2022 年 4 巻 1 号 p. 1-8
2022 年 4 巻 1 号 p. 1-8
Background: Despite the beneficial effects of BCR-ABL1 tyrosine kinase inhibitors (TKIs) in the treatment of chronic myeloid leukemia (CML), they may also cause adverse events (AEs), especially cardiovascular toxicity. The incidence of TKI-induced AEs may vary among ethnic groups, and there is little specific information for Japanese patients.
Methods and Results: Sixty-nine consecutive patients who were started on treatment with dasatinib (n=25) or imatinib (n=44) for CML or gastrointestinal stromal tumor (GIST) between December 2008 and December 2019 were retrospectively recruited to the study. We determined the prevalence of AEs through October 2020 and compared the incidence of AEs between the 2 drugs. Baseline characteristics were comparable between the 2 groups. However, compared with the imatinib-treated group, the dasatinib-treated group had a higher incidence of congestive heart failure (CHF; 20.0% vs. 2.3%; P=0.04), pleural effusion (48% vs. 20.5%; P=0.03), pericardial effusion (24% vs. 4.6%; P=0.02), QT prolongation (4 vs. 0 patients; P=0.02), and pulmonary hypertension (3 vs. 0 patients; P=0.04). In the dasatinib-treated group, CHF tended to be associated with tricuspid valve regurgitation pressure gradient, and pleural effusion was observed in all patients. All-cause mortality and other cardiovascular events did not differ significantly between the 2 groups.
Conclusions: Cardiotoxic AEs occurred more frequently in Japanese patients with CML and GIST treated with dasatinib than imatinib.
BCR-ABL1 tyrosine kinase inhibitors (TKIs) are the standard treatment for patients with chronic myeloid leukemia (CML) in the chronic phase. Imatinib is a first-generation BCR-ABL1 TKI with improved efficacy compared with interferon combination therapy. The International Randomized Study of Interferon and STI571 (IRIS) showed that the 5-year survival rate of patients with newly diagnosed CML increased from around 40–50% to 90% with imatinib.1 Dasatinib, a second-generation BCR-ABL1 TKI, was initially approved as salvage treatment and subsequently as first-line CML therapy based on superior 12-month complete cytogenetic response rates compared with imatinib.2 However, the international Phase 3 DASatinib versus Imatinib Study in treatment-Naïve CML patients (DASISION) revealed that pulmonary hypertension (PH), arterial ischemic events, and pleural effusion occurred more frequently with dasatinib than imatinib.3 Furthermore, in a subanalysis of the final 5-year report of DASISION, Japanese patients were found to have higher molecular responses, a higher incidence of pleural effusion, and fewer arterial ischemic events than the entire study population.4
Given that the annual incidence of CML differs between Asian countries and the US,5,6 the safety of BCR-ABL1 TKIs in the Japanese population may differ from that in other ethnic populations. However, there are few relevant reports addressing this issue. Thus, in the present study we compared the incidence of imatinib- and dasatinib-associated adverse events (AEs) in Japanese patients.
Between December 2008 and December 2019, 71 patients were started on dasatinib or imatinib treatment for CML or gastrointestinal stromal tumor (GIST) at Nagasaki University Hospital. Two patients aged <20 years were excluded from the present retrospective study, but the remaining 69 patients were enrolled. CML patients were treated with dasatinib or imatinib, and GIST patients were treated with imatinib. The treatment was at the discretion of the attending physician. Patients were divided into 2 groups, a dasatinib-treated (DAS) group (n=25; all patients with CML) and an imatinib-treated (IMA) group (n=44; 19 patients with CML, 25 patients with GIST; Figure). Six patients in the DAS group had been previously treated with imatinib. Of these 6 patients, 2 were refractory to imatinib treatment and 4 had AEs associated with imatinib, such as rash, liver dysfunction, and nausea.
