Abstract
Background: Few data are available regarding the impact of atrial fibrillation (AF) at diagnosis and type of AF during the follow-up period on long-term outcomes in patients with heart failure with preserved ejection fraction (HFpEF).
Methods and Results: In all, 1,697 patients diagnosed as HFpEF between March 2010 and December 2017 were included in this study. At enrollment, 698 (41.1%) patients had AF. Over a median follow-up of 1,017 days, there were no significant differences between patients with and without AF in the adjusted hazard ratio (HR) for all-cause death or admission for heart failure. However, those with AF had a higher risk of stroke (HR 1.831; P=0.003). Of 998 patients with sinus rhythm at enrollment, 139 (13.9%) developed new-onset AF. Predictors of new-onset AF were pulse, hemoglobin, left ventricular end-diastolic dimension, and B-type natriuretic peptide. Compared with sinus rhythm, paroxysmal AF had a similar risk for all-cause death, admission for HF, and stroke; persistent AF had a lower risk of all-cause death (HR 0.701; P=0.015), but a higher risk for admission for HF (HR 1.608; P=0.002); and new-onset AF had a lower risk for all-cause death (HR 0.654; P=0.040), but a higher risk of admission for HF (HR 2.475; P<0.001).
Conclusions: In patients with HFpEF, long-term outcome may differ by type of AF. Physicians need to consider individual risk with regard to AF type.
Heart failure with preserved ejection fraction (HFpEF) is recognized worldwide as a different entity to heart failure that with reduced ejection fraction (HFrEF), and accounts for more than 50% of all heart failure patients. HFpEF can be complicated by atrial fibrillation (AF).1 Although some patients maintain sinus rhythm throughout follow-up, others are found to experience various types of AF, including paroxysmal, persistent, and new-onset AF, during follow-up. However, few studies have comprehensively investigated AF in patients with HFpEF. Thus, the aims of this study were to investigate: (1) the incidence and long-term outcomes of AF at enrollment; (2) the incidence and determinants of new-onset AF; and (3) the relationship between AF type (paroxysmal, persistent, and new onset) and long-term outcome in patients with HFpEF.
Methods
Study Population
Patient data were extracted from the electronic medical records of Osaka University Hospital between March 1, 2010 and December 31, 2017. Patients were enrolled in the study if they had been diagnosed with chronic heart failure according to International Statisitical Classifications of Diseases (ICD)-10 codes, had B-type natriuretic peptide (BNP) concentrations ≥100 pg/mL, and an ejection fraction as assessed by echocardiography ≥50% and these measures had been obtained within 30 days of each other. Patients were excluded if they were aged ≤20 years, had acute coronary syndrome at enrollment, had severe valvular disease (e.g., aortic valve stenosis or regurgitation, mitral valve stenosis or regurgitation), hypertrophic cardiomyopathy,2 congenital heart disease, cardiac sarcoidosis, or amyloidosis, or had undergone heart transplantation.
The study protocol was approved by the Institutional Review Board of Osaka University Hospital. With regard to informed consent, the opt-out method was adopted.3
Data Collection
Patient demographics, electrocardiograms (ECG), laboratory data, echocardiograms, and medication data were collected from electronic medical records at Osaka University Hospital. AF was defined as documentation of AF on 12-lead ECG or Holter ECG during the follow-up period. Persistent AF was defined as AF observed throughout the entire follow-up period. Paroxysmal AF was defined as documented AF once and sinus rhythm observed on the latest ECG. New-onset AF was defined as sinus rhythm at enrollment and documented AF on the latest ECG. For follow-up data, Day 0 was set as the day when the inclusion criteria were fulfilled and the exclusion criteria were not met simultaneously. The clinical endpoint in this study was all-cause death, admission for heart failure, myocardial infarction, or stroke (defined as ischemic, lacunar, or hemorrhagic stroke), whichever occurred first.
Statistical Analysis
Continuous values are expressed as the mean±SD or as the median with the interquartile range if the distribution of data was skewed. Categorical data are presented as frequencies. Baseline characteristics between the groups were compared using t tests or repeated-measures analysis of variance for comparisons of continuous variables, and the Chi-squared or Fisher’s exact test for categorical variables. Multivariate Cox regression analysis was used to identify factors associated with new-onset AF and to investigate the hazard ratio (HR) of AF at enrollment and each type of AF vs. sinus rhythm. Variables inserted into the models were variables that affected clinical outcomes. Outcomes data were described by the Kaplan-Meier method and compared by the log-rank test.
All analyses were performed using SPSS 23.0 (SPSS Japan, Tokyo, Japan). Statistical significance was defined as P<0.05 or a confidence interval (CI) that did not include 1.0.
Results
Of the 1,845 patients who fulfilled the inclusion criteria for this study, 6 patients aged <20 years, 19 with acute coronary syndrome, 46 with congenital heart disease, 3 with cardiac amyloidosis, 6 with cardiac sarcoidosis, and 68 whose rhythm was unknown were excluded. This left 1,697 patients, with a mean age of 71.4 years, as study subjects.
