2022 年 4 巻 7 号 p. 322-329
2022 年 4 巻 7 号 p. 322-329
Background: The overlap of multiple lifestyle-related diseases increases the risk of vascular diseases. This study investigated the effects of a mobile health (mHealth)-based disease management program on blood pressure and the safety of this program in people with multiple lifestyle-related diseases at risk of developing vascular disease.
Methods and Results: This retrospective observational study was conducted using secondary data collected by PREVENT Inc. People with a full history of hypertension, diabetes, and dyslipidemia and who participated in a 6-month mHealth-based disease management program were included in the study. The primary outcome was blood pressure. Adverse events during the program were investigated to evaluate safety. In total, 125 participants (mean [±SD] age 55.3±6.2 years) were examined. Systolic and diastolic blood pressure were significantly lower after the intervention than at baseline (systolic blood pressure, 128.0±12.3 vs. 131.9±12.7 mmHg [P<0.001]; diastolic blood pressure, 81.2±9.3 vs. 83.6±8.9 mmHg; P=0.003). No serious adverse events occurred during the program.
Conclusions: The present results indicate that the mHealth-based disease management program may reduce blood pressure in people with multiple lifestyle-related diseases at risk of developing vascular disease and that the program is safe. These findings will help shape future health instructions using mHealth-based interventions.
Lifestyle-related diseases, such as hypertension, diabetes, and dyslipidemia, and metabolic syndrome have been identified as risk factors for atherosclerosis.1–3 The appropriate management of these lifestyle-related diseases is important for preventing the onset or recurrence of atherosclerotic diseases, including coronary artery disease and ischemic stroke.2 Blood pressure levels have been decreasing in Japan due to improved treatment and control rates associated with advances in the diagnosis and treatment of hypertension.4,5 However, the treatment rate, particularly in the middle-aged population, is only approximately 50%, and the control rate is not sufficient.4,6 Therefore, health guidance on hypertension for the middle-aged population requires further improvement.
The provision of a disease management program based on structured treatment plans for patients with chronic conditions, including lifestyle-related and vascular diseases, may not only reduce, but also prevent the worsening of symptoms.7,8 Disease management programs for hypertensive and diabetic patients have been shown to primarily promote adherence to medication.9,10 Numerous studies have recently been published on mobile health (mHealth)-based disease management programs for hypertension11–13 and diabetes.11,13–17 These studies generally reported positive effects, such as improved blood pressure control13 and blood glucose control,13–16 as well as lifelog data, such as body weight and the number of steps. mHealth-based disease management programs also collect application-related information, such as device usage11 and user engagement,16 which predicts the effectiveness of improvements in outcomes.
Yun et al13 examined the effects of an electronic health management program, including a mobile app, on patients at risk of cardiovascular disease. However, they included patients with at least one indicator of poor disease control among those treated for hypertension, diabetes, or dyslipidemia. The overlap of these lifestyle-related diseases was shown to markedly increase the risk of not only developing coronary artery disease,18,19 but also atherosclerotic mortality, including stroke.20 However, the effects and safety of mHealth-based disease management programs for patients with lifestyle-related diseases at risk of developing vascular disease remain unclear.
The primary aims of the present study were to investigate the effects (with a focus on blood pressure) and safety of an mHealth-based disease management program in people with multiple lifestyle-related diseases at risk of developing vascular disease. The secondary aim was to examine the effect of application-related information from the program on blood pressure change. This study will contribute to the body of evidence for appropriate approaches to prevent vascular disease using mHealth-based disease management programs.
This retrospective observational study was conducted using secondary data collected by PREVENT Inc. (Nagoya, Japan), which conducts medical data analyses and implements disease management programs for chronic conditions. PREVENT Inc. performs predictive simulations for disease onset based on health insurance claims and health checkup data. Individuals at risk of developing vascular disease are selected and asked to participate in a lifestyle-modification support program. The present study was conducted in accordance with the Declaration of Helsinki, and was approved by the Research Ethics Committee of Konan Women’s University (Approval no. 2021008).
