2021 年 3 巻 8 号 p. 423-430
2021 年 3 巻 8 号 p. 423-430
Background: Hospitalization-associated disability (HAD) is associated with prolonged functional decline and increased mortality after discharge. Therefore, we examined the incidence and risk factors associated with HAD in elderly patients undergoing cardiac surgery in Japan.
Methods and Results: We retrospectively examined 2,262 elderly patients who underwent elective cardiac surgery at Sakakibara Heart Institute. HAD was defined as a functional decline between time of admission and discharge measured by the Barthel Index. We analyzed clinical characteristics using machine learning algorithms to identify the risk factors associated with HAD. After excluding 203 patients, 2,059 patients remained, of whom 108 (5.2%) developed HAD after cardiac surgery. The risk factors identified were age, serum albumin concentration, estimated glomerular filtration rate, Revised Hasegawa’s Dementia Scale, N-terminal pro B-type natriuretic peptide, vital capacity, preoperative Short Physical Performance Battery (SPPB) score, operation times, cardiopulmonary bypass times, ventilator times, length of postoperative intensive care unit stay, and postoperative ambulation start day. The highest incidence of HAD was found in patients with an SPPB score ≤9 and in those who started ambulation >6 days after surgery (76.9%).
Conclusions: Several risk factors for HAD are components of frailty, suggesting that preoperative rehabilitation to reduce the risk of HAD is feasible. Furthermore, the association between HAD and a delayed start of ambulation reaffirms the importance of early mobilization and rehabilitation.
Acute-phase cardiac rehabilitation after cardiac surgery is recommended to prevent postoperative complications and declines in physical function, as well as to regain patients’ preoperative functional level in activities of daily living (ADL) as early as possible.1 Many Japanese hospitals have recently initiated early postoperative mobilization, and the rehabilitation teams are actively involved in perioperative management.
However, in elderly patients with several comorbidities, postoperative rehabilitation is often delayed. In addition, some patients are discharged from hospital with disabilities. Studies have reported that approximately one-third of elderly patients (age >70 years) hospitalized with an acute medical illness show a persistent decline in their ability to maintain ADL.2 The loss of independence in ADL following a hospitalization episode that is not caused by the underlying disease is referred to as hospitalization-associated disability (HAD).2 HAD is associated not only with prolonged length of in-hospital stay and non-home discharge, but also with prolonged functional decline and increased mortality after discharge.3–6
HAD is a particularly important issue in countries with an aging population, such as Japan. The proportion of hospitalized patients aged ≥65 years in Japan increased from 40% in 1984 to 70% in 2017 due to population aging.7 In addition, based on the annual report of the Japanese Association for Thoracic Surgery, the frequency of cardiovascular surgeries has been growing and reached approximately 70,000 in 2017.8 In addition, the expansion of surgical indications in elderly patients and advances in surgical technology are increasing the frequency of elderly patients undergoing surgery. HAD after cardiac surgery affects health economics in Japan. Nevertheless, there is a lack of information regarding HAD after cardiac surgery. Therefore, the aims of this study were to determine the incidence of and explore the risk factors associated with HAD in elderly Japanese patients undergoing cardiac surgery.
This was a single-center retrospective chart review study conducted at Sakakibara Heart Institute (Tokyo, Japan). This study was conducted in compliance with the Declaration of Helsinki and the Guidelines for Medical and Health Research Involving Human Subjects of the Ministry of Health, Labor and Welfare in Japan. The study protocol was approved by the Ethics Committee of Sakakibara Heart Institute (No. 17-027). This study used the opt-out consent form approved by the Ethics Committee.
ParticipantsWe retrospectively examined 2,262 consecutive elderly patients (age ≥65 years) who underwent elective cardiac surgery at Sakakibara Heart Institute between April 2013 and March 2019. The primary diseases for elective cardiac surgeries were ischemic heart disease (48.3%), valvular heart disease (66.0%), and others (18.7%). To minimize the influence of the underlying disease, only patients who underwent elective surgery in a stable condition were included in the study. The exclusion criteria were: (1) preoperative disability (Barthel Index [BI] <100 points); (2) postoperative in-hospital death; (3) postoperative stroke; and (4) being transferred to another hospital due to other diseases.
