2021 年 3 巻 9 号 p. 504-510
2021 年 3 巻 9 号 p. 504-510
Background: Antiplatelet therapy following stent implantation in patients requiring oral anticoagulation (OAC) is controversial because triple therapy (i.e., dual antiplatelet therapy [DAPT] with OAC) is associated with a high risk of bleeding.
Methods and Results: In this study, 21 rabbits were divided into 5 groups: prasugrel and warfarin (Prasugrel+OAC group); aspirin and warfarin (Aspirin+OAC group); prasugrel, aspirin, and warfarin group (Triple group); prasugrel and aspirin (Conventional DAPT group); and no medication (Control group). The treated groups were administered medication for 1 week. An arteriovenous shunt loop was established from the rabbit carotid artery to the jugular vein and 2 bare metal stents were deployed in a silicone tube. After 1 h of circulation, the volume of thrombi was evaluated quantitatively by measuring the amount of protein. Bleeding time was measured at the same time. The volume of the thrombus (amount of protein) around stent struts was lowest in the Triple group, followed by the Prasugrel+OAC and Conventional DAPT groups, and was highest in the Control group. Bleeding time was the longest in the Triple group, followed by the Aspirin+OAC, Prasugrel+OAC, Conventional DAPT, and Control groups.
Conclusions: This study suggests that prasugrel with OAC may be a feasible antithrombotic regimen following stent implantation in patients who require OAC therapy.
Dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 receptor inhibitor has become the gold standard after percutaneous coronary intervention (PCI) to prevent stent thrombosis (ST).1 With the number of patients with atrial fibrillation (AF) increasing, it was recently reported that approximately 10% of patients who underwent PCI had AF.2 Triple therapy, consisting of DAPT plus oral anticoagulants (OAC), had been recommended to prevent both ST and cardiogenic embolism. However, recent randomized control studies (RCTs) comparing triple therapy and dual therapy with an OAC and P2Y12 receptor inhibitor have demonstrated a significant reduction in bleeding events as well as similar risk of ST.3–7 Therefore, the latest Japanese guideline recommends triple therapy during the periprocedural period (within 2 weeks after PCI) followed by dual therapy with OAC and clopidogrel (Class IC).8
The originally recommended P2Y12 receptor inhibitor after PCI was clopidogrel, with a 300-mg loading dose and a 75-mg daily maintenance dose.1 However, recent studies demonstrated that polymorphisms of cytochrome P450 family 2 subfamily C member 19 (CYP2C19), which reduces the activity of clopidogrel, are common in East Asian, including Japanese, populations.9 Conversely, prasugrel is less affected by CYP2C19 resulting in stronger antiplatelet effects compared with clopidogrel.10,11 Because East Asian, including Japanese, patients are known to have a higher bleeding risk with a low thrombotic risk than patients from other regions,9 reduced doses of prasugrel (20-mg loading dose, 3.75-mg daily maintenance dose) are approved in Japan. The dose of prasugrel used in Japan is approximately one-third of that approved for use globally. The PRASFIT-ACS trial revealed that the reduced-dose prasugrel regimen was associated with a lower rate of cardiovascular events than clopidogrel, with similar major bleeding events, in Japanese patients.12
Recently, the STOPDAPT-2 trial demonstrated a significantly lower rate of bleeding events with similar thrombotic events following 1 month of DAPT followed by clopidogrel monotherapy compared with 12 months of DAPT in Japanese patients.13 The STOPDAPT-2 trial showed that bleeding risk would be more lethal than thrombotic risk in the Japanese PCI population, suggesting that a shorter duration of combination therapy may provide benefit, especially in patients with AF who need triple therapy. The antithrombogenic effect of the Xience (Abbott Vascular, Santa Clara, CA, USA) drug-eluting stent (DES), which was shown to be greater than that of other DES in several ex vivo arteriovenous shunt models,14–18 is considered to be one of the reasons for the lower risk of ST in the STOPDAPT-2 trial. Therefore, the aim of the present study was to investigate the antithrombotic effect of dual therapy with prasugrel and OAC compared with other regimens, such as triple therapy with prasugrel, aspirin, and OAC, dual therapy with prasugrel and aspirin, and dual therapy with aspirin and OAC, in a rabbit arteriovenous shunt model.