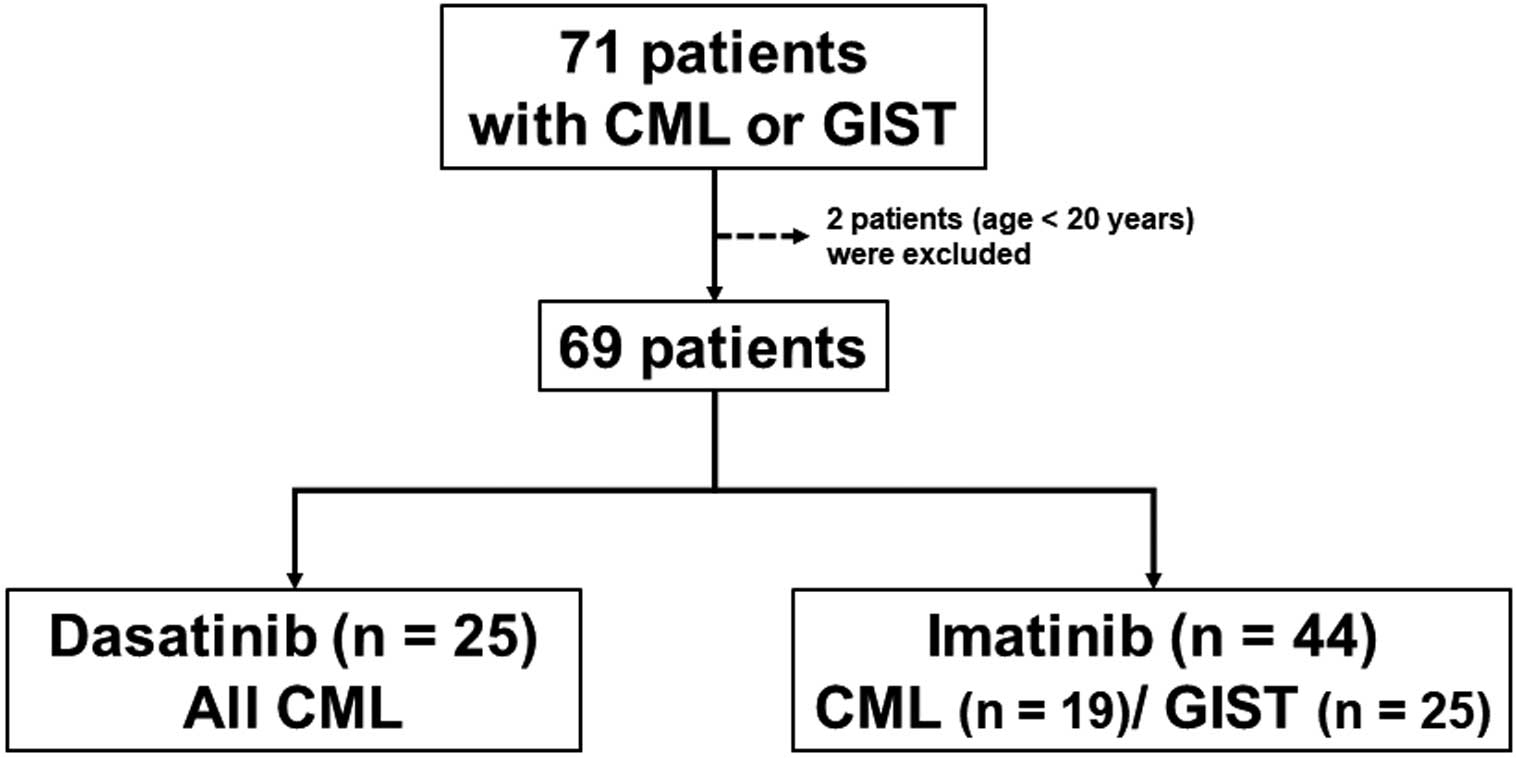
Study flow chart. Records for 71 consecutive patients who were started on treatment with dasatinib or imatinib for chronic myeloid leukemia (CML) or gastrointestinal stromal tumor (GIST) between December 2008 and December 2019 were retrospectively reviewed. Two patients aged <20 years were excluded, and the remaining 69 patients were enrolled in the study. Nineteen CML and 25 GIST patients were included in the imatinib-treated group.
