Abstract
Background:
Recent studies have revealed the benefits of using colchicine, a drug with anti-inflammatory properties, in coronary artery disease (CAD). This study systematically reviewed the benefits and risks of low-dose colchicine in patients with CAD.
Methods and Results:
We searched for randomized controlled trials (RCTs) in MEDLINE, the Cochrane Central Register of Controlled Trials (CENTRAL), and Web of Science databases (March 2020). Efficacy and safety outcomes were evaluated. Estimates are expressed as risk ratios (RRs) and 95% confidence intervals (95% CIs). Heterogeneity was assessed with I2
test. Confidence in the pooled evidence was appraised using the GRADE framework. Colchicine reduced the rate of major adverse cardiovascular events (RR 0.65; 95% CI 0.49–0.86; 6 RCTs; I2=50%; 11,718 patients; GRADE, moderate confidence), acute coronary syndrome (RR 0.64; 95% CI 0.46–0.90; I2=47%; 7 RCTs; 11,955 patients; GRADE, very low confidence), stroke (RR 0.49; 95% CI 0.30–0.78; I2=0%; 6 RCTs; 11,896 patients; GRADE, moderate confidence), and cardiovascular interventions (RR 0.61; 95% CI 0.42–0.89; I2=40%; 4 RCTs; 11,284 patients; GRADE, high confidence). Colchicine did not increase the risk of adverse events, except for gastrointestinal events (RR 1.54; 95% CI 1.11–2.13; I2=72%; 9 RCTs; 12,374 patients; GRADE, very low confidence).
Conclusions:
Low-dose colchicine in patients with CAD is associated with beneficial effects on prognosis, although an increased risk of gastrointestinal events was confirmed.
Cardiovascular diseases are a major cause of morbidity and mortality worldwide.1
An important share of the burden of cardiovascular diseases relates to atherosclerosis, which is a process that involves inflammatory cells. Inflammation can accelerate and trigger complications of the atherosclerotic process.2
Therefore, it is not unexpected that old drugs targeting inflammation, such as colchicine, could be investigated for this cardiovascular indication.
Colchicine has anti-inflammatory properties3
and is widely used in the treatment of acute gout, as well as in Behçet’s disease, pericarditis, primary biliary cirrhosis, and familiar Mediterranean fever.4
Orally administered colchicine has an estimated bioavailability of 44%, reaches peak plasma concentration in 1 h, and has a predominantly hepatic elimination.5
While in the circulation, colchicine concentrates in leukocytes, where it interferes with the kinetics of the cytoskeletal microtubules by inhibiting mitosis, and so inhibiting leukocyte motility, impairing inflammation mediated by these cells.
Results regarding the benefits of low-dose colchicine in patients with coronary artery disease (CAD) are contentious.3
Therefore, the aim of this study was to systematically review randomized controlled trials (RCTs) evaluating the prognostic effects of low-dose colchicine in patients with CAD.
Methods
This systematic review followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)6
and was registered in PROSPERO (Reference CRD42020178878).
Search Methods
We first searched for relevant material in March 2020 in the MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), PsycINFO, Web of Science Core Collection, SciELO Citation Index, Korean Journal Database, and the Russian Science Citation Index databases. The search was updated in January 2021.
The search methods used are summarized in
Supplementary Data 1. In addition, we analyzed the references presented in the included studies and review articles to extract significant data.
Study Selection Criteria and Outcomes
We selected all available RCTs whose patients presented with CAD, including acute coronary syndrome (ACS) or myocardial infarction (MI) and chronic coronary syndrome or stable coronary disease, regardless of the presence of other comorbidities (including other atherosclerotic or ischemic events). These studies had to include a low-dose colchicine (≤1.5 mg/day) arm and a control group (placebo, standard treatment, or other alternative treatment). We did not exclude studies with cointerventions if they were administered to both groups of the study. In randomized cross-over trials, only the first stage was considered. We defined a minimum follow-up period of 1 month. To broadly evaluate the clinical impact of colchicine, we considered cardiovascular mortality and major adverse cardiovascular events (MACE) as the primary outcomes. Different definitions of MACE were considered. Secondary outcomes were MI, stroke, heart failure, hospitalization (all causes), cardiovascular interventions (e.g., urgent revascularization), and adverse events, namely gastrointestinal adverse events.
