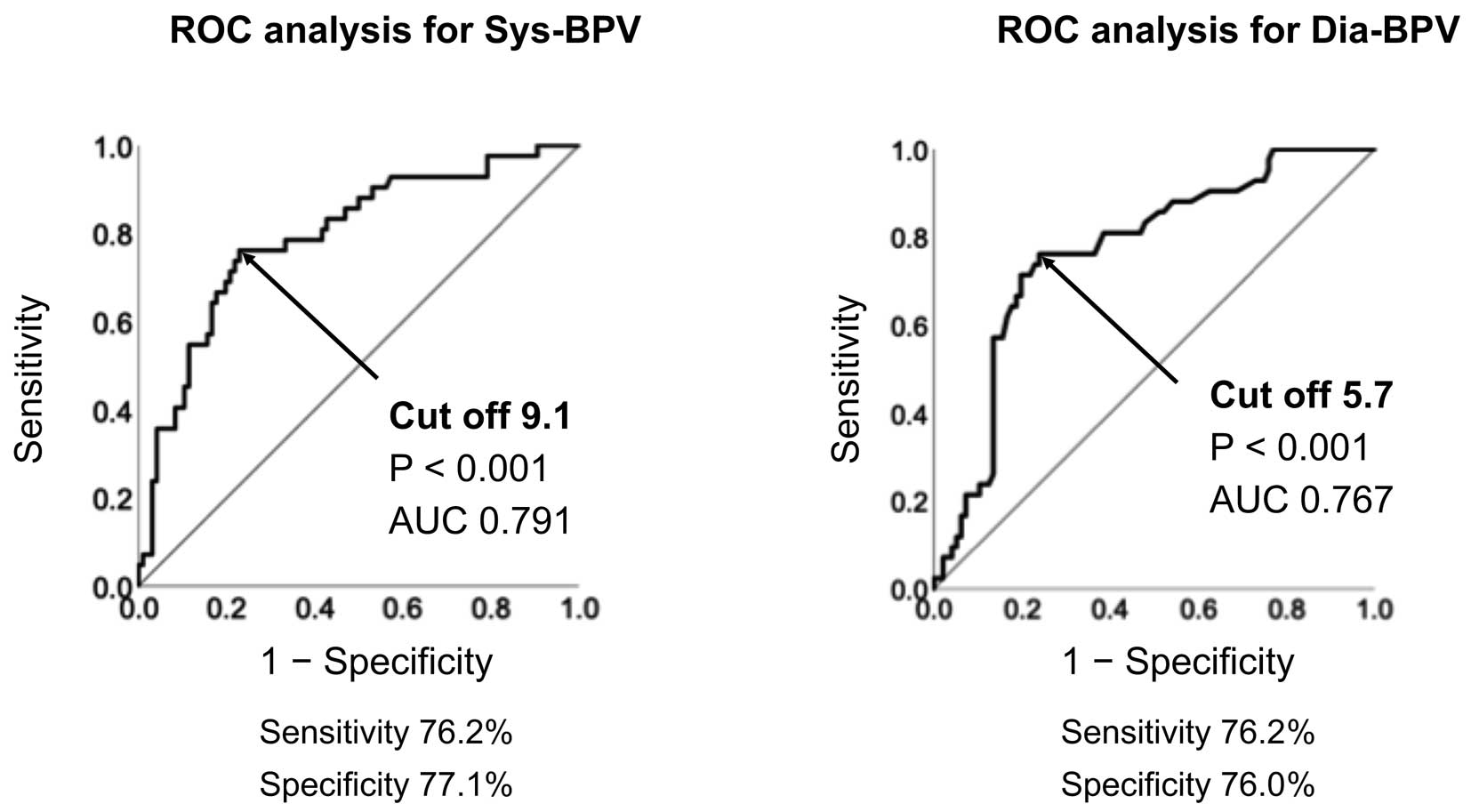
Cut-off values for (Left) systolic blood pressure variability (Sys-BPV) and (Right) diastolic blood pressure variability (Dia-BPV) for the prediction of atrial fibrillation recurrence determined using receiver operating curve (ROC) analysis. AUC, area under the curve.