Prasugrel hydrochloride (prasugrel; CS-747S; molecular formula C20H20FNO3S·HCl) was obtained from Ube Industries, aspirin (molecular formula C9H8O4) was purchased from Cayman Chemical, and warfarin sodium (molecular formula C19H15NaO4) and Gum Arabic (vehicle) were purchased from Wako Pure Chemical Industries.
Dosage of Antiplatelets and Anticoagulants in Healthy RabbitsTo investigate the optimal dosage of antiplatelets and anticoagulants in healthy rabbits, prasugrel (1 or 3 mg/kg), aspirin (10 or 30 mg/kg), warfarin (0.3 or 1 mg/kg), or vehicle were orally administered to male Kbl:JW rabbits (Kitayama Labes) once daily for 7 consecutive days. Blood samples were collected from the auricular artery after final dosing using 3.8% sodium citrate. Platelet aggregation in platelet-rich plasma was monitored for 8 min after the application of ADP (20 μmol/L) or collagen (6 μg/mL) and recorded as the maximum platelet aggregation using an automated platelet aggregometer (MCM HEMA TRACER 313M; MC Medical). The prothrombin time in platelet-poor plasma was analyzed using an automated coagulation analyzer (CA-7000; Sysmex).
Experimental Setup and Test GroupsA rabbit arteriovenous shunt model was established to investigate the volume of the thrombus attached around stent struts and bleeding time in the 5 treatment regimens: prasugrel+aspirin (Conventional DAPT; n=4), Prasugrel+OAC (n=4), prasugrel+OAC+aspirin (Triple group; n=4), Aspirin+OAC (n=4), and a control group (n=4). To create the shunt model, 2 bare metal stents (Multi-Link Vision, 3.0/28 mm+2.25/28 mm; Abbott Vascular, Santa Clara, CA, USA) were implanted in an overlapping manner in a custom-made silicone tube (inner diameter 3.0 mm; Shin-etsu Silicone, Tokyo, Japan; Figure 1). Specifically, a 2.25-mm stent was implanted at high pressure (22 atm), followed by implantation of a 3.0-mm stent with a nominal pressure at the distal portion of a 2.25-mm stent with the edges of stents overlapping 10 mm in the tube. After implantation of the 2 stents, both stents were dilated with a 3.0-mm balloon.

The rabbit arteriovenous shunt model, which involved overlapping stents in a silicone tube, was used to evaluate thrombogenicity after 1 h of circulating blood.
The study protocol was reviewed and approved by the Education and Research Support Center in the Department of Animal Care at Tokai University (Reference no. 141091). In the rabbit arteriovenous shunt model, rabbits (Japanese white rabbits; Tokyo Laboratory Animals Science, Tokyo, Japan) started treatment with antiplatelets and/or anticoagulants 7 days prior to the procedures.
At the time of the procedure, rabbits were anesthetized with 2.5% isoflurane via a facemask. Blood pressure and heart rate were monitored and controlled by an experienced staff member to ensure blood flow in the silicone tube was stable. Heparin was not used through the entire process. Surgical access was obtained via the carotid artery using a general sterile technique; 4-Fr vascular sheaths were inserted through the left carotid artery and a 5-Fr sheath was inserted through the jugular vein. The vascular sheaths were then connected to the silicone tube, establishing an arteriovenous carotid artery to jugular vein shunt.
The extent of platelet aggregation to the struts, especially the overlapping portion of the stents, was evaluated after exposure to circulating blood for 1 h. During the experiments, the stented silicone tube was maintained in a 37℃ water bath (Figure 2).