HAD DefinitionIn this study, HAD was defined as a functional decline between the time of hospital admission and discharge as measured by the BI. The BI consists of 10 items (feeding, transfer, grooming, toilet use, bathing, ambulation, stair climbing, dressing, urination, and defecation management) to assess the functional ability to perform basic ADL.9 Each item was scored according to the degree of independence. Total scores range from 0 to 100 points, with lower scores indicated a greater dependency. In this study, physical therapists and trained nurses recorded the BI during admission and at discharge.
Clinical Characteristics of PatientsData for the following preoperative clinical characteristics were obtained from the medical records of all patients included in this study: age, sex, body mass index, New York Heart Association (NYHA) functional classification, medical history (comorbidity), European System for Cardiac Operative Risk Evaluation II (EuroSCORE II),10 laboratory data (hemoglobin, estimated glomerular filtration rate [eGFR], serum sodium and albumin concentrations, N-terminal pro B-type natriuretic peptide [NT-proBNP] concentrations), left ventricular ejection fraction, vital capacity (VC) as a percentage of predicted VC (%VC), forced expiratory volume % in one second, Revised Hasegawa’s Dementia Scale (HDS-R),11 and the Short Physical Performance Battery (SPPB) score.12 The SPPB is a standardized physical functional test and the most frequently used in elderly individuals. It consists of tests for standing balance, gait speed, and the time it takes to rise from a chair. Each test scores 0 to 4 points and total SPPB scores range from 0 to 12 points. Higher scores indicate a better physical functional status.
Further, as postoperative clinical characteristics, the following data were collected: type of surgery, operation time, use of cardiopulmonary bypass (CPB), CPB time, bleeding volume, ventilator time, the progress of postoperative mobilization (sitting, standing, and ambulation start day), length of postoperative intensive care unit (ICU) stay, continuous renal replacement therapy, postoperative delirium, rate of discharge to home, and the length of in-hospital stay.
Postoperative Rehabilitation ProgramThe postoperative rehabilitation program was implemented according to the general condition and physical function based on the Guidelines for Rehabilitation in Patients with Cardiovascular Disease.13
Statistical AnalysesData analyses consisted of 3 steps. First, we used conventional descriptive statistics to examine patients’ basic and clinical characteristics. Next, crude differences in baseline characteristics between HAD and non-HAD patients were analyzed using t-tests or Chi-squared tests depending on the type of variable. For these analyses, 2-sided P<0.05 was considered statistically significant. We further investigated data from the BI to identify which activity was commonly impaired.
Second, we explored the important variables associated with the decline in BI using Boruta, a novel feature selection algorithm.14 Boruta detects all relevant variables using a random forest (RF) classification algorithm, which iteratively removes the variables using a statistical test.15 The model determines the importance of the variables by classifying each variable as “confirmed”, “tentative”, or “rejected”. This approach was selected over a conventional statistical analysis, such as logistic regression, mainly because it requires no formal distributional assumptions and there is no limit on the number of independent variables.16 In this analysis, to obtain reliable and stable results, the method was configured to perform 1,000 runs to avoid “tentative” conclusions. In addition, we used Gini impurity importance as a measure of variable importance for feature selection instead of Z-scores of the mean decrease accuracy measure, because accuracy does not consider the imbalanced nature of the given dataset. To be more conservative, for this analysis significance was set at 2-sided P<0.01.
In addition, to use all the available information contained in the dataset, we used the Multivariate Imputation by Chained Equations (MICE) approach.17 This method fills in plausible values for any missing data. We created 20 datasets using this method. Predictive mean matching was used for numeric data, logistic regression imputation was used for binary data, polytomous regression imputation was used for categorical data, and a proportional odds model was used for ordered categorical data. For each imputed dataset, the feature selection process using the Boruta algorithm was executed. In the end, 20 different sets of selected features were generated. The final chosen selected features were those that appeared as “confirmed” at least 11 times in the 20 different Boruta sets.
Boruta generates variable importance and is subsequently able to extract important variables from a large number of predictor variables; however, the results were less interpretable due to the nature of the machine learning algorithm, called “black-box”. Thus, the third step was to explore the risk profiles of HAD using the conditional inference tree (Ctree) technique as an exploratory analysis. Ctree is a non-parametric machine learning method that uses repeated binary partitioning of the value spaces of explanatory variables so that each partition corresponds to as homogeneous an outcome as possible.18 This analysis offers intuitive prediction rules with a decision tree, which cannot be achieved by the typical machine learning algorithm. Ctree is similar to the more well-known classification and regression tree (CART) but uses a statistical significance test as the splitting criterion rather than Gini impurity or information gain.18 We included all the available data in this analysis. To avoid overfitting, a minimum of 200 observations in a node was required to enable further splitting, with a minimum criterion requiring P<0.001. In addition, the maximum tree depth was set to 3.