At enrollment, the prevalence of AF was 41.1% (n=698). Patient characteristics stratified by the presence or absence of AF at enrollment are presented in Table 1. Patients with AF were more likely to be male, older, to have had a prior stroke, and experienced admission for heart failure. There were no significant differences in the prevalence of a CHADS2
score ≥2 or in BNP concentrations between the 2 groups. Echocardiogram data revealed that patients with AF were more likely to have a larger left atrial and ventricular dimensions and to have a lower ejection fraction. In terms of medication at enrollment, patients with AF were more likely to be receiving β-blockers, diuretics, and anticoagulants. Over a median follow-up period of 1,017 days (IQR 411–1,661 days), there was no significant difference in the occurrence of all-cause death (Figure 1A), admission for heart failure (Figure 1B), myocardial infarction (Figure 1C), or stroke (Figure 1D) between the 2 groups. After adjustment for male sex, CHADS2
score, the use of angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARBs), β-blockers, statins, loop diuretics, and BNP divided by 100, AF at enrollment was not associated with an increased risk of all-cause death, admission for HF, or myocardial infarction, but was associated with increased risk of stroke, with an adjusted HR of 1.831 (95% CI 1.233–2.717; P=0.003; Table 2). Although there was no significant difference in cardiac death between the 2 groups, non-cardiac death was significantly higher in AF than sinus group (P=0.043; Supplementary Table 1).
Table 1. Patient Characteristics Stratified by the Presence or Absence of AF at Enrollment and During Follow-up
| |
Sinus rhythm
(n=999) |
AF present
(n=698) |
All
(n=1,697) |
P value |
| Baseline |
| Age (years) |
69.7±14.5 |
73.8±10.4 |
71.4±13.1 |
<0.001 |
| Age ≥75 years (%) |
45.6 |
50.7 |
47.7 |
0.040 |
| Male sex (%) |
47.9 |
59.2 |
52.6 |
<0.001 |
| BMI (kg/m2) |
22.2±3.8 |
22.8±3.9 |
22.5±3.9 |
0.001 |
| SBP (mmHg) |
132±24 |
126±22 |
129±23 |
<0.001 |
| DBP (mmHg) |
70±14 |
69±14 |
69±14 |
0.134 |
| Pulse (beats/min) |
72±16 |
74±18 |
73±17 |
0.029 |
| Diabetes (%) |
47.5 |
44.4 |
46.3 |
0.215 |
| Hypertension (%) |
78.9 |
81.6 |
80 |
0.173 |
| Dyslipidemia (%) |
66 |
63.3 |
64.9 |
0.256 |
| Current smoker (%) |
39.8 |
42.2 |
40.8 |
0.331 |
| Prior PCI (%) |
51.4 |
56.6 |
53.1 |
0.315 |
| Prior CABG (%) |
22.6 |
25.9 |
23.7 |
0.459 |
| History of stroke (%) |
21.5 |
29.8 |
24.9 |
<0.001 |
| History of HF admission (%) |
25.9 |
34.4 |
29.3 |
<0.001 |
| CHADS2 score ≥2 (%) |
82.4 |
84.4 |
83.2 |
0.278 |
| Hemoglobin (g/dL) |
11.7±2.1 |
12.4±2.3 |
12±2.2 |
<0.001 |
| Hematocrit (%) |
35.7±6.1 |
37.9±6.6 |
36.7±6.4 |
<0.001 |
| Total cholesterol (mg/dL) |
179±42 |
176±38 |
178±40 |
0.270 |
| LDL-C (mg/dL) |
100±31 |
97±29 |
99±30 |
0.117 |
| HDL-C (mg/dL) |
52±16 |
52±15 |
52±16 |
0.979 |
| Triglyceride (mg/dL) |
125±72 |
126±82 |
125±76 |
0.807 |
| eGFR (mL/min/1.73 m2) |
53±30 |
52±22 |
53±27 |
0.496 |
| CRP (mg/dL) |
0.14 [0.04–0.71] |
0.14 [0.04–0.73] |
0.14 [0.05–0.64] |
0.331 |
| BNP (pg/mL) |
179 [129–304] |
192 [135–295] |
185 [130–302] |
0.438 |
| LA diameter (mm) |
40.2±7.5 |
48.2±10 |
43.5±9.4 |
<0.001 |
| LVEDd (mm) |
46.4±6.8 |
47.5±6.6 |
46.8±6.7 |
<0.001 |
| LVESd (mm) |
28.9±5.3 |
30±5.1 |
29.4±5.2 |
<0.001 |
| Ejection fraction (%) |
62.2±6.8 |
61±6.6 |
61.7±6.7 |
<0.001 |
| TR pressure gradient (mmHg) |
28±14 |
28±11 |
28±13 |
0.641 |
| ACEI (%) |
8.4 |
10.0 |
9.1 |
0.253 |
| ARB (%) |
27.8 |
28.7 |
28.2 |
0.710 |
| ACEI or ARB (%) |
35 |
39.1 |
36.7 |
0.086 |
| β-blocker (%) |
26.7 |
36.8 |
30.9 |
<0.001 |
| Statin (%) |
22.2 |
21.8 |
22.0 |
0.827 |
| Loop diuretics (%) |
30.5 |
38.3 |
33.7 |
0.001 |
| Anti-arrhythmic drugs (%) |
3.6 |
21.1 |
10.8 |
<0.001 |
| Anticoagulation+antiplatelet (%) |
1.0 |
9.3 |
4.4 |
<0.001 |
| Antiplatelet (%) |
32.7 |
26.5 |
30.2 |
0.006 |
| Anticoagulation (%) |
2.6 |
32.5 |
14.9 |
<0.001 |
| Prior ablation (%) |
1.3 |
1.0 |
1.2 |
0.575 |
| During follow-up |
| ACEI (%) |
21.3 |
23.8 |
22.3 |
0.231 |
| ARB (%) |
56.1 |
56.3 |
56.2 |
0.919 |
| ACEI or ARB (%) |
63.7 |
68.1 |
65.5 |
0.061 |
| β-blocker (%) |
54.3 |
65.3 |
58.8 |
<0.001 |
| Anticoagulation (%) |
20.6 |
77.4 |
44.0 |
<0.001 |
| Ablation (%) |
4.2 |
5.4 |
4.7 |
0.236 |
Unless indicated otherwise, data are given as the mean±SD or median (interquartile range). ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, B-type natriuretic peptide; CABG, coronary artery bypass grafting; CRP, C-reactive protein; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL-C, high density lipoprotein cholesterol; HF, heart failure; LA, left atrium; LDL-C, low density lipoprotein cholesterol; LVEDd, left ventricle end-diastolic diameter; LVESd, left ventricle end-systolic diameter; PCI, percutaneous coronary intervention; SBP, systolic blood pressure; TR, tricuspid regurgitation.
