2022 年 4 巻 6 号 p. 264-273
2022 年 4 巻 6 号 p. 264-273
Background: Although cardiac resynchronization therapy (CRT) is effective for patients with chronic heart failure (CHF) with reduced left ventricular ejection fraction and wide QRS (≥120 ms), data on the use of or long-term outcomes after CRT implantation in Japan are limited.
Methods and Results: We examined proper CRT utilization and outcomes in 3,447 consecutive symptomatic CHF patients registered in the CHART-2 Study. We identified 167 potentially eligible patients and divided them into 4 groups according to the presence (+) or absence (−) of an indication for and implantation of CRT: Group A (reference group), (+)indication/(+)CRT; Group B, (+)indication/(−)CRT; Group C, (−)indication/(+)CRT; and Group D, (−)indication/(−)CRT. Based on the Japanese Circulation Society guidelines, 91 patients met the eligibility for CRT implantation, with 43 (47%) of them undergoing CRT implantation. After adjusting for confounders, age was significantly associated with no CRT use (odds ratio per 5-year increase 1.46; 95% confidence interval 1.11–2.05; P=0.012). Among the 4 groups, the cumulative incidence of cardiovascular death and CHF admission were highest in Group B and lowest in Group D (P=0.029).
Conclusions: In this study, only half the eligible CHF patients properly received CRT. Aging was a significant risk factor for no CRT use. Patients without CRT despite having an indication could be at higher risk of mortality and CHF admission.
Device therapy, including implantable cardioverter defibrillator (ICD) and cardiac resynchronization therapy (CRT), has been shown to improve morbidity and mortality in patients with heart failure with reduced ejection fraction (HFrEF).1,2 In the ASIAN-HF [Asian Sudden Cardiac Death in Heart Failure] registry, an observational study with 5,276 HF patients from 11 Asian regions and across 3 income regions, prophylactic ICD utilization was approximately 55%.3 In our previous study, prophylactic ICD utilization for patients with Class I or IIa indications in the Japanese Circulation Society (JCS) guidelines was 37%.4 These studies demonstrate that fewer HF patients were referred for these device therapies despite the recommendations in the guidelines. The number of patients with chronic heart failure (CHF) has been rapidly increasing globally,5,6 and QRS prolongation (i.e., QRS duration >120 ms) and/or left bundle branch block (LBBB) is present in 24–47% of HFrEF patients.7 Thus, the utilization rate of CRT devices, as an established therapy for HFrEF, would be expected to increase linearly with the increasing number of CHF patients. However, recent studies in European countries have shown that the utilization rate of CRT for eligible CHF patients was only approximately 30%.8,9 In Japan, although the number of CHF patients has been increasing, as in the Western countries,10,11 there has been no change in the number of new implantations or replacements of CRT.12 In Japan, limited data are available on CRT utilization and long-term outcomes after CRT implantation in CHF patients. Thus, the aims of the present study were to clarify the utilization of CRT and evaluate the efficacy of CRT among Japanese heart failure (HF) patients registered in the CHART-2 [Chronic Heart Failure Analysis and Registry in the Tohoku District-2] Study, one of the largest observational CHF studies in Japan.13
The CHART-2 study is a multicenter prospective observational cohort study in Japan.13 The study was approved by the local ethics committees in the 24 participating hospitals and informed consent was obtained from all patients (ClinicalTrials.gov identifier: NCT00418041). This study was conducted according to the principles outlined in the Declaration of Helsinki. The details of the CHART-2 study have been reported in detail elsewhere.13–16 Briefly, patients aged ≥20 years with either significant coronary artery disease or HF Stages B, C, or D as defined by the American College of Cardiology/American Heart Association guidelines17 were enrolled. Patient enrollment started in October 2006 and ended in March 2010. In all, 10,219 patients were enrolled at Tohoku University and 23 affiliated hospitals.
According to the revised JCS guidelines, which changed substantially from 2006 to 2011, we chose the year 2015 to assess the appropriate CRT implantation rate based on the assumption that it would take time for physicians to become familiar with the new guidelines. We identified 3,447 patients with Stage C/D HF who were alive on January 1, 2015 (Figure 1).