All analyses were conducted using R version 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
Of the 2,262 medical records investigated in this study, 203 were excluded based on the exclusion criteria, resulting in a sample size of 2,059 (Figure 1). Table 1 summarizes the patients’ clinical characteristics and the incidence of HAD. Of the 2,059 study patients, 108 (5.2%) developed HAD after cardiac surgery. The HAD patients had a mean decline in BI of 16.1 (median 10; interquartile range 5–20; Table 2). The most frequently deteriorated BI item at discharge was stair climbing (n=100; 92.6%), followed by bathing (n=58; 53.7%), ambulation (n=36; 33.3%), transfer (n=20; 18.5%), and dressing (n=16; 14.8%; Table 3).
Flow diagram of the patient selection process in this study. ARDS, acute respiratory distress syndrome; BI, Barthel Index; HAD, hospitalization-associated disability.
| Overall (n=2,059) |
HAD group (n=108) |
Non-HAD group (n=1,951) |
P value | |
|---|---|---|---|---|
| Age (years) | 74.1±5.7 | 79.0±5.6 | 73.8±5.5 | <0.001 |
| Female sex | 822 (39.9) | 57 (52.7) | 765 (39.2) | 0.005 |
| BMI (kg/m2) | 22.7±3.3 | 21.9±3.8 | 22.7±3.3 | 0.014 |
| NYHA Class ≥III | 186 (9.0) | 22 (20.3) | 164 (8.4) | <0.001 |
| Comorbidity | ||||
| Hypertension | 1,264 (61.4) | 67 (62.0) | 1,197 (61.3) | 0.887 |
| Diabetes | 531 (25.8) | 39 (36.1) | 492 (25.2) | 0.012 |
| Dyslipidemia | 903 (43.9) | 44 (40.7) | 859 (44.0) | 0.503 |
| Renal dysfunction | 1,097 (53.3) | 72 (66.6) | 1,025 (53.8) | 0.004 |
| Coronary artery disease | 382 (18.6) | 28 (25.9) | 354 (18.1) | 0.043 |
| History of hospital admission for CHF | 321 (15.6) | 22 (20.3) | 299 (15.3) | 0.159 |
| History of cardiac surgery | 141 (6.8) | 9 (8.3) | 132 (6.7) | 0.530 |
| Atrial fibrillation | 377 (18.3) | 22 (20.3) | 355 (18.1) | 0.569 |
| COPD | 48 (2.3) | 4 (3.7) | 44 (2.2) | 0.332 |
| Muscle skeletal disease | 289 (14.0) | 19 (17.5) | 270 (13.8) | 0.274 |
| Cerebral vascular accident | 216 (10.5) | 21 (19.4) | 195 (9.9) | 0.002 |
| EuroSCORE II (%) | 3.27±3.95 | 5.88±7.67 | 3.13±3.59 | <0.001 |
| Laboratory data | ||||
| Hemoglobin (g/dL) | 12.6±1.5 | 12.0±1.3 | 12.6±1.5 | <0.001 |
| eGFR (mL/min/1.73 m2) | 57.3±18.0 | 49.8±23.1 | 57.8±17.6 | 0.001 |
| Serum sodium (mEq/L) | 139±2 | 138±2.8 | 139±2.7 | 0.008 |
| Serum albumin (g/dL) | 4.0±0.3 | 3.8±0.5 | 4.0±0.3 | <0.001 |
| NT-proBNP (pg/mg) | 2,105±7,709 | 6,943±24,100 | 1,856±5,633 | 0.065 |
| LVEF (%) | 58±10 | 57±11 | 58±9 | 0.253 |
| %VC (%) | 88.7±18.0 | 77.6±19.7 | 89.3±17.7 | <0.001 |
| FEV1.0% (%) | 79.7±8.2 | 80.6±8.5 | 79.7±8.2 | 0.294 |
| SPPB score | 11.3±1.4 | 9.0±2.6 | 11.4±1.2 | <0.001 |
| HDS-R score | 27.6±2.6 | 27.6±2.5 | 25.7±4.0 | <0.001 |
Unless indicated otherwise, data are presented as the mean±SD or n (%). The significance of differences between the hospitalization-associated disability (HAD) and non-HAD groups was analyzed using t-tests or the Chi-squared test. BMI, body mass index; CHF, chronic heart failure; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; EuroSCORE II, European System for Cardiac Operative Risk Evaluation II; FEV1.0%, forced expiratory volume % in one second; HDS-R, Revised Hasegawa’s Dementia Scale; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; SPPB, Short Physical Performance Battery; VC, vital capacity.