Data Extraction
The titles and abstracts yielded by the searches against the inclusion criteria were first screened by 2 independent reviewers (A.M.A., B.N.-G.). In the second stage, the reviewers read the full-text reports and determined whether the studies met the inclusion criteria. The reasons for the exclusion of articles were recorded at both screening stages. Disagreements were resolved by consensus or with the help of a third reviewer (D.C.). Regarding the same study, the most recent data were considered.
The reviewers (A.M.A., B.N.-G.) then extracted data from the individual studies identified for inclusion into a pre-piloted form that included information about the description of the intervention and controls, population characteristics, outcome measurements, statistical data, and results.
Data Evaluation, Synthesis, and Analysis
The risk of bias in the included studies was assessed using Cochrane risk of bias tool.7
This tool evaluates random sequence generation, allocation concealment, selective reporting, blinding of participants and personnel, blinding of outcome assessment, and incomplete outcome data. The criteria were applied by 2 independent reviewers (A.M.A., B.N.-G.) and disagreements were resolved by consensus or with the help of another 2 reviewers (M.A., D.C.).
A meta-analysis of the data retrieved from the included studies was conducted using RevMan version 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark; The Cochrane Collaboration, 2014; https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download).
Statistical heterogeneity was used to define the method of analysis. Heterogeneity was determined through I2
statistics and deemed to be substantial if greater than 50%. This index reflects the percentage of total variation among studies that is due to heterogeneity rather than random. The DerSimonian and Laird random effects model was used. Publication bias was assessed through examinations of funnel plots if more than 10 studies were included.
Assessment of Confidence in the Cumulative Evidence
The evaluation of primary outcomes was performed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework regarding the risk of bias, imprecision, inconsistency, indirectness, and publication bias.8
The pooled evidence was then classified as having very low, low, moderate, or high confidence.
Results
Included Studies
The search yielded 314 articles. From those, 194 were excluded after screening the titles and abstracts. From the remaining 118 articles subjected to full-text assessment, 9 met the inclusion criteria.3,9–16
The reasons for exclusion are detailed in
Supplementary Data 2.
From the 9 RCTs included in this study (Table 1), 4 involved patients with ACS or MI10,12–14
and 5 included patients with chronic coronary syndrome or stable coronary disease.3,9,11,15,16
One study enrolled patients with acute ischemic stroke in addition to patients with ACS.10
Table 1.
Summary of the Main Characteristics of the Included Studies
| Reference |
Location |
Condition |
Main endpoint |
Total no.
participants
(no. colchicine,
control groups) |
Mean
age
(years) |
No. (%)
women |
Mean
BMI
(kg/m2) |
Smokers |
Hypertension |
Diabetes |
Hyperlipidemia |
History
of angina
or MI |
History
of PCI |
History
of CABG |
History
of heart
failure |
History
of stroke
or TIA |
Colchicine
intervention |
Control |
Follow-up
(months) |
O’Keefe
et al9 |
US |
CAD |
Angiographic
restenosis |
197 (colchicine
130, placebo 67) |
Colchicine
59.0,
placebo
62.0 |
Colchicine
19 (14.6),
placebo
9 (13.4) |
NA |
NA |
NA |
Colchicine
16 (12.0),
placebo
8 (12.0) |
NA |
Colchicine
52 (40.0),
placebo
26 (39.0) |
NA |
Colchicine
34 (26.0),
placebo
17 (25.0) |
NA |
NA |
0.6 mg, b.i.d. |
Placebo |
6 |
Raju
et al10 |
Australia |
ACS or
AIS |
Δhs-CRP |
80 (colchicine 40A,
placebo 40B) |
Colchicine
57.2,
placebo
57.2 |
Colchicine
6 (15.0),
placebo
3 (0.8) |
NA |
Colchicine
31 (77.5),
placebo
32 (80.0) |
Colchicine 19
(47.5),
placebo 15