The following cardiovascular events were recorded and graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE; version 5.0): pleural effusion, pericardial effusion, face or ankle edema, congestive heart failure (CHF), left ventricular (LV) dysfunction, PH, QT prolongation, ischemic heart disease, arteriosclerosis obliterans, myocarditis, pulmonary thromboembolism, and stroke. Pleural effusion was assessed by chest X-ray or computed tomography (CT). Pericardial effusion was assessed by echocardiography or CT. CHF was defined according to the Framingham criteria7 and with Grade ≥3 according to CTCAE version 5.0. Cardiomegaly, a component of the Framingham criteria, was defined by chest X-ray as a cardiothoracic ratio ≥50%. LV dysfunction was defined as a LV ejection fraction (LVEF) <50% or a >10% decrease in LVEF after the administration of TKIs. PH was defined as a tricuspid valve regurgitation pressure gradient >40 mmHg on echocardiography. QT prolongation was defined as corrected QT interval (QTc) >480 ms. If patients had these findings at baseline, any worsening after the start of treatment, regardless of an improvement in the underlying disease, was defined as a drug-related AE. All-cause mortality and non-cardiovascular events, including cytopenia, worsening renal function, liver dysfunction, gastrointestinal (GI) bleeding and symptoms (nausea, vomiting, and diarrhea), and respiratory AEs were also recorded. Worsening renal function was defined as a ≥0.3-mg/dL increase in serum creatinine. The follow-up period was until October 2020.
This study was approved by the Nagasaki University Hospital Ethics Committee (Approval no. 20051807, 2020) and was conducted in accordance with the ethical principles in the Declaration of Helsinki.
Statistical AnalysisAll statistical analyses were performed using JMP Pro version 15.0 for Mac (SAS Institute, Cary, NC, USA). Continuous variables are expressed as the mean±SD for normally distributed variables and as the median with interquartile range for skewed variables. Categorical variables are expressed as numbers with percentages. The significance of differences in continuous variables, including clinical characteristics and laboratory data, was analyzed using an unpaired t-test or the Wilcoxon rank-sum test. The significance of differences in categorical variables was assessed using the Chi-squared or Fisher’s exact test. Logistic regression analyses were used to identify prognostic factors for cardiovascular events. Two-sided P<0.05 was considered significant.
Baseline demographics, including medical history, medications, laboratory data, and echocardiographic findings, are presented in Table 1. The mean patient age was 58±15 years, and there were 34 men in the study cohort (49.3%). Patients in the IMA group had a significantly lower white blood cell count and tended to have higher hemoglobin and hematocrit levels than those in the DAS group. There was no significant difference in the follow-up period between the 2 groups.
| All (n=69) |
Dasatinib (n=25) |
Imatinib (n=44) |
P value | |
|---|---|---|---|---|
| Age (years) | 58±15 | 57±17 | 58±15 | 0.64 |
| Male sex | 34 (49.3) | 13 (52.0) | 21 (47.7) | 0.73 |
| BMI (kg/m2) | 21.8±3.9 | 21.6±3.7 | 22.0±4.1 | 0.70 |
| Medical history | ||||
| Myocardial ischemia | 3 (4.3) | 1 (4.0) | 2 (4.6) | 0.91 |
| Valvular disease | 1 (1.5) | 1 (4.0) | 0 (0) | 0.18 |
| Arterial diseases | 1 (1.5) | 0 (0) | 1 (2.3) | 0.45 |
| Hypertension | 22 (31.9) | 6 (24.0) | 16 (36.4) | 0.29 |