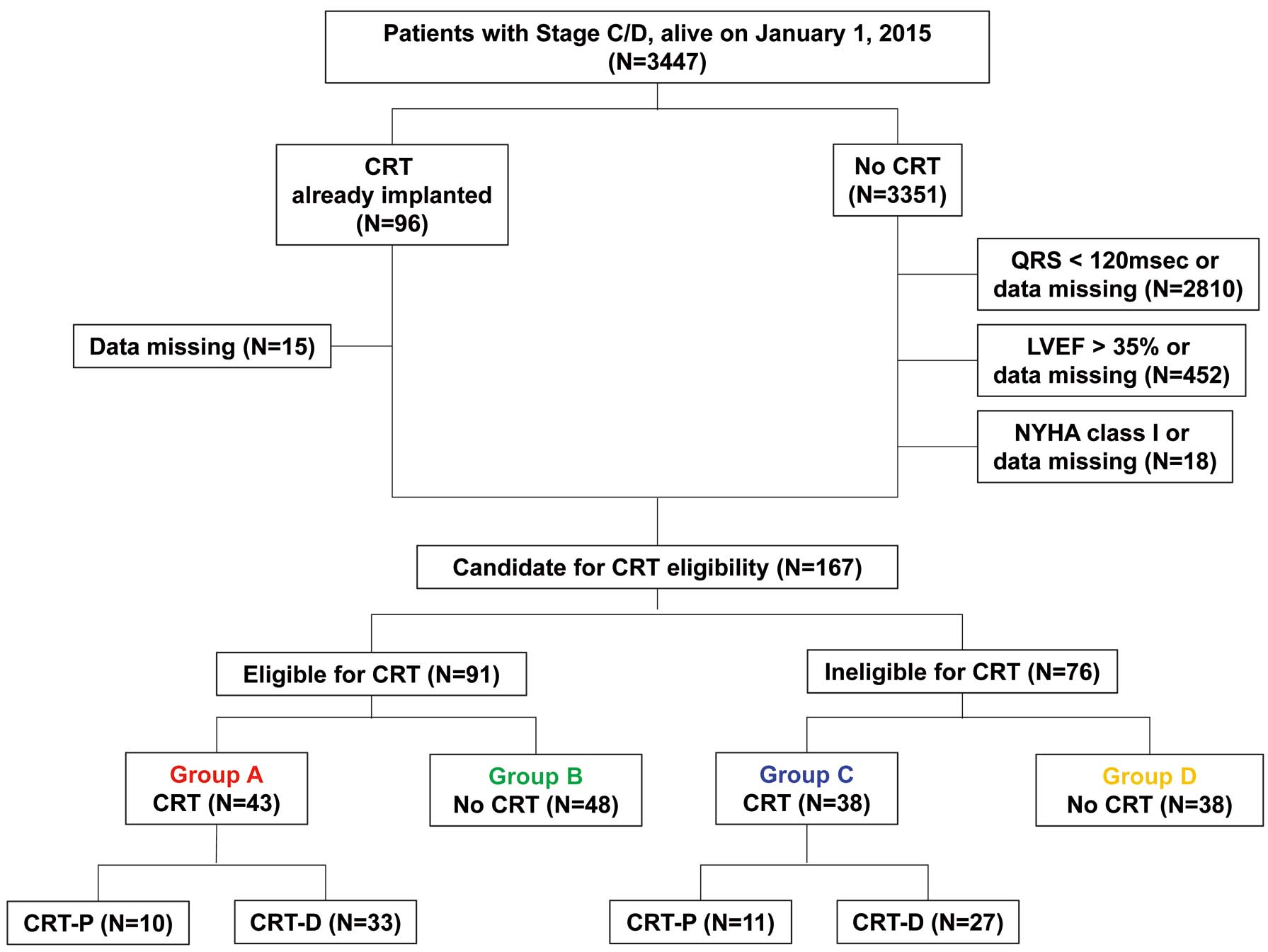
Flow chart of patient selection. CRT, cardiac resynchronization therapy; CRT-D, CRT defibrillator; CRT-P, CRT pacemaker; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
LVEF was evaluated once a year. In the present study, in order to compare baseline patient characteristics at the time when CRT implantation was considered, LVEF and laboratory data in 2015 were used for patients without CRT implantation, whereas LVEF and laboratory data before CRT implantation were used for those with CRT. The difference of the time from baseline to the onset of HF between groups with and without CRT was not clear, but the observation period was started from 2015 in both groups. Patients with symptomatic CHF (New York Heart Association [NYHA] Class II–IV) were enrolled. Then, CHF patients with a QRS duration <120 ms or missing data, those who had an left ventricular ejection fraction (LVEF) >35% or missing LVEF data based on the JCS guidelines,18,19 or those in NYHA Class I or with missing NYHA class data were excluded. This left 167 patients who were evaluated for CRT eligibility, with 91 meeting the eligibility criteria for CRT implantation based on the JCS guidelines.18,19 After assessing CRT eligibility, we divided the 167 patients into the following 4 groups according to the presence (+) or absence (−) of an indication for and implantation of CRT (Figure 1): Group A (reference), (+)indication/(+)CRT; Group B, (+)indication/(−)CRT; Group C, (−)indication/(+)CRT; Group D, (−)indication/(−)CRT. The indications for CRT implantation were based on the 2006 or 2011 JCS guidelines on non-pharmacotherapy of cardiac arrhythmias (Table 1).18,19 The differences between the 2006 and 2011 JCS guidelines were as follows: minimum QRS duration was shortened from 130 to 120 ms, the indication for CRT changed depending on heart rhythm, and patients with NYHA Class II, LVEF ≤30%, and QRS duration ≥150 ms were included in the indications for CRT implantation.
| 2006 JCS guidelines18 | 2011 JCS guidelines19 | ||
|---|---|---|---|
| NYHA Class III or IV | NYHA Class III or IV | NYHA Class II | |
| Class I | LVEF ≤35% QRS ≥130 ms and conduction delay |
LVEF ≤35% QRS ≥120 ms Sinus rhythm |
|
| LVEF ≤35% Pacing for bradycardia |
|||
| Class IIa | LVEF ≤35% Ventricular pacing |
LVEF ≤35% QRS ≥120 ms Atrial fibrillation |
LVEF ≤30% QRS ≥150 ms Sinus rhythm |
| LVEF ≤35% ICD implanted |
|||
| Year of CRT implantation or evaluation of indication |
CRT implanted before 2006 |
CRT implanted between 2012 and 2015 | |
| CRT implanted between 2007 and 2011 |
No CRT implantation | ||
CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; JCS, Japanese Circulation Society; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
The study outcomes included all-cause death, cardiovascular (CV) death, HF death, and CHF admission.13 For studying outcomes, we standardized the patient observation period to begin in 2015. CHF admission was defined as hospitalization for HF mainly for its treatment or when HF was a major reason for admission. A patient admitted for this reason had to show signs and symptoms of worsening HF and require treatment with intravenous diuretics. Evidence of worsening HF had to include at least one of the following components: increasing dyspnea on exertion, orthopnea, nocturnal dyspnea, pulmonary edema, increasing peripheral edema, increasing fatigue, or decreasing exercise tolerance; worsening renal function, raised jugular venous pressure, and radiological signs of CHF; and additional treatment for CHF, such as administration of inotropic drugs including phosphodiesterase III inhibitors, vasodilator, renin-angiotensin-aldosterone inhibitors, diuretics, or hemodialysis.