(37.5) |
Colchicine
7 (17.5),
placebo
6 (15.0) |
Colchicine
19 (47.5),
placebo
19 (47.5) |
Colchicine
8 (20.0),
placebo
6 (15.0) |
NA |
NA |
Colchicine
1 (2.5),
placebo
0 (0.0) |
Colchicine
3 (7.5),
placebo
0 (0) |
1 mg/day |
Placebo |
1 |
Deftereos
et al3 |
NA |
CAD |
Angio-ISR and
IVUS-ISR |
222 (colchicine
112, placebo
110) |
Colchicine
63.7,
placebo
63.5 |
Colchicine
37 (37.0),
placebo
31 (32.3) |
Colchicine
27.4,
placebo
27.5 |
Colchicine
36 (36.0),
placebo
38 (40.0) |
Colchicine 48
(48),
placebo 47
(49.0) |
Colchicine
100 (100),
placebo
96 (100) |
NA |
NA |
NA |
NA |
NA |
NA |
0.5 mg, b.i.d. |
Placebo |
6 |
Nidorf
et al11 |
Australia |
CAD |
Composite
incidence of ACS,
out-of-hospital
cardiac arrest,
non-cardioembolic
ischemic stroke |
532 (colchicine
282, placebo
250) |
Colchicine
66.0,
placebo
67.0 |
Colchicine
31 (11.0),
placebo
28 (11.2) |
NA |
Colchicine
10 (4.0),
placebo
14 (6.0) |
NA |
Colchicine
92 (33.0),
placebo
69 (28.0) |
NA |
Colchicine
64 (23.0),
placebo
61 (24.0) |
Colchicine
169 (60.0),
placebo
138 (55.0) |
Colchicine
62 (22.0),
placebo
39 (16.0) |
NA |
NA |
0.5 mg/day |
No colchicine |
36 |
Akodad
et al12 |
France |
ACS |
CRP peak
value during
hospitalization |
44 (colchicine 23,
placebo 21) |
Colchicine
60.1,
placebo
59.7 |
Colchicine
4 (17.4),
placebo
5 (23.8) |
NA |
Colchicine
17 (73.9),
placebo
14 (66.7) |
Colchicine 9
(39.1),
placebo 10
(47.6) |
Colchicine
3 (13.0),
placebo
3 (14.3) |
Colchicine
8 (34.8),
placebo
8 (38.1) |
NA |
Colchicine
1 (4.3),
placebo
1 (4.8) |
Colchicine
0 (0.0),
placebo
1 (4.8) |
NA |
NA |
1 mg/day |
No colchicine |
1 |
| Hennessy13 |
Australia |
ACS |
Residual hs-CRP
≥2 mg/L |
237 (colchicine
119, placebo
118) |
Colchicine
61.0,
placebo
61.0 |
Colchicine
30 (25.2),
placebo
25 (21.2) |
Colchicine
28.0,
placebo
28.0 |
Colchicine
77 (65.0),
placebo
67 (57.0) |
Colchicine 64
(54.0),
placebo 48
(41.0) |
Colchicine
27 (23.0),
placebo
25 (21.0) |
NA |
Colchicine
18 (15.0),
placebo
18 (15.0) |
Colchicine 13 (11.0),
placebo 14 (12.0) |
NA |
NA |
0.5 mg/day |
Placebo |
1 |
Tardif
et al14 |
Canada
(multinational) |
ACS |
Composite of
death from CV
causes, resuscitated
cardiac arrest, ACS,
stroke, or urgent
hospitalization for
angina leading to
coronary revascularization |
4,745 (colchicine
2,366, placebo
2,379) |
Colchicine
60.6,
placebo
60.5 |
Colchicine
472 (19.9),
placebo
437 (18.4) |
Colchicine
28.2,
placebo
28.4 |
Colchicine
708 (29.9),
placebo
708 (29.8) |
Colchicine
1,185 (50.1),
placebo
1,236 (52.0) |
Colchicine
462 (19.5),
placebo
497 (20.9) |
NA |
Colchicine
370 (15.6),
placebo
397 (16.7) |
Colchicine
392 (16.6),
placebo
406 (17.1) |
Colchicine
69 (2.9),
placebo
81 (3.4) |
Colchicine
48 (2.0),
placebo
42 (1.8) |
Colchicine
55 (2.3),
placebo
67 (2.8) |
0.5 mg/day |
Placebo |
22.6 |
Nidorf
et al15 |
Australia and
Netherlands |
CAD |
Composite of CV
death, ACS,
ischemic stroke, or
ischemia-driven
coronary
revascularization |
5,522 (colchicine
2,762, placebo
2,760) |
Colchicine
65.8,
placebo
65.9 |
Colchicine
457 (16.5),
placebo
389 (14.1) |
NA |
Colchicine
318 (11.5),
placebo
330 (12.0) |
Colchicine
1,421 (51.4),
placebo
1,387 (50.3) |
Colchicine
632 (22.9),
placebo
662 (24.0) |
NA |
Colchicine
2,323
(84.1),
placebo
2,335 (84.6) |
Colchicine
2,100
(76.0),
placebo
2,077 (75.3) |
Colchicine
319
(11.5),
placebo
391 (14.2) |
NA |
NA |
0.5 mg/day |
Placebo |
28.6 |
Tong
et al16 |
Australia |
CAD |
Composite of all-
cause mortality,
ACS, ischemia-
driven urgent
revascularization, and non-
cardioembolic
ischemic stroke |
795 (colchicine
396, placebo
399) |
Colchicine
59.7,
placebo
60.0 |
Colchicine
74 (18.7),
placebo
89 (22.3) |
NA |
Colchicine
128 (32),
placebo
149 (37) |
Colchicine
201 (51),
placebo 199
(50) |
Colchicine
75 (19.0),
placebo
76 (19.0) |
Colchicine
180 (46.0),
placebo
185 (46.0) |
Colchicine
59 (15.0),
placebo
59 (15.0) |
Colchicine
51 (13.0),
placebo
50 (13.0) |
Colchicine
15 (4.0),
placebo
19 (5.0) |
NA |
Colchicine
5 (1.0),
placebo
11 (3.0) |
0.5 mg, b.i.d.