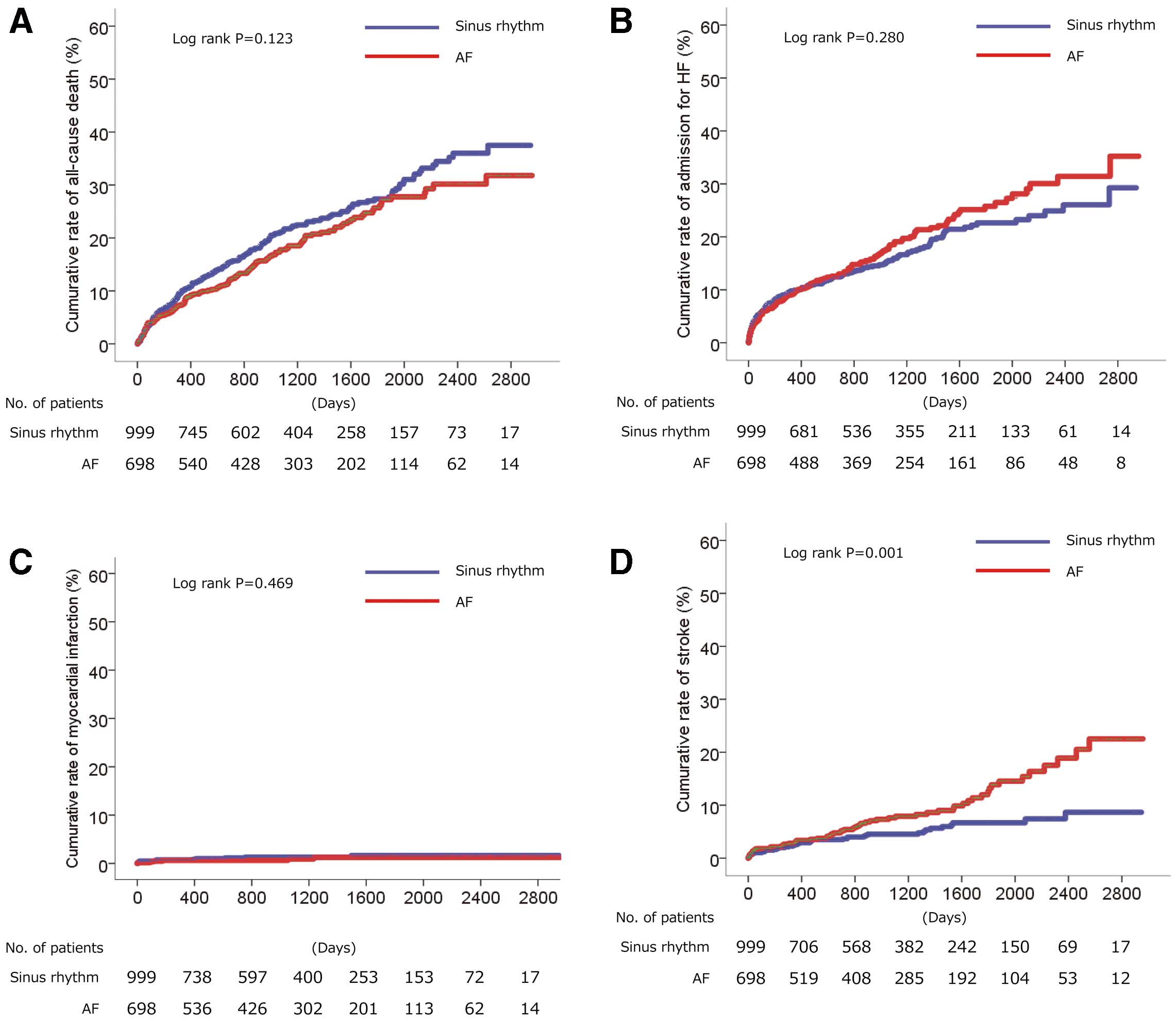
Table 2. Clinical Outcomes and Adjusted HRs for AF at Enrollment
| |
No. subjects |
No. events
(%) |
Adjusted HR
(95% CI) |
P value |
| All cause death |
| Sinus rhythm |
999 |
219 (21.9) |
Reference |
|
| AF |
697 |
134 (19.2) |
0.852 (0.685–1.060) |
0.150 |
| Admission for HF |
| Sinus rhythm |
998 |
158 (15.8) |
Reference |
|
| AF |
696 |
127 (18.2) |
1.132 (0.892–1.438) |
0.307 |
| Myocardial infarction |
| Sinus rhythm |
998 |
12 (1.2) |
Reference |
|
| AF |
696 |
6 (0.9) |
0.635 (0.235–1.716) |
0.371 |
| Stroke |
| Sinus rhythm |
972 |
45 (4.6) |
Reference |
|
| AF |
686 |
62 (9.0) |
1.831 (1.233–2.717) |
0.003 |
Hazard ratios (HRs) were adjusted for male sex, CHADS2 score, the use of ACEI or ARB, β-blockers, statins, and loop diuretics, and BNP divided by 100. CI, confidence interval. Other abbreviations as in Table 1.