Statistical AnalysisContinuous variables are expressed as the mean±SD or as the median with interquartile range, as appropriate, and were compared using Welch’s t-test. Categorical variables are expressed as numerals with percentages and were compared by Fisher’s t-test.
The incidences of all-cause death, CV death, HF death, and CHF admission were estimated using Kaplan-Meier curves with the log-rank test. The incidence rate per 1,000 person-years was compared with the exact binominal test. Relative risks for incidences in Groups B, C, and D compared with Group A (reference) were examined using univariable Cox proportional hazard model analysis.
To determine the predictors for no CRT use, we used univariable and multivariable logistic regression models for each characteristic. A recent study showed that reverse remodeling of the left ventricle by CRT was maintained later after CRT implantation,20 suggesting that eligible patients benefit from early CRT implantation. Thus, we performed detailed analysis of the relationship between the timing of CRT implantation and long-term prognosis stratified by the median time from enrollment to implantation (median time 1.79 years).
Statistical analyses were performed using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria). Two-sided P<0.05 was considered statistically significant.
Among the 3,447 CHF patients who were alive on January 1, 2015, 81 (2.3%) had undergone CRT implantation. According to the JCS guidelines,18,19 91 (2.6%) had Class I or IIa indications for CRT, but only 43 received CRT (Group A; Figure 1). In contrast, despite no indication, 38 had received CRT implantation (overutilization; Group C). The clinical characteristics of the 167 patients are presented in Table 2. Patients in Groups A and C, who received CRT, were significantly younger and had lower systolic blood pressure, higher B-type natriuretic peptide (BNP) concentrations and a higher frequency of a history of ventricular tachycardia. Patients in Group A were more likely to be women and had a longer QRS duration and a higher rate of LBBB. When comparing QRS duration normalized to body surface area between men and women, women had significantly QRS longer duration than men in each group (Supplementary Table). Among the 4 groups, there were no significant differences in systolic or diastolic left ventricular (LV) diameter, but patients with a CRT indication (Groups A and B) had lower LVEF than those without a CRT indication (Groups C and D). The most common etiology of CHF was dilated cardiomyopathy (DCM) in Group A and ischemic heart diseases in the other 3 groups. With regard to medications, patients without CRT implantation were more likely to be taking statins. However, there was no significant difference in the use of β-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or mineralocorticoid receptor antagonists. A history of atrial fibrillation was more common among patients in Groups B and C.
| Group | Group A (n=43) |
Group B (n=48) |
Group C (n=38) |
Group D (n=38) |
P value |
|---|---|---|---|---|---|
| Age | 65.0±12.6 | 72.0±11.0 | 60.4±13.9 | 70.7±10.2 | <0.001 |
| Female sex | 24 (55.8) | 8 (16.7) | 9 (23.7) | 7 (18.4) | <0.001 |
| Systolic BP (mmHg) | 107±19 | 110±16 | 110±20 | 121±17 | 0.007 |
| Diastolic BP (mmHg) | 65±10 | 67±12 | 65±14 | 69±10 | 0.320 |
| BSA (m2) | 1.6±0.2 | 1.7±0.2 | 1.7±0.2 | 1.7±0.2 | 0.002 |
| QRS (ms) | 174±22 | 150±25 | 150±33 | 142±17 | <0.001 |
| LBBB | 28 (65.1) | 14 (29.2) | 11 (28.9) | 16 (42.1) | 0.003 |
| LVDd (mm) | 67.4±9.4 | 63.8±7.9 | 66.2±9.9 | 65.5±8.5 | 0.292 |
| LVDs (mm) | 59.3±9.0 | 55.3±8.2 | 54.8±11.8 | 54.5±10.2 | 0.09 |
| LVEF (%) | 24.8±6.6 | 28.6±6.0 | 33.8±11.3 | 31.4±9.5 | <0.001 |