| Overall (n=2,059) |
HAD group (n=108) |
Non-HAD group (n=1,951) |
P value | |
|---|---|---|---|---|
| Type of surgery | 0.015 | |||
| CABG | 602 (29.2) | 35 (32.4) | 567 (29.1) | |
| Valve surgery | 454 (22.1) | 14 (13.0) | 440 (22.5) | |
| Other | 48 (2.3) | 1 (0.9) | 47 (2.4) | |
| Combined surgery | ||||
| CABG + valve surgery | 318 (15.4) | 28 (25.9) | 290 (14.9) | |
| Combined valve surgery | 303 (14.7) | 14 (13.0) | 289 (14.8) | |
| Other | 334 (16.2) | 16 (14.8) | 318 (16.3) | |
| Operation time (min) | 279±89 | 324±113 | 276±86 | <0.001 |
| Use of CPB | 1,480 (71.8) | 74 (68.5) | 1,406 (72.0) | 0.425 |
| CPB timeA (min) | 137±75 | 191±85 | 152±59 | 0.001 |
| Bleeding volume (mL) | 222±208 | 302±348 | 217±196 | 0.014 |
| Ventilator time (min) | 947±629 | 1,291±874 | 928±608 | <0.001 |
| Progression of mobilization | ||||
| Sitting start (days) | 1.2±0.6 | 1.6±1.3 | 1.2±0.5 | <0.001 |
| Standing start (days) | 1.3±0.7 | 1.9±1.6 | 1.2±0.6 | <0.001 |
| Ambulation start (days) | 2.1±1.9 | 4.7±4.9 | 2.0±1.5 | <0.001 |
| Length of ICU stay (days) | 1.4±1.4 | 2.9±3.7 | 1.3±1.1 | <0.001 |
| Reintubation | 15 (0.7) | 4 (3.7) | 11 (0.5) | <0.001 |
| CRRT | 85 (4.1) | 17 (15.7) | 68 (3.4) | <0.001 |
| Delirium | 167 (8.1) | 26 (24.0) | 141 (7.2) | <0.001 |
| Return to home | 1,989 (96.6) | 67 (62.0) | 1,922 (98.5) | <0.001 |
| Length of in-hospital stay (days) | 13.2±9.3 | 24.1±23.1 | 12.6±7.5 | <0.001 |
| BI score | 99.1±5.3 | 83.9±17.5 | 100.0±0.0 | <0.001 |
Unless indicated otherwise, data are presented as the mean±SD or n (%). APatients who did not undergo CPB were excluded. The significance of differences between the hospitalization-associated disability (HAD) and non-HAD groups was analyzed using t-tests or Chi-squared tests. BI, Barthel Index; CABG, coronary artery bypass grafting; CPB, cardiopulmonary bypass; CRRT, continuous renal replacement therapy; ICU, intensive care unit.
| No. patients (%) | |
|---|---|
| Feeding | 6 (5.5) |
| Transfer | 20 (18.5) |
| Grooming | 13 (12.0) |
| Toilet use | 15 (13.9) |
| Bathing | 58 (53.7) |
| Ambulation | 36 (33.3) |
| Stair climbing | 100 (92.6) |
| Dressing | 16 (14.8) |
| Defecation management | 6 (5.5) |
| Urination management | 7 (6.4) |
Table 1 presents a comparison of the preoperative clinical characteristics between HAD and non-HAD groups. The HAD group was significantly older, with a higher NYHA functional classification and EuroSCORE II, and a higher prevalence of diabetes, renal dysfunction, and cerebral vascular accident than the non-HAD group (all P<0.05). The HAD group also had significantly lower hemoglobin, serum albumin concentrations, %VC, SPPB scores, and HDS-R scores than the non-HAD group (all P<0.05).