| Diabetes | 10 (14.5) | 2 (8.0) | 8 (18.2) | 0.25 |
| Dyslipidemia | 13 (18.8) | 5 (20.0) | 8 (18.2) | 0.85 |
| PAH | 0 (0) | 0 (0) | 0 (0) | – |
| Venous thromboembolism | 2 (2.9) | 1 (4.0) | 1 (2.3) | 0.68 |
| Atrial fibrillation | 1 (1.5) | 1 (4.0) | 0 (0) | 0.28 |
| Chronic kidney disease | 7 (10.1) | 2 (8.0) | 5 (11.4) | 0.66 |
| Respiratory disease | 14 (20.3) | 5 (20.0) | 9 (20.5) | 0.85 |
| Comorbid cancers | 11 (15.9) | 5 (20.0) | 6 (13.6) | 0.49 |
| Medications | ||||
| RAS inhibitors | 16 (23.2) | 4 (16.0) | 12 (27.3) | 0.38 |
| β-blockers | 3 (4.4) | 2 (8.0) | 1 (2.3) | 0.26 |
| Diuretics | 8 (11.6) | 4 (16.0) | 4 (9.1) | 0.45 |
| Digitalis | 1 (1.5) | 0 (0) | 1 (2.3) | 0.45 |
| Statins | 11 (15.9) | 4 (16.0) | 7 (15.9) | 0.99 |
| Steroids | 5 (7.3) | 1 (4.0) | 4 (9.1) | 0.43 |
| Antidiabetic medications | 5 (7.3) | 2 (8.0) | 3 (6.8) | 0.34 |
| Aspirin | 2 (2.9) | 1 (4.0) | 1 (2.3) | 0.68 |
| Anticoagulants | 6 (8.7) | 2 (8.0) | 4 (9.1) | 0.87 |
| Laboratory data | ||||
| WBC (/μL) | 8,200 [5,250–31,450] | 22,900 [7,900–126,800] | 6,950 [5,100–10,875] | 0.004 |
| Hemoglobin (g/dL) | 12.7 [10.9–14.0] | 12.4 [9.45–13.8] | 12.9 [11.2–14.2] | 0.26 |
| Hematocrit (%) | 38.9 [33.9–42.8] | 38.1 [28.9–41.0] | 39.5 [34.4–44.0] | 0.17 |
| Platelet (×104/μL) | 27.1 [20.8–45.5] | 31.3 [21.0–61.8] | 25.4 [19.9–33.5] | 0.26 |
| AST (IU/L) | 22.0 [16.5–30.0] | 24.0 [18.5–30.0] | 20.5 [15.0–28.0] | 0.21 |
| ALT (IU/L) | 18.0 [14.0–26.5] | 17.0 [14.5–26.0] | 18.5 [12.5–28.3] | 0.50 |
| Albumin (g/dL) | 4.0±0.6 | 4.1±0.4 | 4.0±0.7 | 0.41 |
| Creatinine (mg/dL) | 0.75 [0.64–0.89] | 0.75 [0.56–0.88] | 0.74 [0.64–0.90] | 0.60 |
| eGFR (mL/min/1.73 m2) | 74±23 | 79±27 | 71±21 | 0.18 |
| Echocardiographic findings | ||||
| LVDd (mm) | 45.7±5.9 | 45.8±5.4 | 45.6±6.3 | 0.91 |
| LVEF (%) | 68.5±6.3 | 69.5±6.3 | 67.7±6.2 | 0.33 |
| LAD (mm) | 34.0 [29.8–38.0] | 33.0 [29.5–36] | 36.0 [29.5–38.0] | 0.39 |
| TRPG (mmHg) | 20.0 [17.0–25.3] | 20.0 [16.3–27.5] | 21.0 [18.0–25.0] | 0.60 |
| Follow-up period (months) | 44.0 [21.5–73.0] | 40.0 [18.5–73.0] | 44.5 [23.3–73.5] | 0.39 |
Unless indicated otherwise, data are shown as the mean±SD, median [interquartile range], or n (%). ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; eGFR, estimated glomerular filtration rate; LAD, left atrial dimension; LVDd, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; PAH, pulmonary arterial hypertension; RAS, renin-angiotensin system; TRPG, tricuspid valve regurgitation pressure gradient; WBC, white blood cells.
During the follow-up, the DAS group had a higher incidence of CHF (20.0% vs. 2.3%; odds ratio [OR] 10.75, 95% confidence interval [CI] 1.18–98.15; P=0.04), pleural effusion (48.0% vs. 20.5%; OR 3.59, 95% CI 1.22–10.50; P=0.03), pericardial effusion (24.0% vs. 4.6%; OR 6.63, 95% CI 1.22–35.9; P=0.02), PH (12.0% vs. 0%; P=0.04) and QT prolongation (16.0% vs. 0%; P=0.02) than the IMA group (Table 2). The grades of cardiotoxic AEs based on CTCAE version 5.0 are presented in Table 3. Pleural effusion was not so severe, with Grade ≤3 in both groups. In contrast, PAH and QT prolongation were relatively severe AEs, with Grade ≥3 in the DAS group. Peripheral edema did not differ significantly between the 2 groups. There were no cases of new LV dysfunction, ischemic heart disease, arteriosclerosis obliterans, myocarditis, atrial fibrillation, stroke, or pulmonary thromboembolism in either group. There was no significant difference in all-cause mortality between the 2 groups.