| Hb (g/dL) | 12.9±1.7 | 13.5±1.9 | 13.0±1.9 | 13.8±1.3 | 0.075 |
| UA (mg/dL) | 6.8±2.3 | 6.7±1.7 | 8.2±2.0 | 6.4±1.5 | <0.001 |
| HDL-C (mg/dL) | 48.9±14.1 | 51.8±15.0 | 47.9±19.1 | 51.1±13.4 | 0.682 |
| LDL-C (mg/dL) | 105.0±24.1 | 98.4±26.6 | 106.8±31.5 | 96.2±26.6 | 0.311 |
| BUN (mg/dL) | 23 [15–29.8] | 21 [14–25.8] | 22 [15–30] | 18.1 [13.9–22.5] | 0.115 |
| Cre (mg/dL) | 1.0 [0.7–1.3] | 1.1 [0.9–1.6] | 1.1 [0.9–1.4] | 1.0 [0.8–1.2] | 0.377 |
| BNP (pg/mL) | 481 [252–1,006] | 287 [112–472] | 413 [180–728] | 99 [62–192] | <0.001 |
| HF etiology | |||||
| Ischemic heart disease | 12 (27.9) | 23 (47.9) | 12 (31.6) | 20 (52.6) | 0.055 |
| Hypertensive heart disease | 0 (0) | 1 (2.1) | 0 (0) | 1 (2.6) | 0.854 |
| Valvular heart disease | 2 (4.7) | 2 (4.2) | 0 (0) | 3 (7.9) | 0.452 |
| Dilated cardiomyopathy | 18 (41.9) | 14 (29.2) | 10 (26.3) | 8 (21.1) | 0.212 |
| Hypertrophic cardiomyopathy | 1 (2.3) | 0 (0) | 3 (7.9) | 0 (0) | 0.061 |
| Comorbidity | |||||
| Hypertension | 36 (87.8) | 46 (97.9) | 32 (88.9) | 36 (94.7) | 0.228 |
| Diabetes | 19 (44.2) | 21 (43.8) | 18 (47.4) | 24 (63.2) | 0.263 |
| Dyslipidemia | 40 (97.6) | 47 (100) | 36 (100) | 37 (97.4) | 0.583 |
| Hyperuricemia | 35 (85.4) | 45 (95.7) | 34 (94.4) | 27 (71.1) | 0.007 |
| Atrial fibrillation | 7 (17.1) | 12 (25.5) | 8 (22.2) | 7 (18.4) | 0.047 |
| Ventricular tachycardia | 13 (31.7) | 12 (25.5) | 11 (30.6) | 9 (23.7) | 0.001 |
| Stroke | 11 (26.8) | 8 (17) | 5 (13.9) | 4 (10.5) | 0.292 |
| Cancer | 4 (9.8) | 7 (14.9) | 4 (11.1) | 8 (21.1) | 0.528 |
| Medication | |||||
| β-blocker | 40 (93) | 40 (83.3) | 32 (84.2) | 33 (86.8) | 0.529 |
| ACE inhibitor | 23 (53.5) | 26 (54.2) | 24 (63.2) | 20 (52.6) | 0.779 |
| ARB | 19 (44.2) | 13 (27.1) | 15 (39.5) | 16 (42.1) | 0.319 |
| MRA | 23 (53.5) | 21 (43.8) | 23 (60.5) | 21 (55.3) | 0.472 |
| Statin | 16 (37.2) | 27 (56.2) | 11 (28.9) | 26 (68.4) | <0.001 |
| CCB | 6 (14) | 10 (20.8) | 7 (18.4) | 6 (15.8) | 0.856 |
| Diuretics | 38 (88.4) | 37 (77.1) | 31 (81.6) | 28 (73.7) | 0.359 |
Unless indicated otherwise, data are given as the mean±SD, median [interquartile range], or n (%). ARB, angiotensin II receptor blocker; BNP, B-type natriuretic peptide; BP, blood pressure; BSA, body surface area; BUN, blood urea nitrogen; CCB, calcium channel blocker; Cre, creatinine; Group A (reference group), cardiac resynchronization therapy (CRT) indication and implantation; Group B, CRT indication, no CRT implantation; Group C, no indication but CRT implantation; Group D, no indication and no CRT implantation; Hb, hemoglobin; HDL-C, high-density lipoprotein cholesterol; HF, heart failure; LBBB, left bundle branch block; LDL-C, low-density lipoprotein cholesterol; LVDd, left ventricular end-diastolic diameter; LVDs, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; UA, uric acid.
The median time from enrollment to CRT implantation was 1.79 years. CRT utilization increased in the early study period and then decreased. Among patients with CRT, more than half were implanted between 2007 and 2011 based on the 2006 JCS guidelines (Figure 2).18
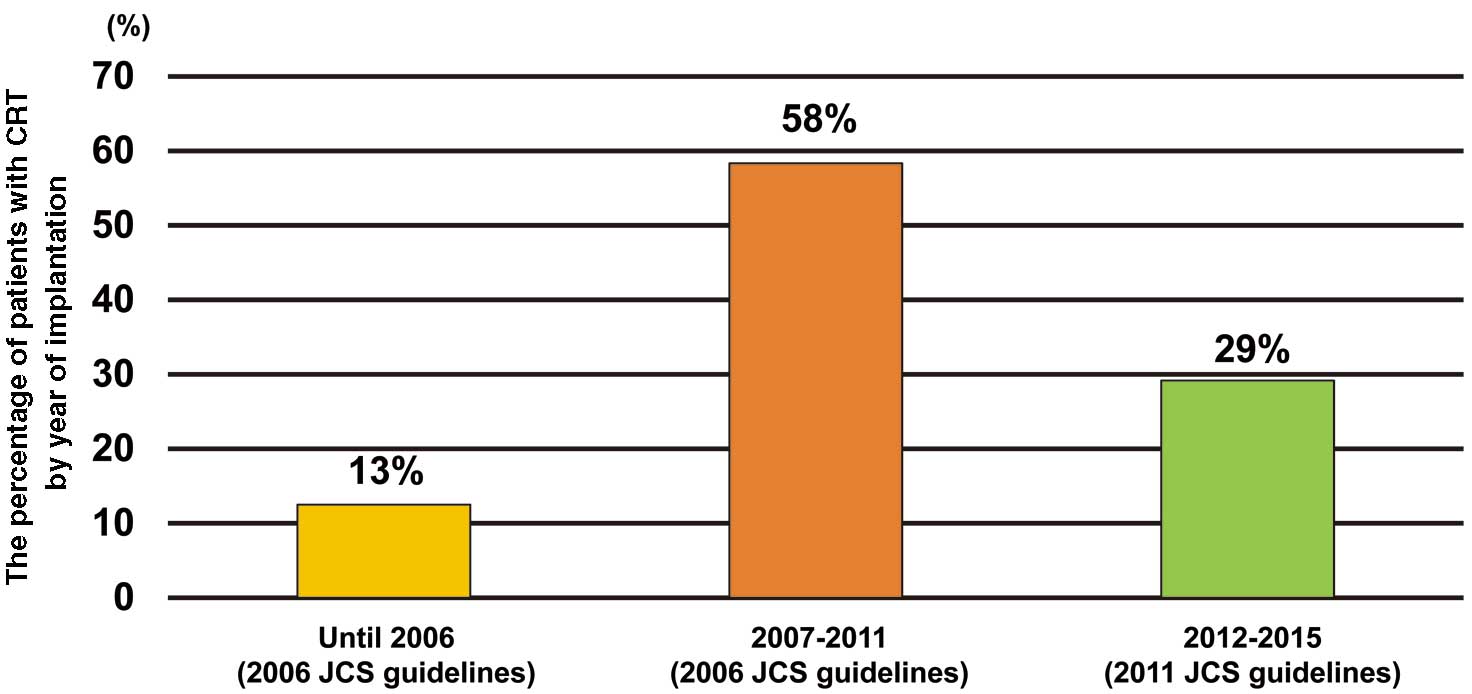
Table 3 presents the results of the multivariable logistic model for no CRT use among patients with a CRT indication. Age (per 5-year increase) was significantly associated with no CRT use (odds ratio [OR] 1.46; 95% confidence interval [CI] 1.11–2.05; P=0.012). LVEF (per 1% increase) was also associated with no CRT use (OR 1.11; 95% CI 1.00–1.25; P=0.069). In contrast, female sex (OR 0.10; 95% CI 0.02–0.40; P=0.002), QRS duration (per 10 ms; OR 0.57; 95% CI 0.41–0.75; P<0.001), DCM (OR 0.25; 95% CI 0.06–0.94; P=0.049), and LBBB (OR 0.23; 95% CI 0.06–0.82; P=0.027) were significantly associated with the use of CRT.