for 1 month,
then 0.5 mg/
day for 11
months |
Placebo |
12 |
Unless indicated otherwise, data show the number of patients with percentages in parentheses. A35 patients with ACS and 5 patients with AIS. B38 patients with ACS and 2 patients with AIS. ACS, acute coronary syndrome; AIS, acute ischemic stroke; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CRP, C-reactive protein; CV, cardiovascular; hs-CRP, high-sensitivity C-reactive protein; ISR, in-stent restenosis; IVUS, intravascular ultrasound; NA, not applicable; PCI, percutaneous coronary intervention; TIA, transient ischemic attack.
The eligible studies included 12,374 patients, 5,894 with ACS, 6,473 with chronic coronary syndrome, and 7 with acute ischemic stroke. The median age of the participants ranged between 57.2 and 67.0 years. All eligible studies enrolled a total of 1,130 (9.1%) women in the colchicine group and 1,016 (8.2%) in the placebo/no colchicine group. In all studies, patients were followed-up for a median period of 12.7 months. Seven studies compared colchicine treatment with placebo3,9,10,13–16
and 2 studies compared colchicine with standard treatment alone.11,12
A summary of the main characteristics of the studies, including cardiovascular risk factors, is presented in
Table 1.
Risk of Bias
Regarding the risk of bias, the Colchicine Cardiovascular Outcomes Trial (COLCOT),14
Colchicine in Patients with Acute Coronary Syndrome (COPS),16
and Low Dose Colchicine for Secondary Prevention of Cardiovascular Disease 2 (LoDoCo2)15
trials were classified as having a low risk of bias in all domains, whereas the LoDoCo-MI13
trial and the trial by O’Keefe et al9
revealed some concerns, especially due to a lack of information about the randomization and blinding process. Four trials exhibited a high overall risk of bias.3,10–12
Detailed information about the risk of bias is provided in
Supplementary Data 3.
Primary Outcomes: Cardiovascular Mortality and MACE
Pooled data (Figure 1) showed no difference in cardiovascular mortality (risk ratio [RR] 0.79; 95% confidence interval [CI] 0.53–1.18; 6 RCTs; I2=0%; 12,016 patients), but low-dose colchicine was associated with a significant risk reduction in MACE (RR 0.65; 95% CI 0.49–0.86; 6 RCTs; I2=50%; 11,718 patients).