The study patients were divided into 2 groups based on Sys-BPV and Dia-BPV cut-off values. Kaplan-Meier analysis was then used to evaluate AT/AF recurrence after CA (Figure 3). AF recurrence was significantly (P=0.001) higher in patients with Sys-BPV >9.1 (n=56) than in those with Sys-BPV ≤9.1 (n=82). Moreover, AT/AF recurrence was significantly (P=0.001) higher in patients with Dia-BPV >5.7 (n=54) than in those with Dia-BPV ≤5.7 (n=84).
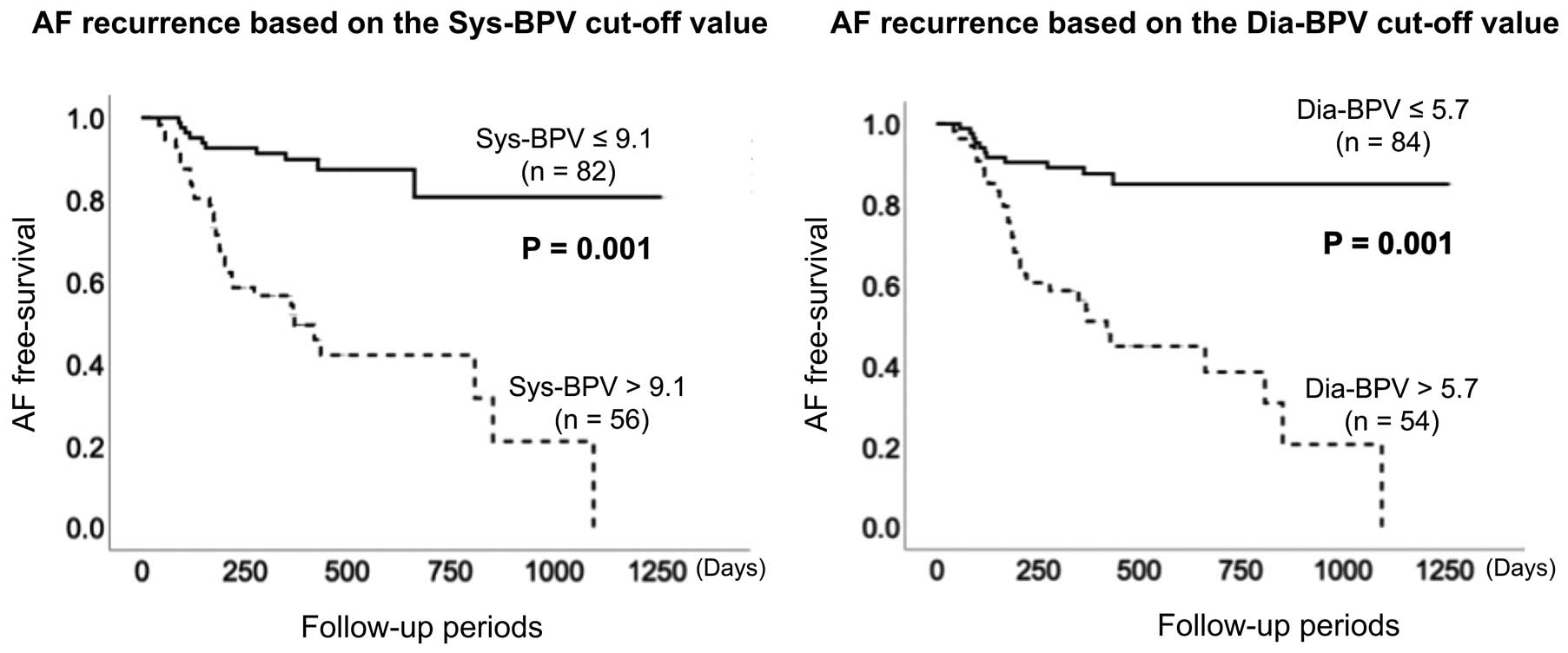
Kaplan-Meier survival curves for atrial fibrillation (AF) recurrence. There were significant differences in the ratio of AF recurrence between groups defined using the systolic blood pressure variability (Sys-BPV; Left) and diastolic blood pressure variability (Dia-BPV; Right) cut-off values (P=0.001 for both).