Study Population and ProcedureEmployees or their dependents enrolled in health insurance associations that participated in the lifestyle-modification support program, called “Mystar”, run by PREVENT Inc., between December 2018 and November 2020 were screened for inclusion in the present study. The Mystar program is targeted at individuals with hypertension, diabetes, and dyslipidemia who are taking medication or have a history of coronary artery disease or stroke. A list of diagnoses (e.g., arrhythmia, cardiomyopathy, end-stage kidney disease, and mental disorders) and the medications (e.g., cardiotonic, immunosuppressive, and antipsychotic agents) that prohibit participation in the Mystar program are available on GitHub (https://github.com/PREVENT-Inc/MyscopeMasterList). In the present study, we defined and included participants who were diagnosed with all 3 lifestyle-related diseases (i.e., hypertension, diabetes, and dyslipidemia) by their attending physician as people at risk of developing vascular disease. We excluded participants that dropped out of the 6-month program. The cost for participation in the program was covered by health insurance associations. Participants agreed to a privacy policy at the start of the program, which stated that the data gathered in the app may be used for future research.
Overview of the Mystar ProgramFigure 1 shows an overview of the Mystar program. Participants may only attend the program after obtaining written approval from their attending physician. The aim of the Mystar program is to reduce the risk of ischemic disease by personalized lifestyle-modification support. One of the features of the Mystar program is that it incorporates the concept of a disease management program, which allows for comprehensive support for participants with multiple lifestyle-related diseases. Medical staff, such as nurses, public health nurses, dietitians, and physical therapists, provide one-on-one instructions, mainly through mobile health-based interventions. The program consists of 12 sessions (approximately 6 months) based on telephone calls every 2 weeks. When participants start the program, they download the Mystar app to their smartphones. In addition to lifelog data, such as body weight, participants actively record their physical activity, pulse rate, and sleep status in the app using a wearable device (Fitbit Alta HR or Fitbit Inspire HR; Fitbit, San Francisco, CA, USA) and a salt-measurement device (GENEN Monitor; Kono ME Institute, Kawasaki, Japan) rented from PREVENT Inc. Every 2 months, medical staff make a progress summary of the program and share program information with the attending physician through the participant.