Among the 998 patients with sinus rhythm at enrollment, excluding 1 patient whose rhythm during the follow-up period was unknown, 139 (13.7%) patients developed new-onset AF during follow-up (Table 3; Figure 2). Patients with new-onset AF were more likely to be older and less likely to have received a previous percutaneous coronary intervention. Although there was no significant difference in anticoagulation treatment at enrollment between patients who maintained sinus rhythm and those who developed new-onset AF, the prevalence of anticoagulation therapy during the entire study period was significantly higher in patients with new-onset AF. On multivariate Cox regression analysis, pulse, hemoglobin, left ventricular end-diastolic dimension, and BNP divided by 100 were extracted as factors associated with the occurrence of new-onset AF in the current population (Table 4, Model 1). These factors remained unchanged when a stepwise selection method was used (Table 4, Model 2).
Table 3. Patient Characteristics Stratified by Sinus Rhythm and New Onset of AF Among Patients With Sinus Rhythm at Enrollment and During Follow-up
| |
Sinus rhythm
(n=859) |
New-onset AF
(n=139) |
All
(n=998) |
P value |
| Baseline |
| Age (years) |
69.4±15 |
72.1±10.1 |
69.7±14.5 |
0.007 |
| Age ≥75 years (%) |
45.9 |
44.6 |
45.7 |
0.782 |
| Male sex (%) |
47.7 |
48.9 |
47.9 |
0.794 |
| BMI (kg/m2) |
22.2±3.8 |
22.6±3.8 |
22.2±3.8 |
0.171 |
| SBP (mmHg) |
131±24 |
133±22 |
132±24 |
0.300 |
| DBP (mmHg) |
70±14 |
69±12 |
70±14 |
0.315 |
| Pulse (beats/min) |
73±16 |
66±15 |
72±16 |
<0.001 |
| Diabetes (%) |
47.4 |
48.2 |
47.5 |
0.871 |
| Hypertension (%) |
78.3 |
82.7 |
78.9 |
0.237 |
| Dyslipidemia (%) |
65.7 |
68.6 |
66.1 |
0.500 |
| Current smoker (%) |
39.1 |
43.6 |
39.7 |
0.320 |
| Prior PCI (%) |
54.1 |
35 |
51.4 |
0.025 |
| Prior CABG (%) |
23.6 |
17.1 |
22.6 |
0.357 |
| History of stroke (%) |
20.5 |
27.3 |
21.5 |
0.070 |
| History of HF admission (%) |
26.8 |
20.4 |
25.9 |
0.116 |
| CHADS2 score ≥2 (%) |
82.5 |
82.0 |
82.5 |
0.880 |
| Hemoglobin (g/dL) |
11.6±2.1 |
12.3±1.8 |
11.7±2.1 |
<0.001 |
| Hematocrit (%) |
35.5±6.2 |
37.4±5 |
35.7±6.1 |
<0.001 |
| Total cholesterol (mg/dL) |
177±43 |
187±33 |
179±42 |
0.059 |
| LDL-C (mg/dL) |
99±32 |
105±25 |
100±31 |
0.210 |
| HDL-C (mg/dL) |
52±16 |
54±17 |
52±16 |
0.389 |
| Triglyceride (mg/dL) |
124±72 |
128±73 |
125±72 |
0.688 |
| eGFR (mL/min/1.73 m2) |
54±30 |
48±26 |
53±30 |
0.114 |
| CRP (mg/dL) |
0.15 [0.05–0.81] |
0.09 [0.02–0.39] |
0.14 [0.04–0.73] |
0.678 |
| BNP (pg/mL) |
178 [128–299] |
184 [130–403] |
179 [129–304] |
0.054 |
| LA diameter (mm) |
39.8±7.4 |
42.9±7.1 |
40.2±7.5 |
<0.001 |
| LVEDd (mm) |
46.1±6.7 |
47.9±7.2 |
46.4±6.8 |
0.003 |
| LVESd (mm) |
28.7±5.2 |
29.9±5.5 |
28.9±5.3 |
0.019 |
| Ejection fraction (%) |
62.2±6.8 |
62.1±6.9 |
62.2±6.8 |
0.890 |
| TR pressure gradient (mmHg) |
28±14 |
28±12 |
28±14 |
0.054 |
| ACEI (%) |
9.1 |
4.3 |
8.4 |
0.061 |
| ARB (%) |
27.4 |
30.9 |
27.9 |
0.383 |
| ACEI or ARB (%) |
34.9 |
36.0 |
35.1 |
0.810 |
| β-blocker (%) |
25.7 |
33.1 |
26.8 |
0.069 |
| Statin (%) |
22.2 |
22.3 |
22.2 |
0.986 |
| Loop diuretics (%) |
30.4 |
31.7 |
30.6 |
0.763 |
| Anti-arrhythmic drugs (%) |
3.1 |
6.5 |
3.6 |
0.051 |
| Anticoagulation+antiplatelet (%) |
1.0 |
0.7 |
1.0 |
0.718 |
| Antiplatelet (%) |
32.8 |
32.4 |
32.8 |
0.916 |
| Anticoagulation (%) |
2.7 |
2.2 |
2.6 |
0.721 |
| Prior ablation (%) |
1.4 |
0.7 |
1.3 |
0.513 |
| During follow-up |
| ACEI (%) |
21.0 |
23.7 |
21.3 |
0.457 |
| ARB (%) |
55.2 |
61.9 |
56.1 |
0.140 |
| ACEI or ARB (%) |
63.1 |
67.6 |
63.7 |
0.303 |
| β-blocker (%) |
52.2 |
67.6 |
54.3 |
0.001 |
| Anticoagulation (%) |
15.4 |
53.2 |
20.6 |
<0.001 |
| Ablation (%) |
4.7 |
1.4 |
4.2 |
0.080 |
Unless indicated otherwise, data are given as the mean±SD or median (interquartile range). Abbreviations as in Table 1.