Secondary Outcomes
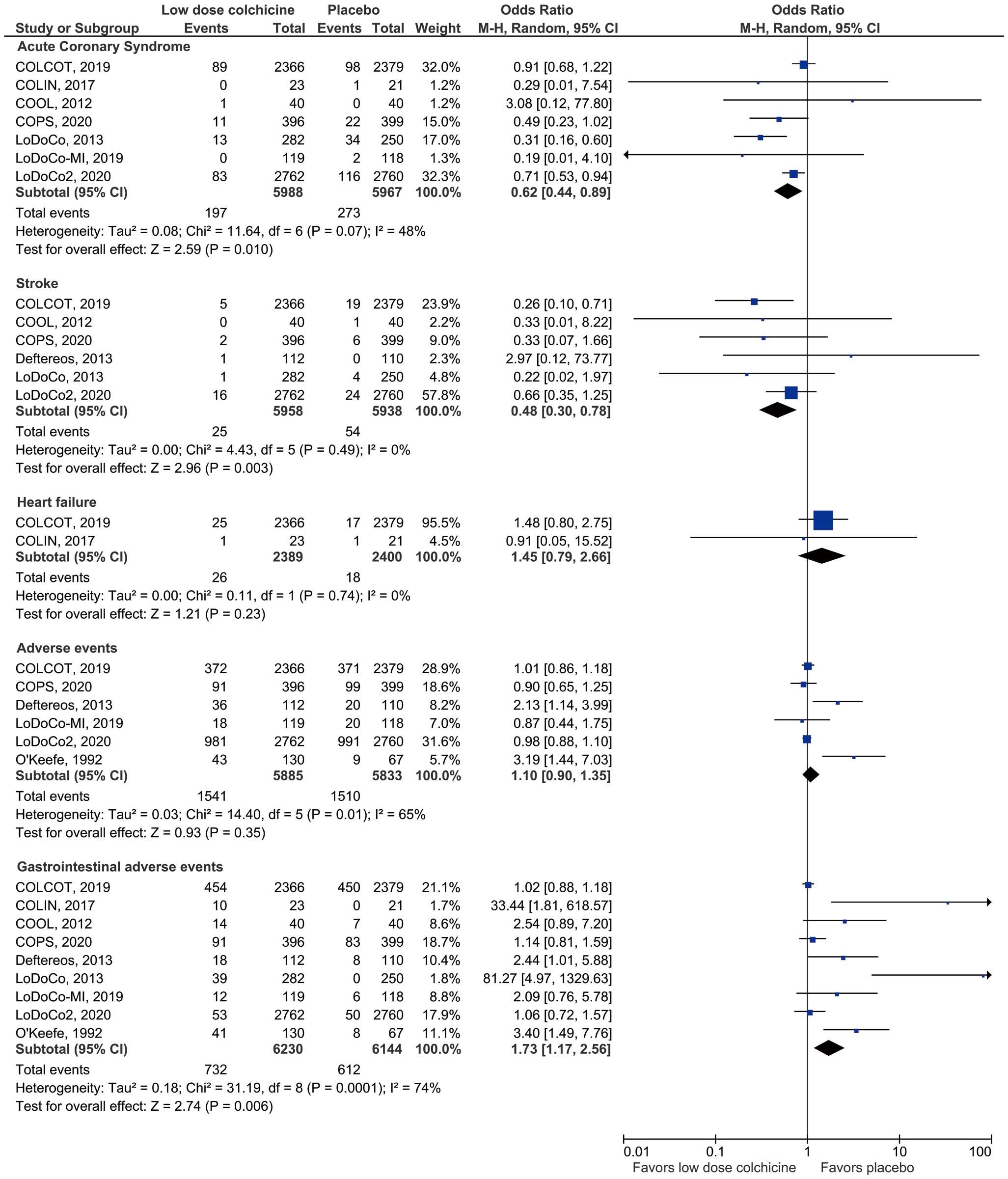
Colchicine administration was associated with a 36% risk reduction in ACS (RR 0.64; 95% CI 0.46–0.90; I2=47%; 7 RCTs; 11,955 patients) and a 51% risk reduction in stroke events (RR 0.49; 95% CI 0.30–0.78; I2=0%; 6 RCTs; 11.896 patients;
Figure 2). The LoDoCo215
and COLCOT14
trials were the major contributors to these outcomes.
Heart failure events (RR 1.44; 95% CI 0.79–2.63; I2=0%; 2 RCTs; 4,789 patients;
Figure 2) and hospitalizations (RR 0.76; 95% CI 0.53–1.10; I2=52%; 4 RCTs; 11,299 patients) did not differ among the groups (Supplementary Data 4).
Urgent coronary revascularizations were also significantly reduced by colchicine administration (RR 0.61; 95% CI 0.42–0.89; I2=40%; 4 RCTs; 11,284 patients;
Supplementary Data 4).
Overall adverse events (RR 1.07; 95% CI 0.92–1.25; I2=62%; 6 RCTs; 11,718 patients) did not differ between the 2 arms, but colchicine significantly increased gastrointestinal events (RR 1.54; 95% CI 1.11–2.13; I2=72%; 9 RCTs; 12,374 patients;
Figure 2).