| Variables | OR | 95% CI | P value |
|---|---|---|---|
| Age (/5 years) | 1.47 | 1.11–2.05 | 0.013 |
| LVEF | 1.11 | 1.00–1.25 | 0.069 |
| QRS (/10 ms) | 0.57 | 0.41–0.75 | <0.001 |
| Female sex | 0.10 | 0.02–0.40 | 0.002 |
| DCM | 0.25 | 0.06–0.94 | 0.049 |
| LBBB | 0.23 | 0.06–0.82 | 0.027 |
The odds ratios (ORs) were adjusted by age (per 5 years), systolic blood pressure, left ventricular end-diastolic dimension, left ventricular ejection fraction (LVEF), B-type natriuretic peptide, QRS (per 10 ms), sex, history of heart failure admission, ischemic heart disease, hypertensive heart disease, valvular heart disease, dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy, left bundle branch block (LBBB), New York Heart Association functional class, atrial fibrillation, and ventricular tachycardia. CI, confidence interval.
During the median follow-up period of 3.6 years, there was no significant difference in the incidence of all-cause death among the 4 groups (Figure 3A). However, the cumulative incidence of CV death and CHF admission, as well as that of HF death and CHF admission, was significantly higher in Group B ((+)indication /(−)CRT; both P=0.029; Figure 3B,C). The incidence rate of all endpoints tended to be lower in Group A ((+)indication/(+)CRT) than in Group B, and higher in Group C ((−)indication/(+)CRT) than in Group D ((−)indication/(−)CRT). In particular, Group C showed a rapid increase in the incidence rate during the early follow-up period compared with the other 3 groups (Figure 3). Compared with Group A, the hazard ratios (HR) for all-cause death in Groups C and D were 0.72 (95% CI 0.34–1.54) and 0.66 (95% CI 0.31–1.41), respectively. However, Group B had a higher HR for all-cause death (1.24; 95% CI 0.66–2.33) than Group A. A similar trend was noted for the HR of the composite endpoints (Figure 4).
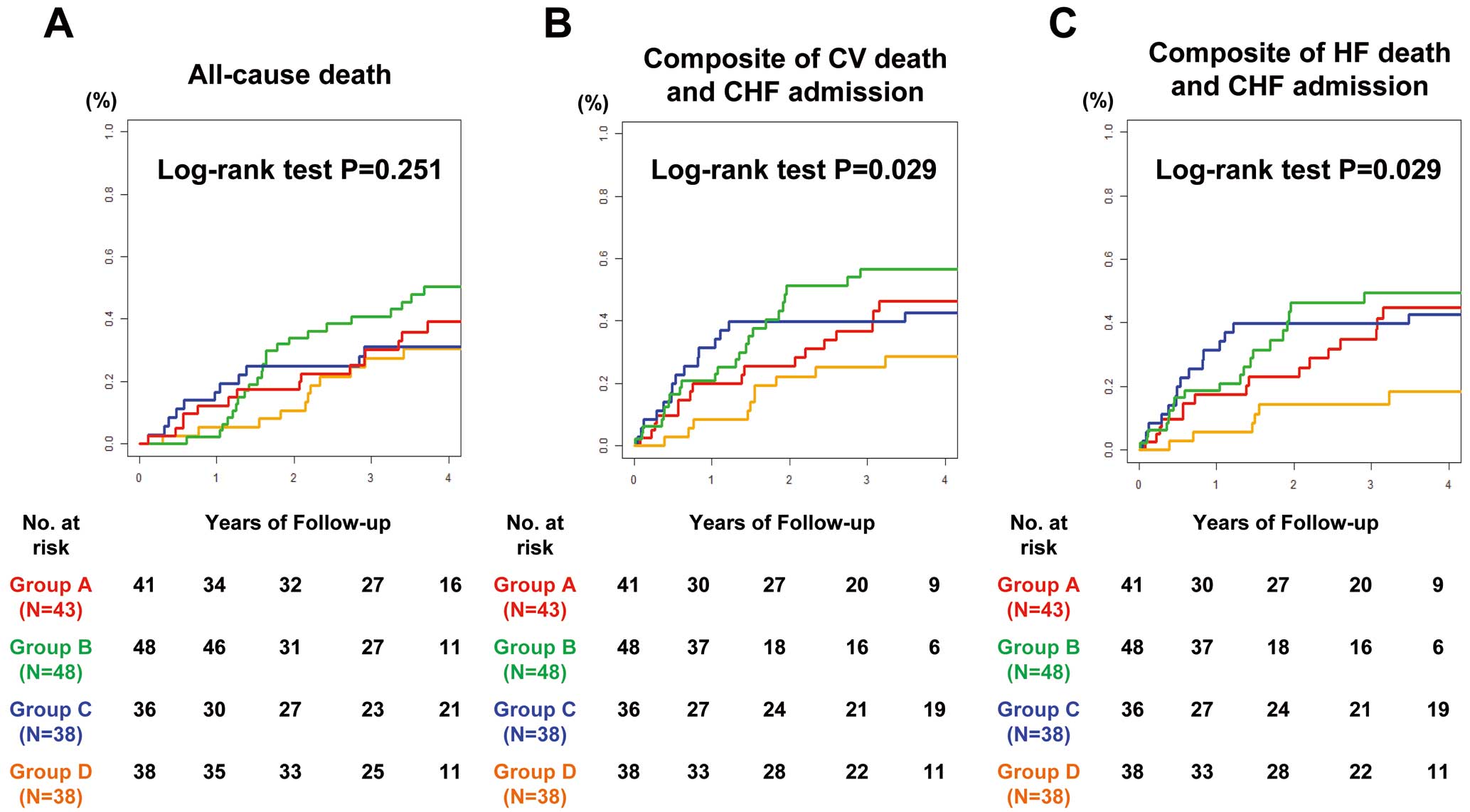
Cumulative incidence of (A) all-cause death, (B) the composite of cardiovascular (CV) death and chronic heart failure (CHF) admission, and (C) the composite of heart failure (HF) death and CHF admission. Group A (reference group), cardiac resynchronization therapy (CRT) indication and implantation; Group B, CRT indication, no CRT implantation; Group C, no indication but CRT implantation; Group D, no indication and no CRT implantation.