Comparisons of Peri- and Postoperative Clinical Characteristics Between HAD and Non-HAD PatientsA comparison of the peri- and postoperative clinical characteristics between HAD and non-HAD groups is shown in Table 2. The HAD group had significantly longer operation, CPB, and ventilator times and a significantly higher bleeding volume than the non-HAD group (all P<0.05). There were significant delays in sitting, standing, and ambulatory start day in the HAD group, and the length of postoperative ICU and postoperative in-hospital stays were significantly longer in the HAD than non-HAD group (P<0.05).
Important Factors Associated With HADFigure 2 illustrates one of the feature selection results using the Boruta algorithm from the 20 multiple imputation datasets. From the 20 datasets, 12 variables were identified as important features: age, serum albumin concentration, eGFR, HDS-R score, NT-proBNP concentration, %VC, preoperative SPPB score, operation time, CPB time, ventilator time, length of postoperative ICU stay, and postoperative ambulation start day.
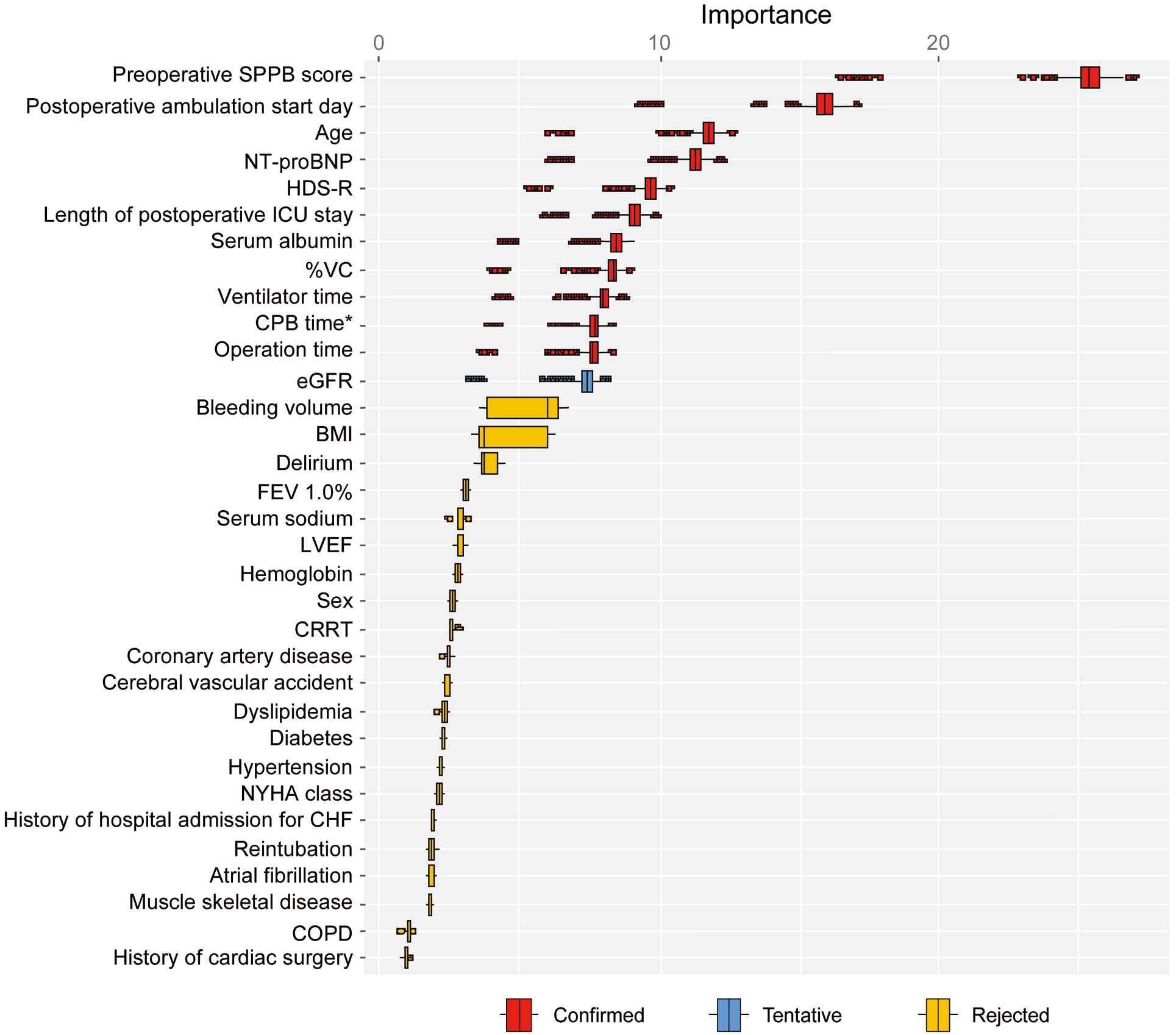
Feature selection using the Boruta algorithm. Box plots depicting the importance of confounding variables on hospitalization-associated disability (HAD). “Confirmed” variables are those that were found to contribute significantly to HAD. *The cardiopulmonary bypass (CPB) time was considered to be 0 for those who did not undergo CPB. BI, Barthel Index; BMI, body mass index; CABG, coronary artery bypass grafting; CHF, chronic heart failure; COPD, chronic obstructive pulmonary disease; CRRT, continuous renal replacement therapy; CVA, cerebral vascular accident; eGFR, estimated glomerular filtration rate; FEV1.0%, forced expiratory volume % in one second; HAD, hospitalization-associated disability; HDS-R, Revised Hasegawa’s Dementia Scale; ICU, intensive care unit; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; SPPB, Short Physical Performance Battery; VC, vital capacity.