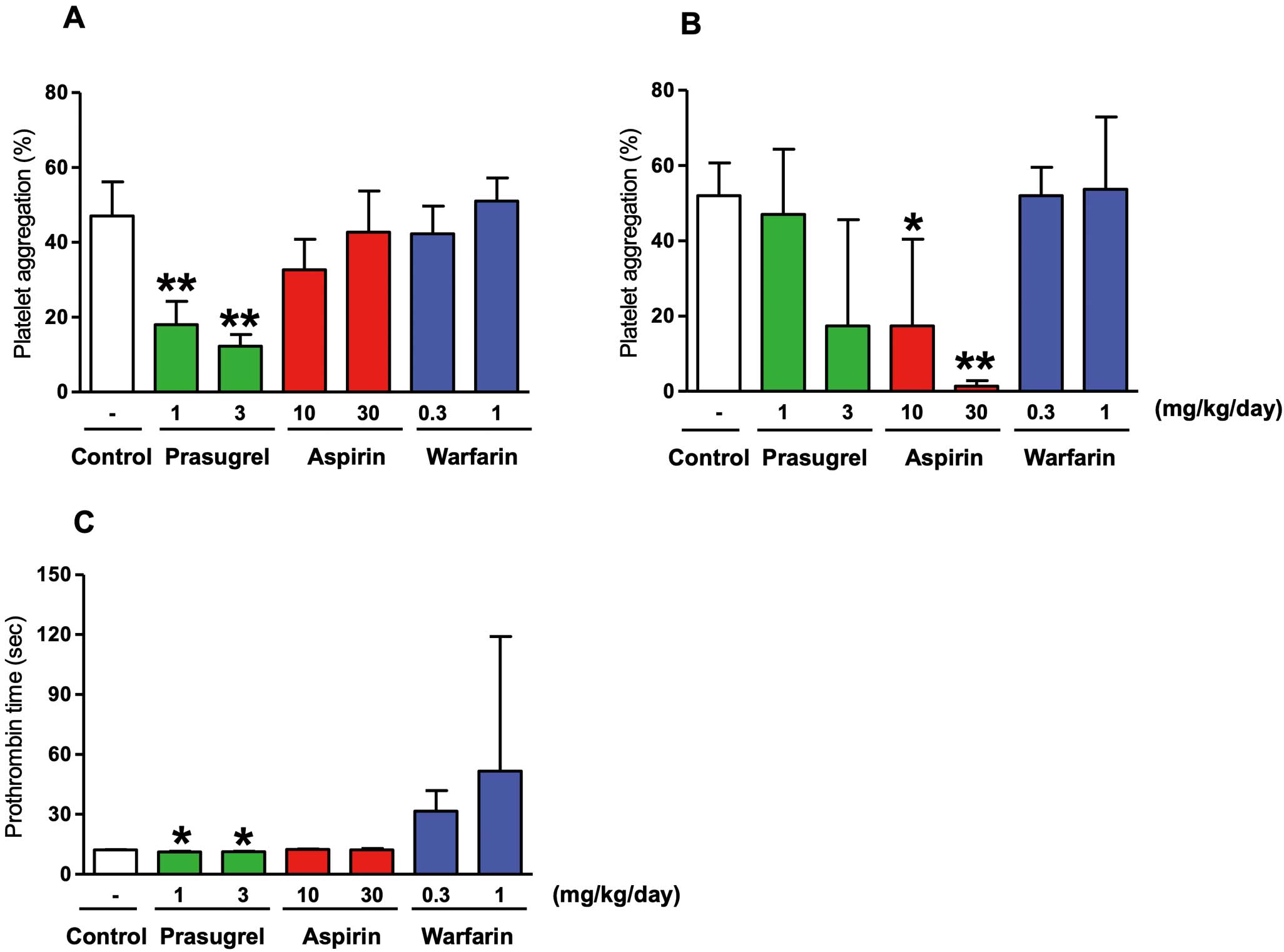
Effects of prasugrel, aspirin, and warfarin on platelet aggregation and prothrombin time in rabbits. Prasugrel (1 or 3 mg/kg), aspirin (10 or 30 mg/kg), warfarin (0.3 or 1 mg/kg), or vehicle (control) were administered orally to male Kbl:JW rabbits once daily for 7 consecutive days. (A) ADP- and (B) collagen-induced platelet aggregation of platelet-rich plasma and (C) the prothrombin time of platelet-poor plasma were evaluated. Data represent the mean±SD (n=3). *P<0.05, **P<0.01 compared with control (Dunnett’s test).