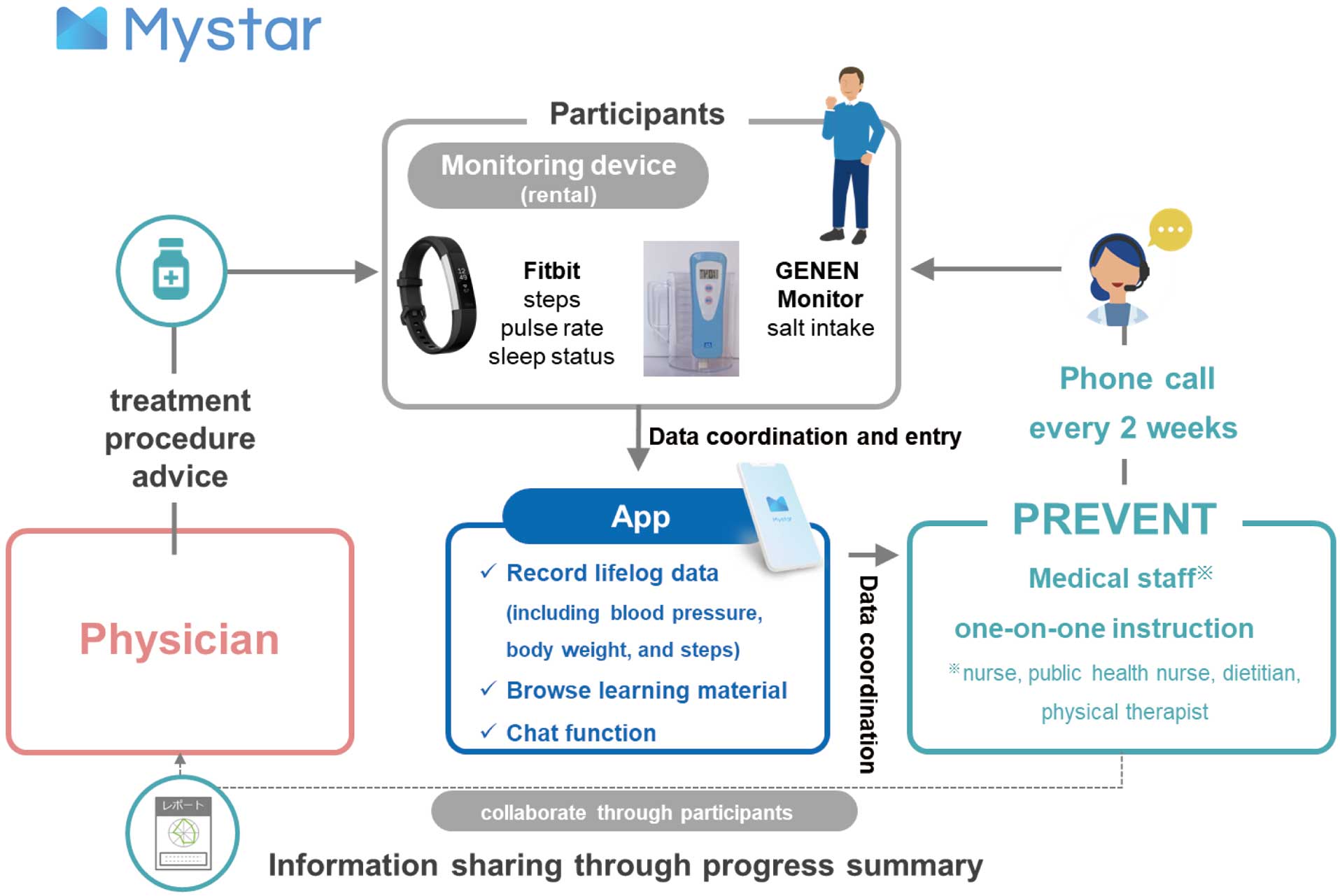
Overview of the Mystar program.
Disease management programs are conducted as directed by the attending physician. Medical staff provide an orientation to participants on the use of the app during the initial telephone call. Medical staff then ask participants about their current lifestyle habits (e.g., exercise, diet, salt intake, alcohol consumption, smoking, sleep, and stress) in detail, and identify with the participants which lifestyle habits they are willing to change in order to modify their lifestyle-related disease management. Medical staff prioritize targeted lifestyle-related diseases based on health checkup data and blood sampling data obtained in advance, as well as lifelog data entered by the participants. Medical staff and participants then collaborate to select long-term goals for completing the program, and individual behavioral goals are set for each telephone call every 2 weeks. In addition, both medical staff and participants may send and receive messages on counseling or consultations at any time using the chat function. Through this chat function, medical staff check the progress of a participant’s health behavior, supplemented by telephone calls. Moreover, medical staff supplement telephone calls at any time by providing learning materials, such as descriptions of lifestyle-related diseases and vascular disease, as well as tips for lifestyle improvements, to individual participants, which is one of the functions of the app.
If the target lifestyle-related disease is hypertension, medical staff provide appropriate exercise support (setting a target number of steps and exercise intensity by using a wearable device) and dietary support (instructions on reductions in salt intake and a healthy nutritional balance based on diet photographs recorded in the app), the cessation of smoking, and decreases in alcohol intake. Medical staff repeat evidence-based clinical reasoning over the course of the program and, in some cases, review target lifestyle-related diseases and goal setting.