Subgroup Analysis
Subgroup analysis with different follow-up periods was performed (Supplementary Data 5). There were no differences in the main outcomes between the 2 groups. There was a significant difference in the rate of hospitalization with a shorter follow-up period, but only 1 study was included in this subgroup analysis. The rate of gastrointestinal adverse events also differed between subgroups: studies with a longer follow-up period had a lower RR than those with a shorter follow-up period, but heterogeneity was high (I2=71%).
Assessment of Confidence in the Cumulative Evidence
Table 2
presents a summary of the findings regarding the certainty of the evidence (GRADE). Although most outcomes exhibited very low certainty, cardiovascular mortality, MACE, and stroke outcomes were of moderate certainty and cardiovascular intervention outcomes had high certainty.
Table 2.
Summary of Findings Using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Approach
| Outcome |
No.
participants
(studies) |
RR
(95% CI) |
Anticipated absolute effects (95% CI) |
Certainty |
What happens |
Without
colchicine
(%) |
With
colchicine
(%) |
DifferenceA
(%) |
| CV mortality |
12,016
(6 RCTs) |
0.79
(0.53, 1.18) |
1.0 |
0.8
(0.5, 1.1) |
−0.2
(−0.5, 0.2) |
   ○ModerateB ○ModerateB |
Colchicine likely reduces
CV mortality slightly |
| MACE |
111,718
(6 RCTs) |
0.65
(0.49, 0.86) |
6.3 |
4.1
(3.1, 5.4) |
−2.2
(−3.2, −0.9) |
   ○ModerateC ○ModerateC |
Colchicine likely reduces
MACE |
| ACS |
11,955
(7 RCTs) |
0.64
(0.46, 0.90) |
4.6 |
2.9
(2.1, 4.1) |
−1.6
(−2.5, −0.5) |
 ○○○Very lowC,D ○○○Very lowC,D |
Colchicine may reduce
ACS, but the evidence is
very uncertain |
| Stroke |
11,896
(6 RCTs) |
0.49
(0.30, 0.78) |
0.9 |
0.4
(0.3, 0.7) |
−0.5
(−0.6, −0.2) |
   ○ModerateE ○ModerateE |
Colchicine likely results in
a reduction in stroke |
| Heart failure |
4,789
(2 RCTs) |
1.44
(0.79, 2.63) |
0.8 |
1.1
(0.6, 2) |
0.3
(−0.2, 1.2) |
 ○○○Very lowB,F,G ○○○Very lowB,F,G |
Colchicine may have little
to no effect on heart
failure, but the evidence
is very uncertain |
| Hospitalizations |
11,299
(4 RCTs) |
0.76
(0.53, 1.10) |
5.9 |
4.4
(3.1, 6.4) |
−1.4
(−2.8, 0.6) |
 ○○○Very lowB,C ○○○Very lowB,C |
Colchicine may reduce or
have little to no effect on
hospitalizations, but the
evidence is very uncertain |
| CV interventions |
11,284
(4 RCTs) |
0.61
(0.42, 0.89) |
4.3 |
2.6
(1.8, 3.8) |
−1.7
(−2.5, −0.5) |
    High High |
Colchicine results in large
reductions in CV
interventions |
| AEs |
11,718
(6 RCTs) |
1.07
(0.92, 1.25) |
25.9 |
27.7
(23.8, 32.4) |
1.8
(−2.1, 6.5) |
 ○○○Very lowB,C,H ○○○Very lowB,C,H |
Colchicine may increase
or have little to no effect
on AEs, but the evidence
is very uncertain |
| GI events |
12,374
(9 RCTs) |
1.54
(1.11, 2.13) |
10.0 |
15.3
(11.1, 21.2) |
5.4
(1.1, 11.3) |
 ○○○Very lowC,I ○○○Very lowC,I |
Colchicine may increase or
have little to no effect on GI
events, but the evidence is
very uncertain |
Discussion
This systematic review showed that low-dose colchicine in patients with CAD is associated with a 35% risk reduction of MACE, a 36% risk reduction of ACS, a 51% risk reduction of stroke, and a 39% risk reduction of cardiovascular interventions. However, there was no significant risk reduction in cardiovascular mortality, heart failure events, or hospitalizations. As expected, the risk of gastrointestinal events was increased with colchicine, but overall adverse events were not different among the groups.