After 1 h circulation of the arteriovenous shunt, the silicone tube was gravity perfused with 50 mL saline. Then, the silicone tube was kept in a dry place for 24 h. Additional dehydration was achieved by freeze drying at −100℃ for 24 h, followed by perfusion of the tube with 200 μL of 0.1 mol/L NAOH and incubation for 30 min. The amount of protein (mainly thrombus) was then measured using a DC protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA).
Bleeding Time to Assess Platelet FunctionBleeding time was also measured to assess platelet function in each treatment group. To this end, the time from creation of an incision in the ear of each rabbit to cessation of bleeding was recorded.
Statistical AnalysisNormally distributed continuous variables are presented as the mean±SD. Variables that were not normally distributed are presented as the median and interquartile range (IQR). Dunnett’s multiple comparison test was used to compare platelet aggregation and prothrombin time among groups. Comparisons of normally distributed continuous variables were made using Student’s t-test. The non-parametric Mann-Whitney U test was used for comparisons of non-normally distributed continuous variables. Categorical variables are expressed as counts and percentages, and were compared using the Chi-squared test or Fisher’s exact test. Two-sided P<0.05 was considered statistically significant. All analyses were performed using SAS system Release 9.2 (SAS Institute) and JMP version 13.0 (SAS Institute).
The effects of prasugrel, aspirin, and warfarin on platelet aggregation and blood clotting time are summarized in Figure 2. At 1 and 3 mg·kg−1·day−1, prasugrel significantly inhibited ADP-induced platelet aggregation, with inhibition of platelet aggregation (IPA) values of 56.2±3.5% and 71.0±5.3%, respectively (Figure 2A). The dose of prasugrel used in the combination study was 1 mg/kg due to submaximal inhibition of platelet aggregation. At 10 and 30 mg·kg−1·day−1, aspirin also significantly inhibited collagen-induce platelet aggregation in a dose-dependent manner, with IPA values of 69.3±20.4% and 97.9±1.4%, respectively (Figure 2B). The dose of aspirin used in the combination study was 10 mg/kg due to submaximal inhibition of platelet aggregation. The dose of warfarin used for the combination study was 0.3 kg/kg due to optimal prolongation of prothrombin time (~3-fold prolongation; Figure 2C).
Assessment of the Volume of the Thrombus Around Stent StrutsRepresentative images of stents in silicone tubes from the 5 different antithrombotic/anticoagulant treatment regimens are shown in Figure 3. The volume of the thrombus (amount of protein) around stent struts was lowest in the Triple group, followed by the Prasugrel+OAC and conventional DAPT groups, and was highest in the Control group (median [IQR] 0.49 [0.38–1.11], 0.74 [0.46–1.34], 0.96 [0.50–1.41], 2.92 [2.14–4.24], and 3.72 [2.30–5.15] mg/mL in the Triple, Prasugrel+OAC, Conventional DAPT, Aspirin+OAC, and Control groups, respectively; Figure 4; Table 1).