| Adverse events | All (n=69) |
Dasatinib (n=25) |
Imatinib (n=44) |
P value |
|---|---|---|---|---|
| Cardiopulmonary events | ||||
| CHF | 6 (8.7) | 5 (20.0) | 1 (2.3) | 0.04 |
| Pleural effusion | 21 (30.4) | 12 (48.0) | 9 (20.5) | 0.03 |
| Pericardial effusion | 8 (11.6) | 6 (24.0) | 2 (4.6) | 0.02 |
| Face or ankle edema | 36 (53) | 12 (48.0) | 24 (54.6) | 0.80 |
| LV dysfunction | 0 (0) | 0 (0) | 0 (0) | – |
| Myocardial ischemia/arterial diseases | 0 (0) | 0 (0) | 0 (0) | – |
| Hypertension | 1 (1.5) | 0 (0) | 1 (2.3) | 0.45 |
| QT prolongation | 4 (5.8) | 4 (16.0) | 0 (0) | 0.02 |
| Pulmonary arterial hypertension | 3 (4.4) | 3 (12.0) | 0 (0) | 0.04 |
| Pulmonary thromboembolism | 0 (0) | 0 (0) | 0 (0) | – |
| Respiratory adverse event | 0 (0) | 0 (0) | 0 (0) | – |
| Stroke | 0 (0) | 0 (0) | 0 (0) | – |
| Non-cardiopulmonary events | ||||
| Rash | 20 (29) | 6 (24.0) | 14 (31.8) | 0.49 |
| Worsening renal function | 19 (27.5) | 2 (8.0) | 17 (38.6) | 0.01 |
| Liver dysfunction | 5 (7.3) | 2 (8.0) | 3 (6.8) | 0.86 |
| Cytopenia | 11 (15.9) | 6 (24.0) | 5 (11.4) | 0.17 |
| Gastrointestinal bleeding | 1 (1.5) | 1 (4.0) | 0 (0) | 0.18 |
| Gastrointestinal symptoms | 10 (14.5) | 0 (0) | 10 (22.7) | 0.01 |
| Mortality | ||||
| All-cause death | 11 (15.9) | 4 (16.0) | 7 (15.9) | 0.99 |
Unless indicated otherwise, values are given as n (%). CHF, congestive heart failure; LV, left ventricular.
| Dasatinib | Imatinib | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Hypertension | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (100) | 0 | 0 |
| Pleural effusion | 0 | 9 (75) | 3 (25) | 0 | 0 | 3 (33) | 5 (56) | 1 (11) | 0 | 0 |
| Pericardial effusion | – | 1 (17) | 5 (83) | 0 | 0 | – | 2 (100) | 0 | 0 | 0 |
| PAH | 0 | 0 | 2 (67) | 1 (33) | 0 | 0 | 0 | 0 | 0 | 0 |
| QT prolongation | 0 | 0 | 3 (75) | 1 (25) | 0 | 0 | 0 | 0 | 0 | 0 |
| CHF | 0 | 0 | 2 (40) | 2 (40) | 1 (20) | 0 | 0 | 1 (100) | 0 | 0 |
Data are given as n (%). CHF, congestive heart failure; CTCAE, Common Terminology Criteria for Adverse Events; PAH, pulmonary arterial hypertension.
Worsening renal function (8.0% vs. 38.6%l OR 0.138, 95% CI 0.03–0.66; P=0.01) and GI symptoms (0% vs. 22.7%; P=0.01) were more frequent in the IMA than DAS group. There were no significant differences in rash, liver dysfunction, cytopenia, GI bleeding, and respiratory AEs, including interstitial pneumonitis and pneumonia, between the 2 groups.
The characteristics of CHF cases are summarized in Table 4. All CHF patients treated with dasatinib presented with dyspnea on exertion and pleural effusion, with the CHF treatment decreasing the cardiothoracic ratio except in 1 fatal case. Logistic regression analysis in DAS did not identify any significant predictors; however, tricuspid valve regurgitation pressure gradient tended to be a predictor of CHF (Table 5).
| Case no. | Days to onset (days) |
Daily dose (mg) |
Framingham criteria | Changes in CTR with CHF treatment |
|
|---|---|---|---|---|---|
| Major criteria | Minor criteria | ||||
| Dasatinib | |||||
| 1 | 19 | 100 | Cardiomegaly | Dyspnea on exertion, pleural effusion, ankle edema |
55% → 51% |
| 2 | 24 | 100 | Cardiomegaly | Dyspnea on exertion, pleural effusion, ankle edema |
63% → 50% |
| 3 | 31 | 100 | Cardiomegaly, orthopnea | Dyspnea on exertion, pleural effusion |
55% → dead |
| 4 | 1,150 | 140 | Cardiomegaly | Dyspnea on exertion, pleural effusion, ankle edema |
52% → 40% |
| 5 | 19 | 100 | Acute pulmonary edema, orthopnea |
Dyspnea on exertion, pleural effusion, ankle edema |
49% → 42% |
| Imatinib | |||||
| 1 | 20 | 300 | Acute pulmonary edema, orthopnea |
Pleural effusion, ankle edema | 45% → 45% |
CHF, congestive heart failure; CTR, cardiothoracic ratio.