Table 4. Factors Associated With New Onset of AF by Multivariate Cox Regression Analysis
| |
Model 1 |
Model 2 |
| HR |
95% CI |
P value |
HR |
95% CI |
P value |
| Age |
1.007 |
0.989–1.025 |
0.435 |
– |
– |
– |
| Male sex |
0.753 |
0.483–1.175 |
0.212 |
– |
– |
– |
| BMI |
1.001 |
0.935–1.071 |
0.983 |
– |
– |
– |
| SBP |
0.996 |
0.986–1.007 |
0.477 |
– |
– |
– |
| Pulse |
0.974 |
0.958–0.991 |
0.003 |
0.974 |
0.958–0.990 |
0.002 |
| Prior stroke |
1.242 |
0.757–2.037 |
0.391 |
– |
– |
– |
| ACEI or ARB |
0.756 |
0.477–1.200 |
0.235 |
– |
– |
– |
| Loop antidiuretics |
0.957 |
0.564–1.623 |
0.869 |
– |
– |
– |
| Hemoglobin |
1.200 |
1.058–1.362 |
0.005 |
1.172 |
1.043–1.317 |
0.008 |
| LA dimension |
1.029 |
0.992–1.068 |
0.126 |
1.032 |
0.997–1.068 |
0.078 |
| LV end-diastolic dimension |
1.060 |
1.021–1.100 |
0.002 |
1.045 |
1.010–1.082 |
0.013 |
| TR pressure gradient |
1.001 |
0.984–1.018 |
0.902 |
– |
– |
– |
| BNP divided by 100 |
1.088 |
1.035–1.144 |
0.001 |
1.089 |
1.045–1.136 |
<0.001 |
Model 1: forced entry method; Model 2, stepwise selection method. LV, left ventricle. Other abbreviations as in Tables 1,2.
Among the 1,648 patients for whom rhythm data were available during the follow-up period, 859 (52.1%), 283 (17.2%), 367 (22.3%), and 139 (8.4%) had sinus rhythm, paroxysmal AF, persistent AF, and new-onset AF, respectively (Supplementary Table 2). Although there was no significant difference in all-cause death among the 4 groups (Figure 3A), there was a significant difference among the groups in admission for heart failure (Figure 3B). In addition, although there was no significant difference in myocardial infarction among the groups (Figure 3C), the incidence of stroke differed (Figure 3D). Table 5 shows clinical events during follow-up and the adjusted HR for each type of AF when the HR for sinus rhythm was set at a reference value of 1. There was no significant risk difference in all cause-death, admission for HF, myocardial infarction, or stroke between sinus rhythm and paroxysmal AF. Although persistent AF had lower risk for all-cause death, it had higher risk for admission for HF and tended to have a higher risk for stroke. Furthermore, although new-onset AF had lower risk for all-cause death, it had a higher risk for admission for HF and no significant difference in myocardial infarction or stroke. These results suggest that the effect on clinical outcome differed for each type of AF. With regard to cause of death, there was no significant difference in cardiac death among the 4 groups (P=0.094). However, there was a significant difference in non-cardiac deaths among the 4 groups (P=0.010), with a lower rate of non-cardiac deaths in the persistent AF than sinus group (P=0.002; Supplementary Table 3).