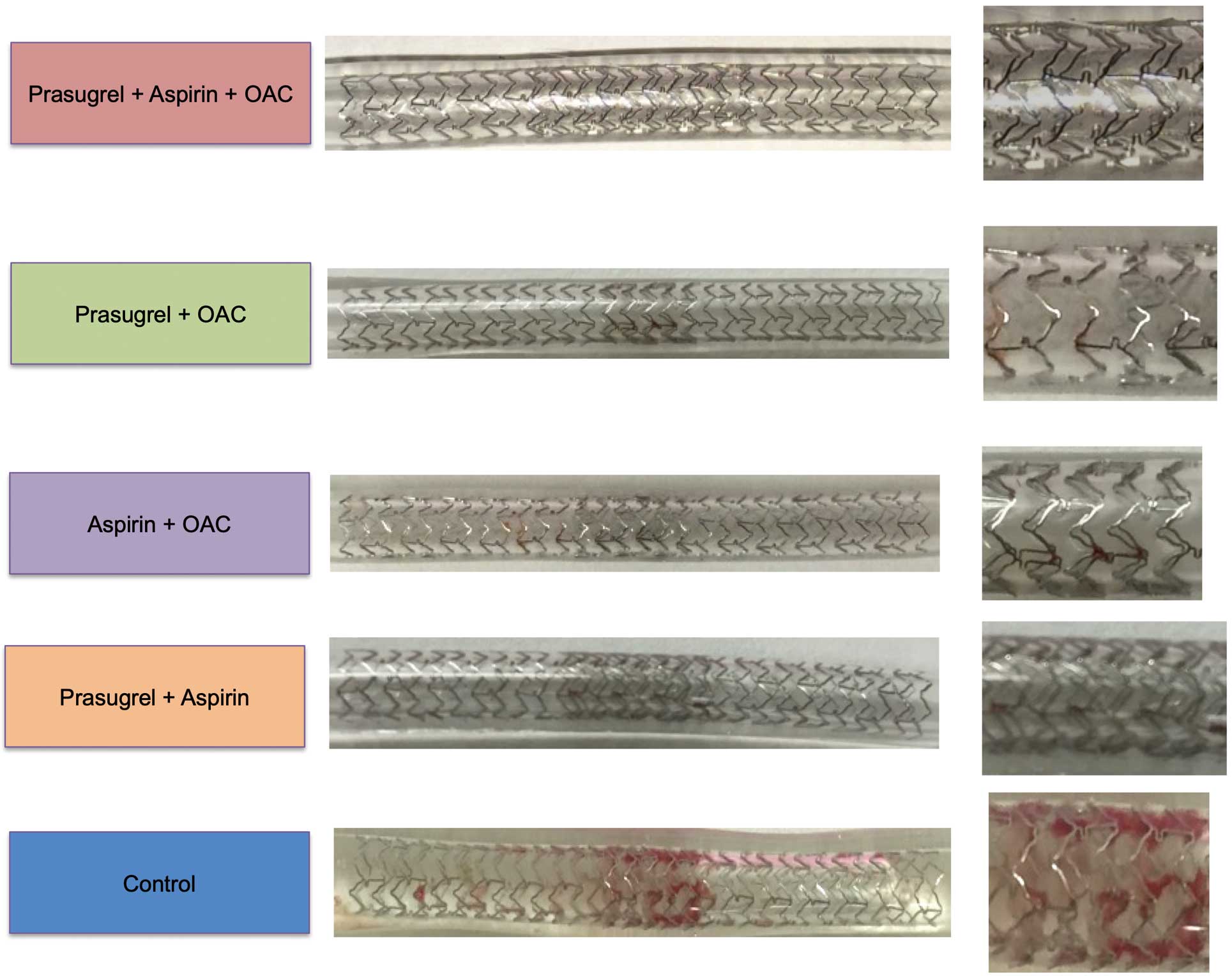
Representative gross images of stents in each of the 5 antithrombotic treatment groups. Note thrombus attachment in the overlapping portion of the stents, which is more prominent in the control group than in the other 4 groups. OAC, oral anticoagulant.