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| Age | 1.01 | 0.95–1.07 | 0.68 |
| Male | 1.5 | 0.20–11.00 | 0.69 |
| Hypertension | 2.67 | 0.33–21.73 | 0.35 |
| Respiratory disease | 3.78 | 0.43–33.08 | 0.22 |
| eGFR | 1.00 | 0.97–1.04 | 0.85 |
| Hemoglobin | 1.02 | 0.70–1.50 | 0.90 |
| LVEF | 0.93 | 0.77–1.11 | 0.41 |
| TRPG | 1.13 | 0.98–1.31 | 0.05 |
CI, confidence interval; OR, odds ratio. Other abbreviations as in Table 1.
Regarding the discontinuation of TKIs after the incidence of cardiovascular AEs, 1 patient with pleural effusion in each of the DAS and IMA groups discontinued the drug (for 26 and 9 days, respectively). Two patients (33%) with pericardial effusion discontinued dasatinib. PH and QT prolongation occurred only in the DAS group. Two-thirds of PH patients discontinued dasatinib, whereas 1 patient with QT prolongation tentatively discontinued dasatinib for 54 days. The drugs were continued in the other patients, along with the management of AEs.
In the present study, treatment of CML and GST with dasatinib was associated with a higher incidence of CHF, pleural effusion, PH, and QT prolongation compared with imatinib treatment. The incidence of these AEs was higher than in previous reports from the US and Europe.3,9 Conversely, no arterial ischemic events were observed in either the DAS or IMA group in the present study. There were no significant differences between these 2 groups in all-cause mortality and other cardiovascular events, such as hypertension, LV dysfunction, and thrombosis. With regard to drug-related non-cardiovascular events, patients in the IMA group had a higher incidence of worsening renal function and GI symptoms. Other non-cardiovascular AEs were comparable between the 2 groups. Overall, few studies comparing AEs between dasatinib and imatinib have been published (Table 6).3,4,8,9 The results of the present study may have some impact on the treatment of Japanese patients with TKIs.
| Present study | DASISION | NordCML0069 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Japanese4 | East Asian8 | 5-year final report3 | ||||||||
| DAS (n=25) |
IMA (n=44) |
DAS (n=26) |
IMA (n=23) |
DAS (n=59) |
IMA (n=48) |
DAS (n=259) |
IMA (n=260) |
DAS (n=22) |
IMA (n=24) |
|
| Pleural effusion | 48 | 20.5 | 42 | 4 | 23.7 | 0 | 28 | 0.8 | 23 | 0 |
| Pericardial effusion | 24 | 4.6 | – | – | – | – | – | – | 5 | 0 |
| Face or Ankle edema | 50 | 54.6 | 19 | 30 | 16.9 | 35.4 | – | – | – | – |
| PAH | 12 | 0 | 3.8 | 0 | – | – | 5 | 0.4 | – | – |
| QT prolongation | 16 | 0 | – | – | – | – | – | – | – | – |
| CHF | 20 | 2.3 | – | – | – | – | 8.5 | 3.9 | – | – |
| Ischemic heart disease | 0 | 0 | – | – | – | – | 5 | 0 | ||
| Arterial ischemic event | 0 | 0 | 0 | 0 | 5 | 3 | – | – | ||
| Hypertension | 0 | 2.3 | – | – | – | – | – | – | – | – |
Values show the percentage of patients with each adverse event. CHF, congestive heart failure; DAS, dasatinib treatment; IMA, imatinib treatment; PAH, pulmonary arterial hypertension.
Regarding imatinib-induced CHF, IRIS demonstrated that cardiotoxicity was low, with only 1 patient developing definite CHF in 5 years.1 Atallah et al reported that 22 (1.7%) patients met the Framingham criteria for CHF among 1,276 patients treated with imatinib.10 In that study, there was no significant difference in the rates of new-onset CHF in IMA patients compared with age- and sex-matched control patients from the Framingham Heart Study.10 A previous study using echocardiography and multiple gated acquisition scanning reported no evidence of cardiac dysfunction over 12 months of imatinib treatment.11 Thus, treatment with imatinib is not related to an increased incidence of CHF.