Table 5. Clinical Outcomes and Adjusted HR of Each Type of AF
| |
No. subjects |
No. events
(%) |
Adjusted HR
(95% CI) |
P value |
| All-cause death |
| Sinus rhythm |
859 |
191 (22.2) |
Reference |
|
| Paroxysmal AF |
282 |
62 (22.0) |
0.948 (0.708–1.269) |
0.718 |
| Persistent AF |
367 |
64 (17.4) |
0.701 (0.525–0.934) |
0.015 |
| New-onset AF |
139 |
28 (20.1) |
0.654 (0.437–0.980) |
0.040 |
| Admission for HF |
| Sinus rhythm |
859 |
109 (12.7) |
Reference |
|
| Paroxysmal AF |
281 |
44 (15.7) |
1.207 (0.844–1.726) |
0.303 |
| Persistent AF |
367 |
77 (21.0) |
1.608 (1.191–2.171) |
0.002 |
| New-onset AF |
139 |
49 (35.3) |
2.475 (1.753–3.495) |
<0.001 |
| Myocardial infarction |
| Sinus rhythm |
859 |
9 (1.0) |
Reference |
|
| Paroxysmal AF |
281 |
4 (1.4) |
1.251 (0.376–4.162) |
0.716 |
| Persistent AF |
367 |
2 (0.5) |
0.398 (0.084–1.887) |
0.246 |
| New-onset AF |
139 |
3 (2.2) |
1.422 (0.347–5.827) |
0.625 |
| Stroke |
| Sinus rhythm |
835 |
40 (4.8) |
Reference |
|
| Paroxysmal AF |
276 |
23 (8.3) |
1.491 (0.881–2.522) |
0.137 |
| Persistent AF |
362 |
33 (9.1) |
1.582 (0.982–2.549) |
0.059 |
| New-onset AF |
137 |
5 (3.6) |
0.555 (0.214–1.439) |
0.226 |
HRs were adjusted for male sex, CHADS2 score, the use of ACEI or ARB, β-blockers, statins, and loop diuretics, and BNP divided by 100. Abbreviations as in Tables 1,2.
Discussion
We retrospectively investigated the prevalence, characteristics, and outcomes of HFpEF complicated with AF at enrollment, as well as the type of AF that occurred during follow-up.
The incidence of AF at enrollment was 41.4%. Previous reports of HFpEF have reported a prevalence of AF ranging from 19% in a randomized controlled trial5 to 65% in an observational study.6 The occurrence of AF has been associated with age and diastolic function, which is impaired with advanced age.7,8 Therefore, the prevalence of AF in HFpEF appears to increase with age.5,6,9,10
The incidence of new-onset AF during a median follow-up period of approximately 1,000 days in the present study was 13.9%. In contrast, the incidence of new-onset AF in a community-based study with a follow-up period of 3.7 years and in the TOPCAT trial, which examined the efficacy of spironolactone with a follow-up period of 3.1 years, was 31.6%11 and 5%,12 respectively. Compared with patients enrolled in randomized studies, patients registered in observational studies are generally more likely to be older and to have higher morbidity. Accordingly, the incidence of new AF may be higher in the present study than in randomized studies. To date, however, few reports have examined factors associated with the determinants of new-onset AF in patients with HFpEF. In their substudy of the TOPCAT trial, Neefs et al showed that spironolactone, ACEI, ARBs, and β-blockers were not associated with a reduced risk of new-onset AF.12 In contrast, in the present study we found that pulse (bradycardia), elevated hemoglobin, enlarged left ventricular end-diastolic dimension, and elevated BNP were associated with an increased risk of new-onset AF. Among cardiac factors, left ventricular overload and impaired sinus node function may have the potential to induce new-onset AF, whereas elevated hemoglobin was extracted as an extracardiac factor for new-onset AF. We occasionally experience elevated hemoglobin in patients with paroxysmal AF in clinical practice.13 One reason for this may be polyuria induced by excess secretion of atrial natriuretic peptide.14 Although no previous reports have described an association between elevated hemoglobin and new-onset AF, sleep apnea syndrome, which induces an increase hemoglobin levels due to hypoxia, could lead to this phenomenon. Further studies are needed to confirm this observation and clarify the mechanism of this relationship.
Findings to date on AF and outcomes in patients with HFpEF are inconsistent. AF was shown to confer a higher risk of all-cause death in some reports,6,11,15 but not in another study.16 In the present study, although we saw no significant difference in all-cause death or admissions for HF and myocardial infarction between AF at enrollment and sinus rhythm, AF at enrollment carried a higher risk of stroke than sinus rhythm. When we compared the cause of death between the 2 groups in the present study (Supplementary Table 1), we found no significant difference in cardiac deaths (2.8% vs 3.2%; P=0.797), but did find that non-cardiac deaths were significantly higher in patients with sinus rhythm than in those with AF (15.9% vs 12.6%; P=0.043). The higher rate of non-cardiac deaths in our patients with sinus rhythm may have been diminished by the difference in the risk of all-cause death between the 2 groups.
We further divided patients by type of rhythm during follow-up. Compared with sinus rhythm, used as the reference group, persistent AF was associated with a lower risk of all-cause death. We acknowledge that the study design prevents us from identifying any cause-effect relationship, and we are unable to propose a mechanism by which persistent AF was associated with a lower rate of all-cause deaths. Nevertheless, we speculate that one possibility is associated with the lower rate of non-cardiac deaths in patients with persistent AF compared with those with sinus rhythm (10.1% vs. 16.4%; P=0.002; Supplementary Table 3). A second possibility may be the recent progress made in the use of β-blockers to control heart rate, which has shown beneficial effects in patients with heart failure.17–20 This may have attenuated the risk of all-cause death in patients with AF vs. those with sinus rhythm. Indeed, there was no significant difference in cardiac deaths among the 4 groups (P=0.684). Conversely, patients with persistent AF and new-onset AF had a significantly higher risk of admission for HF. Again, although we are unable to propose a cause-and-effect relationship between new-onset AF and admission for HF, we propose 2 possibilities: first, new-onset AF could result in admission for HF; second, worsening of HF requiring admission could induce new-onset AF. Although the type of AF was not associated with an increased risk of stroke, the HR was >1.0 in paroxysmal and persistent AF but <1.0 in new-onset of AF, indicating that the risk of stroke may differ among types of AF. Importantly, we consider that these findings mandate the need for careful consideration and treatment of patients based on their individual risk according to type of AF. In addition, similar outcomes were observed when we excluded patients who underwent catheter ablation for AF during follow-up (data not shown).