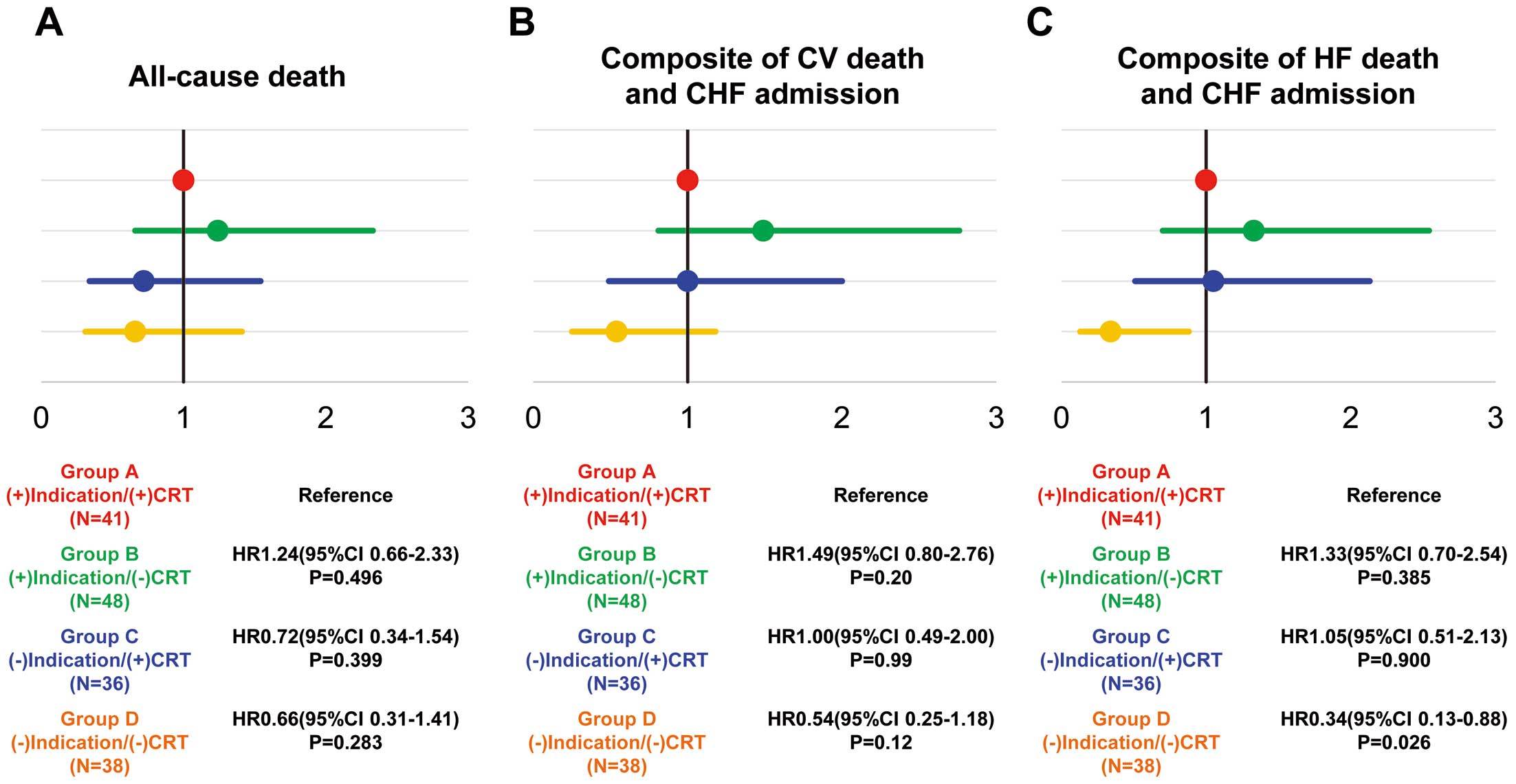
Relative risks of (A) all-cause death, (B) the composite of cardiovascular (CV) death and chronic heart failure (CHF) admission, and (C) the composite of heart failure (HF) death and CHF admission. CRT, cardiac resynchronization therapy.