Colchicine has a well-documented therapeutic use in inflammatory conditions, and so a potential benefit of colchicine in patients with coronary and cerebrovascular disease has been previously hypothesized. Colchicine is a classical antimitotic drug, the primary mechanism of action of which is tubulin disruption. This leads to downregulation of multiple inflammatory pathways and modulates innate immunity. Newly described mechanisms of action of colchicine include blocking the activity of the NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) inflammasome, with suppression of interleukin (IL)-1β and IL-18 release.17,18
NLR3 inflammasomes are activated by cholesterol crystals that form early in disease, leading to the secretion of active proinflammatory Type 1 cytokines IL-1β and IL-18. Both IL-1β and IL-18 have been associated with plaque formation, progression, and rupture.18
In fact, blockade of the IL-1β pathway was recently shown in the Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS) study to effectively reduce cardiovascular events.19
That randomized double blind placebo-controlled trial included more than 10,000 patients with a history of MI and a persistent proinflammatory response, defined as a high-sensitivity C-reactive protein concentration of ≥2 mg/L, and showed that treatment with canakinumab, a monoclonal antibody targeting IL-1β, led to a 15% lower risk of cardiovascular events, independent of decreases in lipid levels.19
One of the major findings of the present study was an important reduction in the risk of stroke with colchicine use. One possible reason for this is related to the possible prevention of atrial fibrillation and the consequent reduction in cardioembolic stroke, as observed in studies using colchicine in patients undergoing cardiac surgery.20
Despite the fact that we could not discriminated between stroke subtypes using the available data, it seems reasonable to assume that the reduction in stroke risk may include not only cardioembolic stroke, but also atherosclerotic stroke.
The putative benefits of colchicine have been not only in RCTs, but also in earlier observational studies enrolling patients with gout, which indicated that colchicine use was associated with a reduced risk of cardiovascular events.21
Together, both RCTs (pooled in our systematic review) and observational data support a beneficial effect of colchicine use in patients with CAD. Furthermore this seems to be a cost-effective intervention in patients who experience an ACS.22
In clinical practice, gastrointestinal adverse events are expected to occur with the use of colchicine, mainly diarrhea, nausea, and vomiting, all of which are generally mild in severity.
Study Limitations
There are some study limitations to consider regarding the results and conclusions of the analysis. All trials have their own limitations, and pooling them together using their meta-data limits the robustness of data interpretation. Across studies, because of the allowed broad spectrum of cardiovascular diseases, the baseline conditions could differ. In addition, a study was included in which there was a negligible proportion of patients with acute ischemic stroke,10
although we are confident that this did not interfere with the main results. Most studies enrolled a modest number of participants, and some of the outcomes were only reported by few studies, making it difficult to identify clinical differences for each outcome. In addition, women constituted a minor proportion of the population included, probably due to a higher percentage of CAD in men. However, this unequal sex representation limits data extrapolation to women.
Another study limitation concerns the different definitions used for MACE, heart failure, and hospitalizations, which could limit the interpretation of the results and possible assumptions (Supplementary Data 6).
Conclusions
The use of low-dose colchicine in patients with CAD seems to be associated with a significant risk reduction in MACE, ACS, stroke, and cardiovascular interventions. However, there is an increased risk of gastrointestinal events, which should be expected in treated patients.
Authors Contributions
A.M.A. and D.C. created the concept and wrote the first draft of the manuscript. A.M.A., B.N.-G. and D.C. were responsible for data acquisition and extraction. All the authors contributed to data analysis, interpretation, draft revision and approved the final version of the submitted manuscript. D.C. is the guarantor.
Sources of Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
M.A. has participated in conferences with Boehringer-Ingelheim, AstraZeneca, Bayer, Bristol Myers Squibb Grünenthal, Tecnimede, and Merck Sharp & Dohme. D.B. reports personal fees from lectures or participation in advisory boards from Amgen, AstraZeneca, Boehringer-Ingelheim, Novartis, Pfizer, Roche Diagnostics, Servier and Vifor Pharma, all outside the submitted work. F.J.P. has received consultant and speaker fees from AstraZeneca, Bayer, BMS, Boehringer-Ingelheim, and Daiichi Sankyo. D.C. has participated in educational meetings and/or attended conferences or symposia (including travel, accommodation) with Daiichi Sankyo, Menarini, Merck Serono and Roche in the past 3 years.