The variables identified in the previous step were included in the Ctree analysis. This decision tree analysis identified preoperative SPPB score as the risk factor with the strongest association with HAD (Figure 3). The next strongest predictor was postoperative ambulation start day, followed by age. The highest incidence of HAD was found in those with an SPPB score ≤9 points and in those who started ambulation >6 days after surgery (76.9%). In the samples with a high preoperative SPPB score, the incidence of HAD tended to increase as the start of ambulation was delayed. The lowest-risk group consisted of patients with a preoperative SPPB score >9 points, who started postoperative ambulation in ≤3 days, and those aged ≤73 years (0.4%).
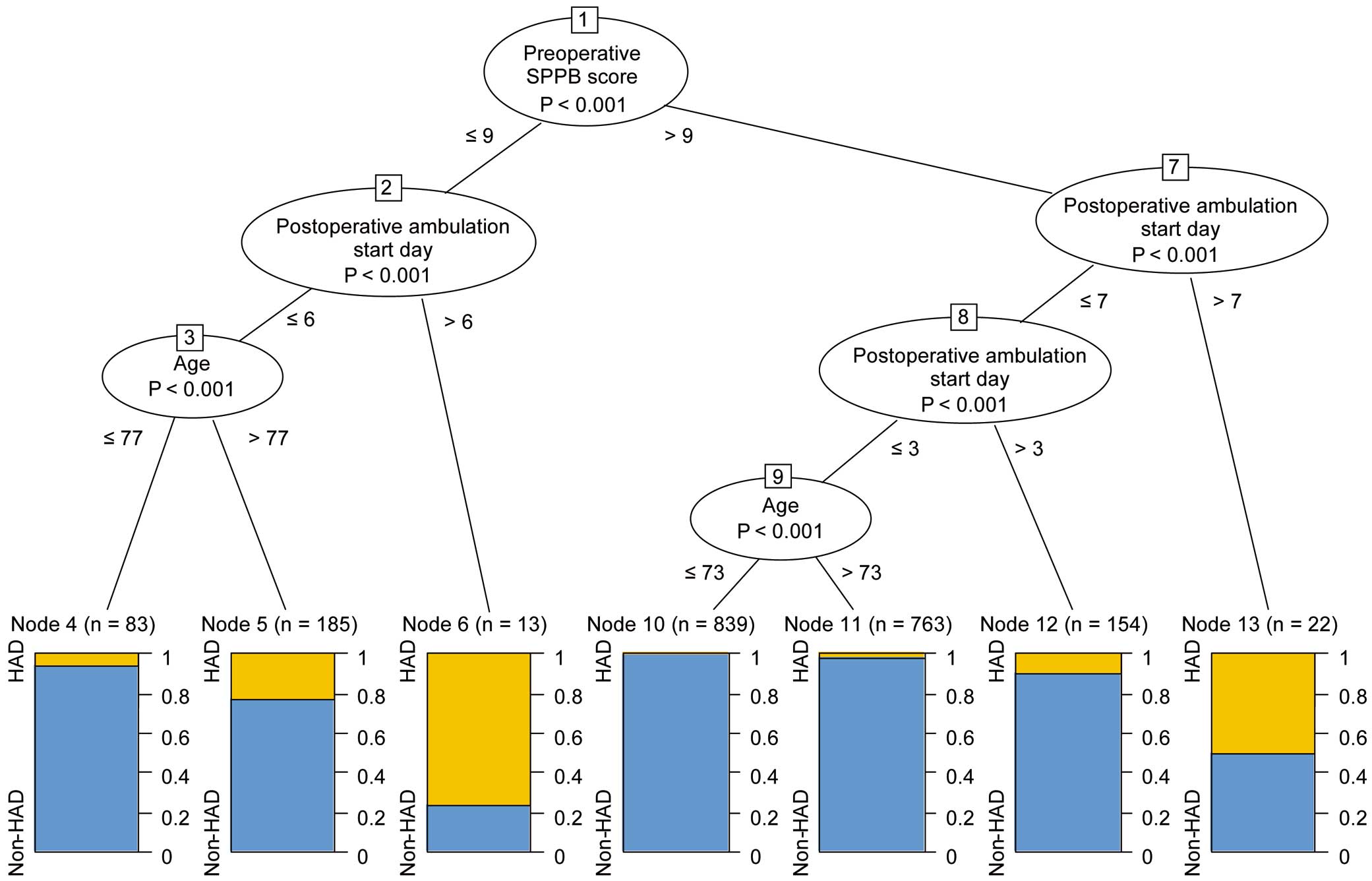
Exploration of the hospitalization-associated disability (HAD) risk profiles using the conditional inference tree. The boxes represent binary splits at the cut-off point (corresponding levels are specified) that maximized the discrepancy in the risk of the 2 subsamples of HAD. SPPB, Short Physical Performance Battery.
Since Convinsky et al described HAD in their study in 2011,2 epidemiological studies have been conducted to identify the incidence, risk factors, and associated outcomes of HAD. However, information related to HAD is limited in Japan, especially in the context of cardiac surgery. This study’s outstanding contribution is that it provides the incidence of HAD and its risk factors, which did not previously exist in the literature in the context of cardiac surgery in Japan. We included a relatively large number of patients and used a machine learning algorithm to generate robust evidence by overcoming statistical limitations.