Volume of the thrombus around stent struts. The volume of the thrombus (as indicated by the amount of proteins) around stent struts was the lowest in the Triple group (warfarin [W]+aspirin [A]+prasugrel [P]), followed by the prasugrel+oral anticoagulant (W+P), and conventional dual antiplatelet therapy (A+P) groups, and was the highest in the control group (n=4 in each group). Vertical lines represent median values.
| Group 1 vs. Group 2 | Thrombus volume: Group 1 vs. Group 2 (mg/mL) |
P value |
|---|---|---|
| Control vs. Triple | 3.73 vs. 0.49 | 0.003 |
| Control vs. Prasugrel+OAC | 3.73 vs. 2.92 | 0.005 |
| Control vs. DAPT | 3.73 vs. 0.74 | 0.007 |
| Control vs. Aspirin+OAC | 3.73 vs. 0.96 | 0.9 |
| Triple vs. Prasugrel+OAC | 0.49 vs. 2.92 | 0.99 |
| Triple vs. DAPT | 0.49 vs. 0.74 | 0.99 |
| Triple vs. Aspirin+OAC | 0.49 vs. 0.96 | 0.02 |
| Prasugrel+OAC vs. DAPT | 2.92 vs. 0.74 | 0.99 |
| Prasugrel+OAC vs. Aspirin+OAC | 2.92 vs. 0.96 | 0.03 |
| DAPT vs. Aspirin+OAC | 0.74 vs. 0.96 | 0.04 |
DAPT, dual antiplatelet therapy; OAC, oral anticoagulant; Triple, treatment with prasugrel, aspirin, and warfarin.
Bleeding time was longest in Triple group, followed by the Aspirin+OAC, Prasugrel+OAC, Conventional DAPT, and Control groups (900 [495–1,365], 405 [300–533], 345 [255–480], 270 [225–270], and 210 [195–450] s, respectively; Figure 5; Table 2).
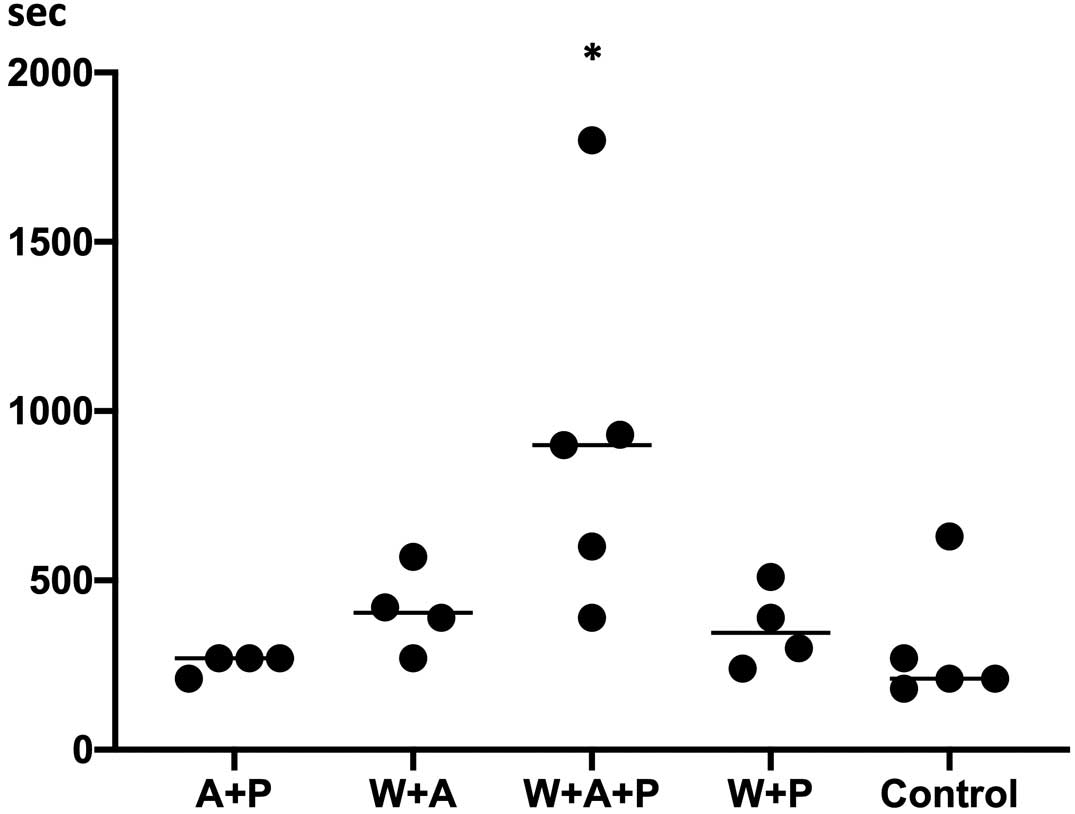
Bleeding time. Bleeding time was the longest in Triple group (warfarin [W]+aspirin [A]+prasugrel [P]) compared with the other 4 groups (n=4 in the A+P, W+A, and W+A+P groups; n=5 in the W+P and control groups). Vertical lines represent median values.