IRB Information
This study was granted an exemption from requiring ethics approval from an ethics committee because the study was a systematic review with meta-analysis.
Supplementary Files
Please find supplementary file(s);
http://dx.doi.org/10.1253/circrep.CR-21-0065
References
- 1.
Cui H, Miao S, Esworthy T, Zhou X, Lee S, Liu C, et al. 3D bioprinting for cardiovascular regeneration and pharmacology. Adv Drug Deliv Rev 2018; 132: 252–269.
- 2.
Costa J, Alarcão J, Araujo F, Ascenção R, Caldeira D, Fiorentino F, et al. The burden of atherosclerosis in Portugal. Eur Heart J Qual Care Clin Outcomes 2021; 7: 154–162.
- 3.
Deftereos S, Giannopoulos G, Raisakis K, Kossyvakis C, Kaoukis A, Panagopoulou V, et al. Colchicine treatment for the prevention of bare-metal stent restenosis in diabetic patients. J Am Coll Cardiol 2013; 61: 1679–1685.
- 4.
Terkeltaub RA. Colchicine update: 2008. Semin Arthritis Rheum 2009; 38: 411–419.
- 5.
Deftereos S. Colchicine and the heart: Pushing the envelope. J Am Coll Cardiol 2013; 62: 1817–1825.
- 6.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009; 339: b2700–b2700.
- 7.
Higgins JP, Green S, editors. Cochrane handbook for systematic reviews of interventions. Chichester: John Wiley & Sons; 2008.
- 8.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ 2004; 328: 1490.
- 9.
O’Keefe JH Jr, McCallister BD, Bateman TM, Kuhnlein DL, Ligon RW, Hartzler GO. Ineffectiveness of colchicine for the prevention of restenosis after coronary angioplasty. J Am Coll Cardiol 1992; 19: 1597–1600.
- 10.
Raju NC, Yi Q, Nidorf M, Fagel ND, Hiralal R, Eikelboom JW. Effect of colchicine compared with placebo on high sensitivity C-reactive protein in patients with acute coronary syndrome or acute stroke: A pilot randomized controlled trial. J Thromb Thrombolysis 2012; 33: 88–94.
- 11.
Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol 2013; 61: 404–410.
- 12.
Akodad M, Lattuca B, Nagot N, Georgescu V, Buisson M, Cristol JP, et al. COLIN trial: Value of colchicine in the treatment of patients with acute myocardial infarction and inflammatory response. Arch Cardiovasc Dis 2017; 110: 395–402.
- 13.
Hennessy T. The Low Dose Colchicine after Myocardial Infarction (LoDoCo-MI) study: A pilot randomized placebo controlled trial of colchicine following acute myocardial infarction. Am Heart J 2019; 215: 62–69.
- 14.
Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med 2019; 381: 2497–2505.
- 15.
Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, et al. Colchicine in patients with chronic coronary disease. N Engl J Med 2020; 383: 1838–1847.
- 16.
Tong DC, Quinn S, Nasis A, Hiew C, Roberts-Thomson P, Adams H, et al. Colchicine in patients with acute coronary syndrome: The Australian COPS randomized clinical trial. Circulation 2020; 142: 1890–1900.
- 17.
Leung YY, Yao Hui LL, Kraus VB. Colchicine: Update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum 2015; 45: 341–350.
- 18.
Imazio M, Andreis A, Brucato A, Adler Y, De Ferrari GM. Colchicine for acute and chronic coronary syndromes. Heart 2020; 106: 1555–1560.
- 19.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377: 1119–1131.
- 20.
Samuel M, Tardif JC, Bouabdallaoui N, Khairy P, Dubé MP, Blondeau L, et al. Colchicine for secondary prevention of cardiovascular disease: A systematic review and meta-analysis of randomized controlled trials. Can J Cardiol 2021; 37: 776–785.
- 21.
Shah B, Toprover M, Crittenden DB, Jeurling S, Pike VC, Krasnokutsky S, et al. Colchicine use and incident coronary artery disease in male patients with gout. Can J Cardiol 2020; 36: 1722–1728.
- 22.
Samuel M, Tardif JC, Khairy P, Roubille F, Waters DD, Grégoire JC, et al. Cost-effectiveness of low-dose colchicine after myocardial infarction in the Colchicine Cardiovascular Outcomes Trial (COLCOT). Eur Heart J Qual Care Clin Outcomes, doi:10.1093/ehjqcco/qcaa045.