Among patients with an indication for CRT, there were no significant differences in the incidence rate of all-cause death, the composite of CV death+CHF admission, or the composite of HF death+CHF admission between those with and without CRT implantation (Group A vs. Group B; Figure 4). However, the incidence of each of these tended to be higher in patients without CRT implantation (Group B; Figure 5A–C). When stratified by timing of CRT implantation (median 1.79 years), earlier CRT implantation was associated with better outcomes than late CRT (Figure 5D–F).

(A–C) Event rates (/1,000 person-years) in Groups A and B for all-cause Death (A), the Composite of cardiovascular (CV) death and chronic heart failure (CHF) admission (B), and the composite of heart failure (HF) death and CHF admission (C). (D–F) Event rates by timing of cardiac resynchronization therapy (CRT) implantation in Groups A and B for all-cause Death (D), the composite of CV death and CHF admission (E) and the composite of HF death and CHF admission (F).
The major findings of the present CHART-2 study, one of the largest CHF cohort studies in Japan, are as follows. First, less than half the patients with an indication for CRT according to the JCS guidelines received CRT. Second, aging was a significant factor for no CRT use. Third, among the 4 groups, there were significant differences in the composite of CV death and CHF admission and the composite of HF death and CHF admission. Furthermore, earlier CRT implantation was associated with better outcomes than late CRT implantation.
Implantation Rate of CRTRecent studies have reported that the number of CHF patients has been rapidly increasing.5,6,10 In Japan, although CRT defibrillator (CRT-D) device implantation for primary prevention and CRT pacemaker (CRT-P) implantation increased up to 2011, the number of implantations has gradually decreased, although CRT-P implantation remained unchanged between 2011 and 2014.21 Similar trends have been noted in other countries. Indeed, the implantation rate from the Swedish Heart Failure Registry, which contained patients registered between 2000 and 2012, was only 21% of eligible patients.8 These results suggest that proper utilization of CRT is not performed despite the recent increase in CHF patients. Many studies indicate that CRT is still underused, with considerable heterogeneity in its implementation.8,22–24 It also should be noted that elderly CHF patients are generally under-represented in clinical trials aiming to evaluate the efficacy of CRT.25
In Japan, although there have been several multi-institutional prospective cohort studies with HF patients,26,27 few data are available regarding CRT utilization in clinical practice. In our CHART-2 study, the diagnosis of CHF was made by attending cardiologists and all patient information, including demographic data, medical history, and laboratory and echocardiography data, was recorded once a year. The mean follow-up period was 8.5 years, which was longer than that of the CHF cohort studies, whereas the mean age (68.3±0.3 years), percentage of men (69.6%), and percentage of patients with ischemic heart disease (56.3%) were similar to those in other CHF studies.8,22–24 Thus, the present findings are important because they demonstrate the current status of CRT utilization and factors against the use of CRT.
Factors Involved in the Underuse of CRTIn the present study, the CRT implantation rate was 47% of eligible patients, which is similar to implantation rates in other studies, namely 42.5% (Netherlands),22 38.8% (US),23 and 26.3% (US).24 In addition, more than half the CRT implantations were performed between 2007 and 2011 based on the 2006 JCS guidelines, suggesting that the recent CRT implantation rate in Japan is not as high as we expected. Because less than half the CHF patients with an indication for CRT in the present study underwent appropriate CRT implantations, we thought it necessary to assess factors preventing the use of CRT. We found that an important factor associated with CRT underuse was age. Indeed, several studies have reported that age is one of the relative risks associated with no CRT use.8,23 Elderly people may be reluctant to undergo CRT implantation, but a recent US registry showed that the beneficial impact of CRT on survival did not differ regardless of age, even in those >80 years of age.28 Moreover, Kron et al reported that patients aged ≥75 years with CRT implantation had improvements in NYHA functional class and LVEF,25,29 and another study reported that the degree of reduction in HF or death in elderly patients was similar to that in younger patients.30 These previous studies suggest that CRT implantation should be considered for elderly patients who meet the indication criteria.
Conversely, in the present study, QRS duration, LBBB, DCM, and female sex were significantly associated with CRT implantation. Because the primary role of CRT is to correct LV dyssynchrony, factors related to LV conduction disturbance, such as long QRS duration and LBBB, could be preferred for the selection of CRT candidates.31–33 In fact, from the American College of Cardiology Foundation/American Heart Association focused update in 2012 and the European Society of Cardiology guidelines in 2012,34,35 more detailed requirements for QRS duration and LBBB have been included in the indications for CRT. The JCS guidelines have also followed and adopted more detailed indications since 2018.36 These guidelines have helped us select more eligible patients for CRT implantation. Patients with non-ischemic cardiomyopathy, such as DCM, experienced greater improvement in LV systolic function and reverse remodeling and tended to show better clinical outcomes than patients with ischemic cardiomyopathy.37 In particular, when candidates for CRT have scars or ischemic lesions on the LV lateral wall, where the LV lead is usually placed, LV reverse remodeling and functional benefit by CRT could be severely impaired.38
In line with the present study, it has been reported that female patients, especially those with non-ischemic cardiomyopathy, had an overall survival advantage and achieved a greater reduction in LV volume and improvement in LVEF than male patients after CRT implantation.39,40 Possible reasons for the greater beneficial effects of CRT in female patients could be: (1) because normal QRS duration values are 5–10 ms shorter in women, for any given QRS duration, female patients may have relatively greater LV conduction disturbance and dyssynchrony, which would be favorable for CRT;41 and (2) in the present study, all patients were treated by cardiologists, who may decide on CRT implantation on the basis of the information detailed above, possibly resulting in more CRT implantations in female patients. Indeed, in the present study, QRS duration normalized to body surface area was longer in female than male patients.