| Group 1 vs. Group 2 | Bleeding time: Group 1 vs. Group 2 (s) |
P value |
|---|---|---|
| Control vs. Triple | 240 vs. 765 | 0.08 |
| Control vs. Prasugrel+OAC | 240 vs. 345 | 0.99 |
| Control vs. DAPT | 240 vs. 270 | 0.99 |
| Control vs. Aspirin+OAC | 240 vs. 405 | 0.99 |
| Triple vs. Prasugrel+OAC | 765 vs. 345 | 0.1 |
| Triple vs. DAPT | 765 vs. 270 | 0.04 |
| Triple vs. Aspirin+OAC | 765 vs. 405 | 0.2 |
| Prasugrel+OAC vs. DAPT | 345 vs. 270 | 0.99 |
| Prasugrel+OAC vs. Aspirin+OAC | 345 vs. 405 | 0.99 |
| DAPT vs. Aspirin+OAC | 270 vs. 405 | 0.9 |
DAPT, dual antiplatelet therapy; OAC, oral anticoagulant; Triple, treatment with prasugrel, aspirin, and warfarin.
To the best of our knowledge, this study is the first preclinical study to investigate the antithrombotic effect of several combinations of antiplatelets and anticoagulants using a rabbit arteriovenous shunt model. In the study, the volume of the thrombus attached to the stent struts was similar in the Triple (prasugrel, aspirin, and OAC), Prasugrel+OAC, and Aspirin+Prasugrel groups. Conversely, bleeding time was longest in Triple group, and the difference was statistically significant compared with the Aspirin+Prasugrel and Control groups. These results suggest that Prasugrel+OAC would be a feasible antithrombotic regimen following stent implantation in patients who require OAC therapy without increasing bleeding risk.
Recently, several ex vivo arteriovenous shunt models have been used to evaluate differences in antiplatelet effects for various type of stents.14–18 Most of these studies used swine, with neither antiplatelets nor anticoagulants administered during the experiment. These models would be suitable for evaluating the antithrombotic effects of each stent, but may be not suitable for comparing the antithrombotic effects of each oral antithrombotic regimen, because the optimal dosage of antiplatelets and anticoagulants in swine has not been investigated. In the present study, the optimal dosage of antiplatelets and anticoagulants was investigated and compared with the control group. Although the results vary in the present study, primarily because of the small number of animals evaluated, there was a tendency for the thrombus volume and bleeding time to be inversely proportional, and this result is consistent with daily clinical practice. Therefore, we believe the current preclinical study is one of the best ways to compare the antithrombotic effects of each regimen.
One of the goals for antiplatelets and anticoagulants after stent implantation in patients with AF is to prevent both ST and embolization of an intracardiac thrombus.8,19 Previous RCTs have clearly shown that the prevalence of ST is significantly higher within 30 days after stent implantation. In addition, 3 factors were responsible for more than 95% of cases of acute (<24 h) and subacute (from 24 h to 30 days) ST: the persistence of uncovered struts, malapposition of struts, and underexpansion.20 All 3 findings highlight that the stent struts were bare within the lumen, and the possibility of thrombus attachment remains until all the struts are covered by neointimal tissue. Because histological and preclinical studies suggest that most of the struts would remain bare particularly within 30 days of DES implantation,15,21,22 antithrombotic effects in that period play a key roll in preventing ST.