HAD Rate and Patients’ Clinical CharacteristicsA meta-analysis of 15 studies reported that approximately 30% of patients developed HAD.19 Jonckers et al also investigated the incidence of HAD in 77 patients who were hospitalized for valvular disease, reporting that 40 (51.9%) developed HAD and that 36 of these patients had received surgical treatment.20 The incidence of HAD observed in the present study was lower than in previous studies. This is likely because we excluded patients whose preoperative BI was <100 points or those who were transferred to another hospital with some other disease, which implies that our study included many patients with a relatively low probability of developing HAD. Further, some studies included in the aforementioned meta-analysis19 used scales such as the Katz Index of Independence in ADL to evaluate HAD. Differences in the evaluation scales used and the definitions of HAD may be one reason why the incidence of HAD in the present study differed from that in previous reports.
Compared with the non-HAD group, the HAD group in the present study was older, had a higher proportion of females, had a higher prevalence of comorbidities, and had significantly lower physical and cognitive function. These are characteristic symptoms of frailty, suggesting that frailty was present in many patients in the HAD group. Regarding the characteristics during the peri- and postoperative periods, a longer operation time, CPB time, ventilator time, time until mobilization, and ICU stay were observed in the HAD group. It was also observed that the HAD group had a higher incidence of postoperative complications, which likely resulted in prolonged perioperative management and delayed postoperative rehabilitation compared with the non-HAD group.
Items Contributing to a Decline in BIThe items most affecting the reduction in the BI included stair climbing, bathing, ambulation, transfer, and dressing. These items involve a relatively high level of exercise intensity, transferring, and mobility, and require exercise tolerance and balance. Accordingly, a reduction in exercise tolerance or physical functions after cardiac surgery is thought to be associated with a decline in ADL.