In contrast, a preclinical study showed that a single intravenous administration of dasatinib, while not reaching therapeutic concentrations, led to impaired LV mechanical function along with a reflex-mediated increase in sympathetic tone.12 The most plausible explanation of the negative inotropic effect of dasatinib is inhibition of non-receptor-type protein kinase ABL1 and ABL2, leading to endoplasmic reticulum stress and finally mitochondrial dysfunction via the activation of serine/threonine-protein kinase/endoribonuclease inositol requiring enzyme 1 (IRE1) and overexpression of protein kinase Cδ.13
The final 5-year analysis of DASISION revealed that CHF occurred in 8.5% and 3.9% of DAS and IMA adult patients with newly diagnosed CML, respectively.14 In the present study, we diagnosed CHF in 5 (20%) patients treated with dasatinib and in 1 (2.3%) patient treated with imatinib. A incidence of CHF was higher in our study, which may be due to differences in the definition of CHF used and ethnicity. We also found that LV dysfunction did not worsen, but that a higher tricuspid valve regurgitation pressure gradient tended to be associated with CHF. Therefore, in the present study, heart failure with preserved ejection fraction or right-sided heart failure may have contributed to the CHF events.
Regarding the duration between the initiation of TKIs and the onset of CHF, the 3-year follow-up of DASISION reported that most of the AEs occurred within the first year, with minimal increases from 1 to 2 years and from 2 to 3 years of therapy.15 IRIS also showed that newly occurring or worsening Grade 3 or 4 AEs at 1 and 2 years were more frequent than those at 2 and 4 years, as well as after 4 years of therapy.1 Consistent with these reports, all patients in the present study except for 1 patient (Case 4 in Table 4) developed CHF early after the administration of the TKI, especially within approximately 1 month. One possible reasons for differences in the duration between the initiation of TKIs and the onset of AEs may be that patients with a higher risk of cardiac events at baseline may experience events earlier than those at a lower risk, and the population at risk for a first AE may then shift over time to lower-risk patients.16 Poor adherence to TKIs can also lead to the development of AEs, which may have contributed to duration differences.17 However, the exact reason for the difference has not yet been clarified. In the present study, a patient who developed AE 1,150 days after the administration of TKI had no cardiovascular risks or pulmonary disease and had taken a low dose of dasatinib (80 mg/day). This may have delayed the occurrence of CHF.
Pleural effusion is a component of CHF and a relatively common AE of dasatinib. In DASISION, drug-related pleural effusion was found in 28% and 0.8% of patients treated with dasatinib and imatinib, respectively.3 The NordCML006 study showed that 23% of patients treated with dasatinib had pleural effusions, whereas pleural effusion was not detected in patients treated with imatinib.9 In addition, the difference in the incidence of pleural effusion between dasatinib and imatinib was more prominent in Japanese CML patients (42% vs. 4%, respectively);4 our findings are consistent with those results, despite IMA patients having a relatively higher incidence of pleural effusion in the present study.
There are several reported predictors of pleural effusion. Older age (>65 years) and the dose of dasatinib are correlated with higher rates of pleural effusion.18 The median age of our patients with pleural effusion was 65.5 years, and the median dose of dasatinib was 100 mg/day. Therefore, these factors were less relevant in our cohort. Other predisposing factors for pleural effusion on dasatinib include prior cardiac history, hypertension, twice-a-day administration of dasatinib, prior skin rash on dasatinib, prior history of autoimmune disease, and hypercholesterolemia.19,20 In the present study, no significant risk factors for pleural effusion were identified.