The latest substudy of the AUGUSTUS trial demonstrated detailed characteristics of patients with ST.23 Main findings of that trial were that combination therapy with apixaban, a non-vitamin K antagonist OAC (NOACs), and a P2Y12 inhibitor resulted in significantly fewer bleeding events without significant affecting the incidence of ischemic events compared with triple therapy after stent implantation in patients with AF.3 These results are consistent with those of other RCTs evaluating other NOACs with a similar regimen.4–7 In the AUGUSTUS substudy, the incidence of ST was low, but there were a trend for a relatively high risk of ST in the dual therapy group (vitamin K antagonist [VKA] / apixaban + P2Y12 inhibitor) compared with triple therapy group (VKA / apixaban + P2Y12 inhibitor + aspirin).23 In the AUGUSTUS trial, 92.6% of patients received clopidogrel as the P2Y12 inhibitor, and prasugrel was used in only 1.2% of patients.23 The results of the AUGUSTUS trial suggest that the antithrombotic effect of clopidogrel is not sufficient, possibly due to CYP2C19 polymorphisms. Conversely, as demonstrated in the present study, the antithrombotic effect was similar between the Prasugrel+OAC and Triple groups, with significantly a significantly shorter bleeding time in the former; thus, prasugrel+OAC therapy may be a feasible regimen in AF patients who undergo PCI.
Study LimitationsThe present study has some limitations. First, the number of the antithrombotic regimens evaluated in each group was 4–5, which is not enough to enable statistical comparisons between groups. Because of the variability in the results, due primarily to the small number of animals evaluated, the results should be interpreted with caution. Second, this study was performed in a healthy rabbit ex vivo shunt model. Therefore, the results cannot be directly applied to diseased human coronary arteries. Nevertheless, to compare the antithrombotic effects of 5 regimens in a diseased human model would be too complicated because there are so many potential variables that could contribute to thrombogenicity. We believe that the simplicity of our model may be one of the best ways to compare the antithrombotic effects of each regimen for AF patients after PCI. Third, warfarin was used as an anticoagulant, which is not recommended in the current guideline for double or triple therapy with OAC and antiplatelet agents,8 but because there are no data for DOAC in a rabbit model, we decided to use warfarin instead of DOAC. Furthermore, the dosing of warfarin was optimized in a preliminary study, so the present study gives certain insights into the regimen of OAC plus antiplatelet agents. Finally, the mechanisms underlying the results of the present study have not been investigated. Further preclinical evaluation is needed to reveal the mechanisms involved.
In the present study in a rabbit arteriovenous shunt model, we demonstrated that the antithrombotic effect of prasugrel plus OAC was comparable to that of triple therapy (prasugrel+OAC+aspirin), with significantly less bleeding risk. The results suggests the feasibility of prasugrel+OAC in patients with AF after PCI.
The authors thank Masayoshi Ito and Sachie Tanaka (Education and Research Support Center, Tokai University) for their valuable technical assistance. The authors also thank Shin Nippon Biomedical Laboratories, Ltd., for their expert technical contributions.
This study was sponsored by Daiichi Sankyo (Tokyo, Japan).
S.T. has received research grants from Abbot Vascular Japan, Boston Scientific Japan, and Medtronic, and honoraria from Boston Scientific Japan. G.N. is a consultant for Boston Scientific, Abbott Vascular, Terumo Corp., Japan Medical Device Technology Co., Ltd, and ZAIKEN, and has received research grants from Boston Scientific, Abbott Vascular, Terumo Corp., and Japan Medical Device Technology Co., Ltd. Y. Ito and A.S. are employees of Daiichi Sankyo Co., Ltd.
Y. Ikari is a member of Circulation Reports’ Editorial Board.
This study was reviewed and approved by the Education and Research Support Center in the Department of Animal Care, Tokai University (Reference no. 141091).