Univariate Cox regression analysis revealed that mean systolic BP, Sys-BPV, Dia-BPV, and uncontrolled BP were associated with AT/AF recurrence (Table 2). In multivariate analysis, after adjustment for covariates, uncontrolled BP (HR 2.420; 95% CI 1.260−4.646; P=0.008), Sys-BPV >9.1 (HR 3.736; 95% CI 1.752−7.964; P=0.001), and Dia-BPV >5.7 (HR 2.958; 95% CI 1.419−6.166; P=0.004) remained independent predictors of AT/AF recurrence.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | 0.983 | 0.948–1.018 | 0.334 | |||
| eGFR | 0.982 | 0.963–1.000 | 0.054 | |||
| LAVI | 1.012 | 0.994–1.031 | 0.188 | |||
| Mean systolic BP | 1.0313 | 1.013–1.070 | 0.004* | |||
| Mean diastolic BP | 1.001 | 0.968–1.035 | 0.956 | |||
| Sys-BPV >9.1 | 6.158 | 3.016–12.575 | <0.001* | 3.736 | 1.752–7.964 | 0.001* |
| Dia-BPV >5.7 | 5.225 | 2.618–10.426 | <0.001* | 2.958 | 1.419–6.166 | 0.004* |
| Uncontrolled BP | 3.415 | 1.803–6.470 | <0.001* | 2.420 | 1.260–4.646 | 0.008* |
CI, confidence interval; OR, odds ratio. Other abbreviations as in Table 1. *P values are statistically significant.
Univariate logistic regression analysis revealed that BMI, calcium channel blockers (CCBs), α-blockers and eGFR were related to Sys-BPV >9.1 (Table 3). In the multivariate analysis, BMI (odds ratio [OR] 1.134; 95% CI 1.011−1.271; P=0.031) and eGFR (OR 0.972; 95% CI 0.949−0.995; P=0.028) were proven to be independent predictors of Sys-BPV >9.1.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Predictors of Sys-BPV >9.1 | ||||||
| Age | 2.473 | 0.849–7.199 | 0.097 | |||
| BMI | 1.120 | 1.002–1.251 | 0.046* | 1.134 | 1.011–1.271 | 0.031* |
| CCB | 0.457 | 0.227–0.918 | 0.028* | |||
| α-blocker | 0.390 | 0.154–0.988 | 0.047* | |||
| eGFR | 0.974 | 0.952–0.997 | 0.028* | 0.972 | 0.949–0.995 | 0.028* |
| Predictors of Dia-BPV >5.7 | ||||||
| Dyslipidemia | 2.226 | 1.071–4.625 | 0.032* | |||
| LAVI | 1.026 | 1.000–1.052 | 0.048* | 1.026 | 1.000–1.052 | 0.048* |
| Uncontrolled BP | 2.091 | 1.011–4.323 | 0.047* | |||
Abbreviations as in Tables 1,2. *P values are statistically significant.
Univariate analysis demonstrated that dyslipidemia as a comorbidity, LAVI, and uncontrolled HTN were associated with Dia-BPV >5.7. After adjustment for the 3 covariates, LAVI remained an independent predictor for Dia-BPV >5.7 (OR 1.026; 95% CI 1.000−1.052; P=0.048).
In the present study, visit-to-visit Sys-BPV and Dia-BPV were significantly higher in patients with than without AF recurrence. The cut-off values of Sys-BPV and Dia-BPV were 9.1 and 5.7, respectively. In addition, Sys-BPV >9.1 and Dia-BPV >5.7 were proven to be independent predictors of AF recurrence after CA in patients with AF and HTN, independent of mean systolic and diastolic BP or uncontrolled HTN. For the prediction of Sys-BPV >9.1 or Dia-BPV >5.7, BMI, and eGFR were proven to be associated with Sys-BPV >9.1, and LAVI was found to be related to Dia-BPV >5.7.
Effects of BPV and Mean BP on AF RecurrenceHTN is a risk factors for cerebrovascular and cardiovascular events, and is known as a risk factor for new onset AF and the progression of AF from paroxysmal to persistent.3,14 Although CA has been established as first-line therapy for AF, the Catheter Ablation Versus Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) trial reported no significant difference in cerebrovascular events, heart failure hospitalization, and total mortality between the ablation group and the AAD group.15 One of the possible reasons for this is a re-elevation of the risk of cardiovascular events due to AF recurrence. We previously reported that patients with AF and uncontrolled HTN had a higher rate of AF recurrence after CA and advanced LA remodeling than those with controlled HTN.7 Thus, an increase in mean BP increases the risk of AF recurrence even if CA is performed in patients with AF and HTN, which, in turn, can cause vascular events such as stroke and heart failure, thus offsetting the effectiveness of CA. Therefore, it is possible that CA cannot suppress the occurrence of cerebrovascular and cardiovascular events.