The Japan Circulation Society guideline recommends dabigatran and apixaban for anticoagulation in patients with AF, and rivaroxaban, edoxiaban, and warfarin in patients with a CHADS2
score ≥1.21 In the present study, we included patients with heart failure, indicating a CHADS2
score ≥1. Despite the fact that the use of anticoagulation therapy during follow-up was relatively low (43.8%, 58.9%, and 7.9% in paroxysmal, persistent, and new-onset of AF, respectively; Supplementary Table 2), care in clinical practice settings should consider a prescription for anticoagulation in patients with HFpEF complicated with AF, if not contraindicated.
This study had several limitations. First, it was a retrospective analysis conducted at a single center, leading to the possibility of bias. Second, our definition of HFpEF differed from that in other studies: we defined HFpEF as both elevated BNP ≥100 pg/mL and an ejection fraction assessed by echocardiography of ≥50%. Other studies have defined HFpEF using the Framingham criteria,22,23 ICD-9 codes,24,25 physicians’ discretion,6,15 or an ejection fraction of ≥40%26,27 or 45%.12,24,25,28 This difference in the definition of HFpEF among studies must be recognized. Third, it may be possible that we missed paroxysmal AF in the sinus rhythm group. Indeed, 2.6% of patients in that group received anticoagulation at enrollment. Fourth, due to a lack of data on bleeding history, we did not calculate the ORBIT bleeding score.29 Fifth, we need to recognize the lower prescription rate of anticoagulant drugs than we expected during the study period in our study population. Therefore, physicians must exercise caution if they use our data in clinical practice.
In conclusion, this retrospective analysis of HFpEF showed that the prevalence of AF at enrollment and any type of AF during follow-up was relatively high, at 41.1% and 47.9%, respectively. New-onset AF was observed in 13.9% of patients. Factors associated with an increased risk of new-onset AF were pulse (bradycardia), elevated hemoglobin, enlarged left ventricular end-diastolic dimension, and elevated BNP. Although there was no significant difference in all-cause deaths between patients with AF at enrollment and those in sinus rhythm, the risk of all-cause death and admission for HF differed between the sinus rhythm group and different types of AF during the follow-up period. Therefore, physicians should approach the care of these patients based on individual risk according to type of AF.
Acknowledgments
The authors thank Taizo Murata (Osaka University Hospital) for extracting data from electrical medical charts, and Sugako Mitsuoka, Nagisa Yoshioka, Satomi Kishimoto, Kyoko Tatsumi, and Noriko Murakami (Osaka University Hospital) for their excellent assistance with data collection, data management, and help with manuscript preparation.
Sources of Funding
This research was conducted as investigator-initiated research with financial support provided by Bristol-Myers Squibb KK.
Disclosures
D.N. has received honoraria from Roche Diagnostics and grants from Bristol-Myers Squibb KK. Y. Sotomi has received honoraria from Daiichi-Sankyo, Bayer, Boehringer Ingelheim, and Bristol-Myers Squibb. H.M. has received personal fees from Daiichi Sankyo, Kowa, Bayer and Pfizer Pharmaceuticals, and grant from Terumo. S.H. has received personal fees from Daiichi Sankyo, Bayer, Bristol-Myers Squibb KK, and Boehringer Ingelheim Japan, and grants from Roche Diagnostics, FUJIFILM Toyama Chemical, and Actelion Pharmaceuticals. Y. Sakata has received personal fees from Otsuka Pharmaceutical, Ono Pharmaceutical, Daiichi Sankyo, Mitsubishi Tanabe Pharma, and Actelion Pharmaceuticals, and grants from Roche Diagnostic, FUJIFILM Toyama Chemical, Abbott Medical Japan, Otsuka Pharmaceutical, Daiichi Sankyo, Mitsubishi Tanabe Pharma, and Biotronik. The remaining authors have no conflicts of interest to disclose.
IRB Information
This study was approved by the Osaka University Research Ethics Committee (Reference no. 16515).
Supplementary Files
Please find supplementary file(s);
http://dx.doi.org/10.1253/circrep.CR-22-0006
References
- 1.
Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation: Vicious twins. J Am Coll Cardiol 2016; 68: 2217–2228.
- 2.
Kitaoka H, Tsutsui H, Kubo T, Ide T, Chikamori T, Fukuda K, et al. JCS/JHFS 2018 guideline on the diagnosis and treatment of cardiomyopathies. Circ J 2021; 85: 1590–1689.
- 3.
Vellinga A, Cormican M, Hanahoe B, Bennett K, Murphy AW. Opt-out as an acceptable method of obtaining consent in medical research: A short report. BMC Med Res Methodol 2011; 11: 40.
- 4.
Dries DL, Exner DV, Gersh BJ, Domanski MJ, Waclawiw MA, Stevenson LW. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: A retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J Am Coll Cardiol 1998; 32: 695–703.