The precise mechanism by which dasatinib induces pleural effusion has not been clarified; however, some potential mechanisms have been proposed. In an off-target effect, dasatinib more potently inhibits platelet-derived growth factor receptor (PDGFR) β, which is expressed in pericytes and is involved in the regulation of angiogenesis,19 than imatinib, whose inhibitory effect on PDGFR is only weak. A case series showed that there is a high lymphocyte concentration in pleural fluid and pleural tissue in DAS patients, suggesting an immune-mediated mechanism given that dasatinib inhibits Src-family kinases LCK and LYN expressed in B and T lymphocytes.21 The disruption of cell adhesion, which is key for the stability of the pleural epithelium and space homeostasis, causes pleural effusion. Src regulates focal adhesions and adherens junctions, which maintain cell adhesion. In addition, vascular permeability, mediated by vascular endothelial growth factor, is directly dependent on Src and the Src-related kinase Yes, both of which are widely expressed in lung tissue and are inhibited by dasatinib.19
The first cases of dasatinib-induced pulmonary arterial hypertension (PAH) were reported in 2009,22 and data published in 2012 from the French Pulmonary Hypertension Registry showed the incidence of PAH to be low (0.45%).23 In DASISION, PH diagnosed based on echocardiographic criteria was documented in 5% of patients.3 Recently, the 6th World Symposium on Pulmonary Hypertension has included dasatinib as a cause of pulmonary arterial hypertension.24 In contrast, imatinib has not been reported to induce pulmonary vasculopathy, and, in fact, it has been studied in a randomized control trial as adjuvant therapy in PAH because of promising case reports in addition to basic and translational data.25 In the present study, all PH cases were associated with the use of dasatinib. This is consistent with previous reports,3,4 although the incidence of PH was higher in the present than previous studies. The vasculopathic effect of dasatinib may be related to a more potent effect on the Src family of tyrosine kinases than imatinib. Inhibition of Src by dasatinib may lead to pulmonary vasoconstriction and increased pulmonary arterial pressure through inhibition of the TWIK-related acid-sensitive potassium-1 channel.26 However, the exact etiology of dasatinib-induced pulmonary toxicities remains unclear.27
QT prolongation was observed in our patients treated with dasatinib, but not in those treated with imatinib. A meta-analysis published in 2017 showed that QTc prolongation due to dasatinib had an 8% weighted incidence rate (range 1.6–73%) and a 1% weighted incidence rate for QTc >500 ms,28 whereas the corresponding incidence rates for imatinib were 3.1% (range <0.5–6.9%) and 0.02%.29 Previous studies suggest that TKIs may inhibit the phosphatidylinositol 3-kinase intracellular signaling pathway, which can lead to downstream upregulation of the late sodium current and downregulation of potassium currents, causing ventricular repolarization abnormalities and QT prolongation.27
Finally, although 5-year follow-up data from DASISION indicated that arterial ischemic events occurred in 5% of patients on dasatinib and in 2% of those on imatinib, no arterial ischemic events were reported with either treatment for the Japanese population in a subanalysis of that trial.4 The results of the present study are consistent with those of the subanalysis.4
The results of the present study suggest that the frequency of AEs mentioned above, especially those induced by dasatinib, is higher in the Japanese population than in other populations. However, the underlying etiology for this difference is unknown.
The present study has some limitations. First, this was a retrospective study performed through a review of medical records in a single center and it included a small study population. Any omissions or errors in the review of the medical records may have led to an underestimation of AEs. Furthermore, routine imaging tests, such as echocardiography and CT, were not conducted, which may also have resulted in underestimation. Second, patients treated with imatinib in the present study were being treated for either CML or GIST. Although these 2 diseases have completely different pathophysiology, the pharmacokinetics of imatinib are similar between GIST and CML patients.30 Thus, the safety profile of imatinib is considered similar in these 2 diseases, except for specific AEs, such as GI symptoms and anemia.31 Severe fluid retention leading to pleural/pericardial effusions or anasarca was rare but more frequently observed in GIST than CML patients (2.8–9% vs. 1–1.7%, respectively).31 We believe that the differences in AEs between the 2 drugs would not be affected, even if the 2 types of diseases treated with imatinib were included in the analysis. Third, some AEs, such as pleural effusion and myelosuppression,32,33 are known to be dependent on the dose of TKIs. In the present study, the mean doses of dasatinib and imatinib were 98.8±26.8 and 356.8±87.3 mg daily, respectively, which correspond to the standard doses for these drugs. Because no alternative, non-standard dose regimens were used, we cannot comment on dose-related differences in AEs between the 2 drugs. Fourth, the follow-up period was relatively shorter than that of previous studies. This may have affected the incidence of AEs.
In Japanese patients, AEs, such as CHF, pleural effusion, PH, and QT prolongation, were more frequent during dasatinib than imatinib treatment. The incidence of these AEs was higher than reported previously in the US and Europe.3,9 Multicenter prospective cohort studies will be required to clarify the prevalence of TKI-associated AEs in the Japanese population, as well as ethnicity-based differences.
This study did not receive any specific funding.
K.M. is a member of Circulation Reports’ Editorial Team. The remaining authors have no conflicts of interest to declare.
This study was approved by the Nagasaki University Hospital Ethics Committee (Approval no. 20051807, 2020)
The deidentified participant data will not be shared.