Conversely, it is known that the contribution of mean BP to the onset of stroke decreases with age, and the importance of BPV as a risk factor independent of mean BP has been reported.16 In the Anglo-Scandinavian Cardiac Outcomes Trial–Blood Pressure Lowering Arm (ASCOT-BPLA), patients with high VV-BPV had a 5-fold increased risk of vascular events, even if HTN was well controlled and fully compliant with antihypertensive drugs.17 Hence, intervention for BPV, as well as mean BP, should be considered in patients with high VV-BPV. We focused on VV-BPV in the present study because VV-BPV has been reported to be more closely associated with the risk of stroke than BPV on ambulatory BP monitoring.11 Moreover, high VV-BPV has been reported to be a risk for new onset AF and a risk for cardiovascular events.10,18 However, the underlying mechanism linking high VV-BPV to a high incidence of cerebrovascular and cardiovascular events remains unclear. In the present study, we proposed one possible answer: that patients with high BPV experienced AF recurrence more frequently despite CA, resulting in the recurrence of cerebrovascular and cardiovascular risk. To the best of our knowledge, this is the first report to show that VV-BPV is a risk factor for AF recurrence independent of mean BP in patients with AF and HTN.
Background of BPVThere is still much that is unknown about the detailed mechanism causing increases in BPV. However, it has been reported that damage to the central nervous system due to subclinical cerebral ischemia may increase BPV.19 In a study using stroke-prone spontaneously hypertensive rats, BPV was reported to be increased by sinoaortic denervation.20 Although cerebrovascular events were not investigated in the present study, an increase in BMI and a decrease in eGFR (as predictors of Sys-BPV) and an increase in LAVI (as a predictor of Dia-BPV) were shown to be associated with the development of cerebrovascular events in addition to HTN. These factors are closely related not only to cerebral infarction and heart failure, but also to AF recurrence.21–26 The use of CCBs to reduce BPV has been reported.17 In the present study, the use of CCBs was a negative predictor for increasing Sys-BPV in the univariate analysis. In ASCOT-BPLA, amlodipine lowered mean BP and BPV, but was less involved in reducing cerebrovascular events, suggesting that the effects of CCBs on BPV are limited.27 Furthermore, the significance of CCBs was absent from the multivariate analysis, and it must be said that the effect of CCBs on BPV was limited in the present study. When considering actual event suppression, the combined use of amlodipine with statins has been shown to reduce BPV and the risk of stroke.27 Therefore, BP control and systemic risk control, including the predictors proven in the present study, are needed to reduce BPV, which, in turn, can lead to a reduction in cerebrovascular and cardiovascular events.
Clinical ImplicationsIn the present study we demonstrated that high Sys-BPV and Dia-BPV were predictors of AF recurrence, independent of mean BP and uncontrolled HTN in patients with AF and HTN. Therefore, it is important to recognize that lowering mean BP alone is not enough, and lowering BPV is also needed to decrease the risk of AF recurrence after CA. Furthermore, it is important to manage not only BP, but also systemic risk factors such as BMI, eGFR, and LAVI, which can affect BPV, from the perspective of suppressing the risk of AF recurrence and subsequent cerebrovascular and cardiovascular events.
Study LimitationsThe present study has several limitations. First, because it was a single-center study, the study population was relatively small. Study power calculations using an α error of 0.05 and a β risk of 0.2 indicated that a sample size of 144 patients was needed; at n=138, the sample size of the present study is 96% of the calculated sample size. This may have affected the analysis in the present study. A larger scale multicenter study is needed to confirm our results. Second, no data were available regarding medication adherence throughout the follow-up period. Third, the present study had no detailed information on the study subjects, such as emotional or situational status at the time of BP measurement, which could have affected the BPV results (e.g., white coat effect, depression, or anxiety).
High Sys-BPV and Dia-BPV are risk factors for AF recurrence after CA in patients with AF and HTN, independent of both mean BP and uncontrolled HTN. LA enlargement, high BMI, and decreased eGFR are important indicators for increased BPV. Therefore, not only BP control but also systemic risk management, which can affect Sys-BPV and Dia-BPV, is essential to suppress cerebrovascular and cardiovascular events secondary to AF recurrence.
This study did not receive any specific funding.
The authors declare that they have no conflicts of interest.
The study protocol was approved by the Ethics Committee of Fukushima Medical University (No. 1808).