- 5.
Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJ, et al. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: Results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol 2006; 47: 1997–2004.
- 6.
Sartipy U, Dahlström U, Fu M, Lund LH. Atrial fibrillation in heart failure with preserved, mid-range, and reduced ejection fraction. JACC Heart Fail 2017; 5: 565–574.
- 7.
Tsang TS, Gersh BJ, Appleton CP, Tajik AJ, Barnes ME, Bailey KR, et al. Left ventricular diastolic dysfunction as a predictor of the first diagnosed nonvalvular atrial fibrillation in 840 elderly men and women. J Am Coll Cardiol 2002; 40: 1636–1644.
- 8.
Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Risks for atrial fibrillation and congestive heart failure in patients ≥65 years of age with abnormal left ventricular diastolic relaxation. Am J Cardiol 2004; 93: 54–58.
- 9.
Reddy YNV, Obokata M, Gersh BJ, Borlaug BA. High prevalence of occult heart failure with preserved ejection fraction among patients with atrial fibrillation and dyspnea. Circulation 2018; 137: 534–535.
- 10.
Lam CSP, Rienstra M, Tay WT, Liu LCY, Hummel YM, van der Meer P, et al. Atrial fibrillation in heart failure with preserved ejection fraction: Association with exercise capacity, left ventricular filling pressures, natriuretic peptides, and left atrial volume. JACC Heart Fail 2017; 5: 92–98.
- 11.
Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: A community-based study. Circulation 2013; 128: 1085–1093.
- 12.
Neefs J, van den Berg NWE, Krul SPJ, Boekholdt SM, de Groot JR. Effect of spironolactone on atrial fibrillation in patients with heart failure with preserved ejection fraction: Post-hoc analysis of the randomized, placebo-controlled TOPCAT trial. Am J Cardiovasc Drugs 2020; 20: 73–80.
- 13.
Imataka K, Nakaoka H, Kitahara Y, Fujii J, Ishibashi M, Yamaji T. Blood hematocrit changes during paroxysmal atrial fibrillation. Am J Cardiol 1987; 59: 172–173.
- 14.
Nakaoka H, Imataka K, Kitahara Y, Fujii J, Ishibashi M, Yamaji T, et al. Plasma levels of atrial natriuretic factor in patients with cardiac arrhythmias and congestive heart failure. J Cardiovasc Pharmacol 1986; 8: 1297.
- 15.
Sartipy U, Savarese G, Dahlström U, Fu M, Lund LH. Association of heart rate with mortality in sinus rhythm and atrial fibrillation in heart failure with preserved ejection fraction. Eur J Heart Fail 2019; 21: 471–479.
- 16.
Rusinaru D, Leborgne L, Peltier M, Tribouilloy C. Effect of atrial fibrillation on long-term survival in patients hospitalised for heart failure with preserved ejection fraction. Eur J Heart Fail 2008; 10: 566–572.
- 17.
Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996; 334: 1349–1355.
- 18.
CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): A randomised trial. Lancet 1999; 353: 9–13.
- 19.
MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353: 2001–2007.
- 20.
Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344: 1651–1658.
- 21.
JCS Joint Working Group. Guidelines for pharmacotherapy of atrial fibrillation (JCS 2013). Circ J 2014; 78: 1997–2021.
- 22.
Satomura H, Wada H, Sakakura K, Kubo N, Ikeda N, Sugawara Y, et al. Congestive heart failure in the elderly: Comparison between reduced ejection fraction and preserved ejection fraction. J Cardiol 2012; 59: 215–219.
- 23.
Yanagihara K, Kinugasa Y, Sugihara S, Hirai M, Yamada K, Ishida K, et al. Discharge use of carvedilol is associated with higher survival in Japanese elderly patients with heart failure regardless of left ventricular ejection fraction. J Cardiovasc Pharmacol 2013; 62: 485–490.
- 24.
Stein GY, Kremer A, Shochat T, Bental T, Korenfeld R, Abramson E, et al. The diversity of heart failure in a hospitalized population: The role of age. J Card Fail 2012; 18: 645–653.
- 25.
Stein GY, Ben-Gal T, Kremer A, Bental T, Alon D, Korenfeld R, et al. Gender-related differences in hospitalized heart failure patients. Eur J Heart Fail 2013; 15: 734–741.
- 26.
Mangla A, Kane J, Beaty E, Richardson D, Powell LH, Calvin JE Jr. Comparison of predictors of heart failure-related hospitalization or death in patients with versus without preserved left ventricular ejection fraction. Am J Cardiol 2013; 112: 1907–1912.
- 27.
Vullaganti S, Goldsmith J, Teruya S, Alvarez J, Helmke S, Maurer MS. Cardiovascular effects of hemoglobin response in patients receiving epoetin alfa and oral iron in heart failure with a preserved ejection fraction. J Geriatr Cardiol 2014; 11: 100–105.
- 28.
Adabag S, Smith LG, Anand IS, Berger AK, Luepker RV. Sudden cardiac death in heart failure patients with preserved ejection fraction. J Card Fail 2012; 18: 749–754.
- 29.
O’Brien EC, Simon DN, Thomas LE, Hylek EM, Gersh BJ, Ansell JE, et al. The ORBIT bleeding score: A simple bedside score to assess bleeding risk in atrial fibrillation. Eur Heart J 2015; 36: 3258–3264.