Prognostic Implications of CRTCRT has been shown to reduce morbidity and mortality in symptomatic CHF patients with LV systolic dysfunction and wide QRS.42,43 From the American Heart Association’s Get With The Guidelines-Heart Failure (GWTG-HF) program, patients with CRT implanted during the index hospitalization had lower mortality (adjusted HR 0.65; 95% CI 0.59–0.71) and were less likely to be readmitted for HF than those without CRT (adjusted HR 0.64; 95% CI 0.58–0.71).24 In the present study, the cumulative incidence of CV death, HF death, and CHF admission were significantly higher in patients with a CRT indication but without CRT implantation (Group B) than in the other 3 groups. Among patients with a CRT indication, there was no significant difference in the incidence rate of all-cause death, CV death, HF death, or CHF admission between those with and without CRT implantation (Groups A and B). However, the relative HRs in patients without CRT implantation (Group B) were higher than in those with CRT (Group A) for all outcomes. Furthermore, even patients in Group A, LVEF was lower and BNP concentrations were higher than in Group B at baseline, whereby the former demonstrated relatively better clinical outcomes than the latter. These results indicate that eligibility for CRT may not only be useful for prognosis, but may also be beneficial for evaluating clinical course.
A previous US study on the prognosis of patients with or without CRT reported that CRT reduced adverse events by more than 50%.24 Furthermore, our detailed analysis showed that early CRT implantation reduced adverse events by more than 50%, which is consistent with the previous US study.24 These results suggest that not only CRT use for eligible patients, but also appropriate timing of CRT implantation would have beneficial effects on long-term prognosis. Conversely, among patients without an indication for CRT, there was no significant difference in the incidence rate of all the clinical outcomes between those with (Group C) and without (Group D) CRT implantation. The clinical characteristics of patients in which CRT was overutilized (Group C) were similar to those in which CRT was appropriately utilized (Group A), showing relatively younger age, higher BNP concentrations, and a higher frequency of ventricular tachycardia; these findings suggest that those who received CRT were likely to have advanced CHF with relatively rapid progression, regardless of the presence (Group B) or absence (Group C) of the CRT indication. According to the current guidelines, the indication for CRT needs to satisfy all 3 criteria regarding LVEF, QRS duration, and NYHA class.36 In the present study, although patients in the overutilized CRT group received CRT in the absence of full indications, most of them met 2 of the 3 criteria. These findings indicate that CRT-implanted patients without an indication for CRT (Group C) aged 60 years old, on average, may have had no treatment option for advanced HF other than CRT, before considering heart transplantation (the availability of which is severely limited in Japan).
Study LimitationsThis study has several limitations. First, because the CHART-2 Study is an observational study in Japan, caution is needed when generalizing the data to other countries. Second, because we assessed the CRT indication only at baseline and some patients would have me the indication criteria during follow-up, the CRT utilization rate may have been underestimated. Third, because of the sample size, the present study may be underpowered to evaluate the prognostic significance of CHF patients with a CRT indication or the effect of CRT-P vs. CRT-D on mortality. Fourth, the guidelines need to be updated and the current indications for CRT will also be changed in the near future. Fifth, in the present study, no data were available on frailty, cognitive function, or comorbidities in elderly patients for predicting prognosis. Thus, further research is needed to clarify the prognostic significance of these factors. Sixth, because the CHART-2 Study was launched in 2006, we were unable to assess the efficacy of recent medications (e.g., sodium-glucose cotransporter 2 inhibitors and angiotensin receptor-neprilysin inhibitor) with proven protective effects against sudden cardiac death.
In the present study we were able to demonstrate that less than half of the eligible patients received CRT and that age is a strong risk for CRT underutilization. Patients with CRT indications are at high risk of mortality and CHF admission, and access to CRT at the appropriate time would improve the prognosis of CHF patients.
The authors thank all the members of the Tohoku Heart Failure Association, the CHART-2 Investigators, and the staff of the Departments of Cardiovascular Medicine and Evidence-based Cardiovascular Medicine, Tohoku University Graduate School of Medicine, for their kind contributions.
This study was supported, in part, by Grants-in Aid from the Ministry of Health, Labour, and Welfare, the Ministry of Education, Culture, Sports, Science, and Technology, and the Agency for Medical Research and Development (15ek210043 h1, 16ek210056 h1, and 16ek210043 h2), Tokyo, Japan. This research was also supported, in part, by Medtronic.
The Department of Evidence-based Cardiovascular Medicine, Tohoku University Graduate School of Medicine, is supported, in part, by unrestricted research grants from Daiichi Sankyo (Tokyo, Japan), Bayer Yakuhin (Osaka, Japan), Kyowa Hakko Kirin (Tokyo, Japan), Kowa Pharmaceutical (Tokyo, Japan), Novartis Pharma (Tokyo, Japan), Dainippon Sumitomo Pharma (Osaka, Japan), Astellas Pharma (Tokyo, Japan), AstraZeneca (Osaka, Japan), Chugai Pharmaceutical (Tokyo, Japan), GlaxoSmithKline (Tokyo, Japan), MSD (Tokyo, Japan), Nippon Boehringer Ingelheim (Tokyo, Japan), Otsuka Pharmaceutical (Tokyo, Japan), Shionogi (Osaka, Japan), and Takeda Pharmaceutical (Osaka, Japan). H.S. has received lecture fees from Bayer Yakuhin (Osaka, Japan), Daiichi Sankyo (Tokyo, Japan) and Novartis Pharma (Tokyo, Japan).
S.Y., Y.S., and H.S. are members of Circulation Reports’ Editorial Team. The remaining authors have no conflicts of interest to be disclosed.
This study was approved by the Ethics Committee of Tohoku University Graduate School of Medicine (Reference no. 2021-1-634).
The CHART-2 Study data are available upon reasonable request to the corresponding author.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circrep.CR-22-0036