Factors Associated With HADOf the HAD-related factors extracted by the RF algorithm, age, serum albumin concentration, eGFR, NT-proBNP concentration, preoperative SPPB score, %VC, and HDS-R were preoperative factors found to be associated with HAD, and are characteristics associated with frailty as discussed above. Frailty is defined as a state of being more vulnerable to stress due to reduced physiological reserves.21 Studies on post-cardiac surgery outcomes have shown that frailty inhibits the progress of postoperative rehabilitation and is associated with longer postoperative in-hospital stays and increased mortality,22–25 which supports the results of the present study. The NT-proBNP concentration reflects the severity of heart failure but is affected by eGFR. Reportedly, eGFR is a defining factor in postoperative declines in mobility,26,27 and may have affected the results of the present study. Several preoperative factors, such as hypoalbuminemia, low physical function, and low lung capacity, are components of frailty, which is a reversible condition with appropriate interventions. This suggests that interventions to reduce the risk of HAD, such as preoperative rehabilitation, are feasible. There is substantial evidence that preoperative rehabilitation may improve outcomes after cardiac surgery.28
The peri- and postoperative factors extracted in our analysis were longer operation time, CPB time, ventilator time, postoperative ICU stay, and postoperative ambulation start day. Studies have reported that delays in the start of ambulation or rehabilitation were associated with prolonged operation, ventilator, and CPB times.29,30 Because HAD is seen as the result of physical inactivity in hospital,2 these factors may have indirectly led to HAD by delaying the start of postoperative ambulation and consequently reducing the amount of physical activity while in hospital. Further, the use of CPB causes a systemic inflammatory response after surgery,31 and increased inflammatory cytokines and protein catabolism are associated with postoperative muscle weakness.32 We surmise that these reactions may also be associated with HAD after cardiac surgery. However, more prospective studies are required to elucidate the relationships between these factors and the incidence of HAD.
In the decision tree analysis, we found that the SPPB, a simple method of evaluating physical functions in the elderly,12 was the most influential factor in predicting HAD. In previous research, the total SPPB score has been found to be a factor predicting negative outcomes after cardiac surgery,33 and studies on the association between SPPB scores and negative outcomes often use an SPPB score of 9–10 points as the cut-off value.34,35 As mentioned above, patients with HAD in the present study were often frail. One study found that an SPPB score of 9 points was the cut-off value for differentiating frailty,36 which also supports the present results. That said, even patients with a preoperative SPPB score >9 points had a 50% probability of developing HAD if they started ambulation after surgery on Day 7 or later. Fast-track recovery programs after cardiac surgery have taken root recently. Standing and ambulation start the day after surgery, and independent ambulation in the ward 4–5 days after surgery is a common goal in rehabilitation.1 This reaffirms the importance of early mobilization and rehabilitation in preventing HAD, even for patients who maintain high levels of physical function.
Study LimitationsThe present study has certain limitations to consider. First, the study data were based on a chart review, which was inevitably incomplete. Although we used multiple imputations to generate conservative evidence, some key information was missing, such as the reason for delayed ambulation, in-hospital physical activity level, and inflammatory cytokine concentrations. Second, we used the BI to define HAD, whereas other studies have used other measures, such as the Katz Index of Independence in ADL. This may have resulted in differences in the incidence of HAD; the incidence of HAD may have been higher in the present study, if the 138 patients with a BI <100 points had been included. Further, the present study was a single-center study, and thus the generalizability of the study results remains limited.
We determined the incidence of HAD after cardiac surgery and its risk factors. Several preoperative factors suggest that interventions, such as preoperative rehabilitation to reduce the risk of HAD, are feasible. Furthermore, the association between HAD and postoperative ambulation reaffirmed the importance of early mobilization and rehabilitation.
The authors thank the Sakakibara Physical Therapy and Rehabilitation Research Team (SPRINT) and all members of the Sakakibara Heart Institute.
This study was supported by a research grant from Sakakibara Heart Institute (Tokyo, Japan).
T.S. is a member of Circulation Reports’ Editorial Team. K.U. is currently employed at Syneos Health and all relevant work in this manuscript was completed during his previous employment at the Hospital for Sick Children and Laurentian University. The remaining authors have no conflicts of interest to declare.
The study protocol was approved by the Ethics Committee at Sakakibara Heart Institute (No. 17-027).
The deidentified participant data will not be shared.