The main instruction concept of the lifestyle-modification support program consists of techniques for behavioral changes.21 Medical staff thoroughly assess the stages of behavioral changes related to a participant’s lifestyle and provide guidance based on the stage.22 For example, medical staff use motivational interviews to encourage participants in the precontemplation stage, and provide learning materials that promote an understanding of the importance of disease management. For participants in the preparation and action stages, medical staff promote greater participant self-efficacy for their target behavior and encourage participants to change their target behavior.
To ensure the safety of the mHealth-based interventions, medical staff may coordinate with the attending physician through the participant when lifestyle-related disease control values are poor. In addition, any medication changes are immediately communicated by the participant to medical staff.
Outcome MeasurementsThe primary outcome of this study was blood pressure. Demographic data (i.e., age, sex, height, body weight, and body mass index [BMI]), medical information, lifelog data, app-related information, and safety were collected from health checkup data, including blood sampling data in the most recent period of the program, data entered into the app by participants, and records of telephone calls entered by medical staff.
In this study, obesity was defined as a BMI >25 kg/m2, with the percentage of obese participants being surveyed. Medical information collected included a history of vascular events, such as coronary artery disease (angina pectoris and myocardial infarction) or stroke, medication, and blood sampling data related to lifestyle-related diseases, including HbA1c, the low-density lipoprotein cholesterol/high-density lipoprotein cholesterol ratio, and creatinine.
The lifelog data collected included morning home systolic blood pressure (SBP) and diastolic blood pressure (DBP), weight during the program, number of steps (measured by Fitbit devices), and daily salt intake (measured by the GENEN Monitor). The reliability and validity of the GENEN Monitor have been demonstrated previously.23 Prior to bedtime, participants were instructed to void completely and discard the urine. Night urine was collected from a cup of urine. After waking up, participants voided and placed the urine in the urine cup, adding any urine they had voided overnight. Participants were instructed to take their urine, set the salt monitor and record the displayed value.
App usage time was measured as the total time that the app was in the foreground from the app database. Total app usage time during the early (first 3 months), late (second 3 months), and all stages (6 months) of the program was examined. In addition, the program duration was calculated for each participant from the app logs.
The safety of the program was evaluated on the basis of adverse events. An adverse event was defined as any unfavorable medical event, such as cardiovascular death or new vascular events requiring hospitalization, during the Mystar program.
Statistical AnalysisContinuous variables are presented as the mean±SD, whereas categorical variables are presented as counts and percentages. App usage time is presented as the median with interquartile range (IQR) because of a skewed distribution. To investigate the effects of the mHealth-based disease management program, paired t-tests were used to compare baseline and postintervention blood pressure and other lifelog data. Based on the frequency of telephone calls, we defined baseline data as the average of 2 weeks after the initial telephone call and postintervention data as the average of 2 weeks prior to the last telephone call.
Multivariate linear regression analyses were used to examine the relationship between blood pressure changes and app usage times. Distributions of app usage time were highly skewed, so data were log transformed in the statistical models to improve normality. App usage time was entered into separate models for the early, late, and all stages of the program. From a clinical viewpoint, the covariates selected to adjust for bias were age, sex, obesity, SBP or DBP at baseline, the number of antihypertensive medications, and program duration. Multicollinearity was assessed using the variance inflation factor (VIF): a VIF value 1–10 was considered to indicate the absence of multicollinearity. Two-sided P<0.05 was considered significant. Statistical analyses were performed with R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria).
Among the 226 participants with a history of hypertension, diabetes, and dyslipidemia included in the study, only 1 participant dropped out for no reason. Thus, 225 participants completed the 6-month program, with 100 being excluded from the analysis because of missing values, namely: blood pressure values before or at the end of the program (n=43), app-related information (n=13), medication status (n=19), and other missing baseline values (n=25). Therefore, 125 participants were ultimately included in the present study (Figure 2). The mean program duration for these participants was 171.9±26.3 days.
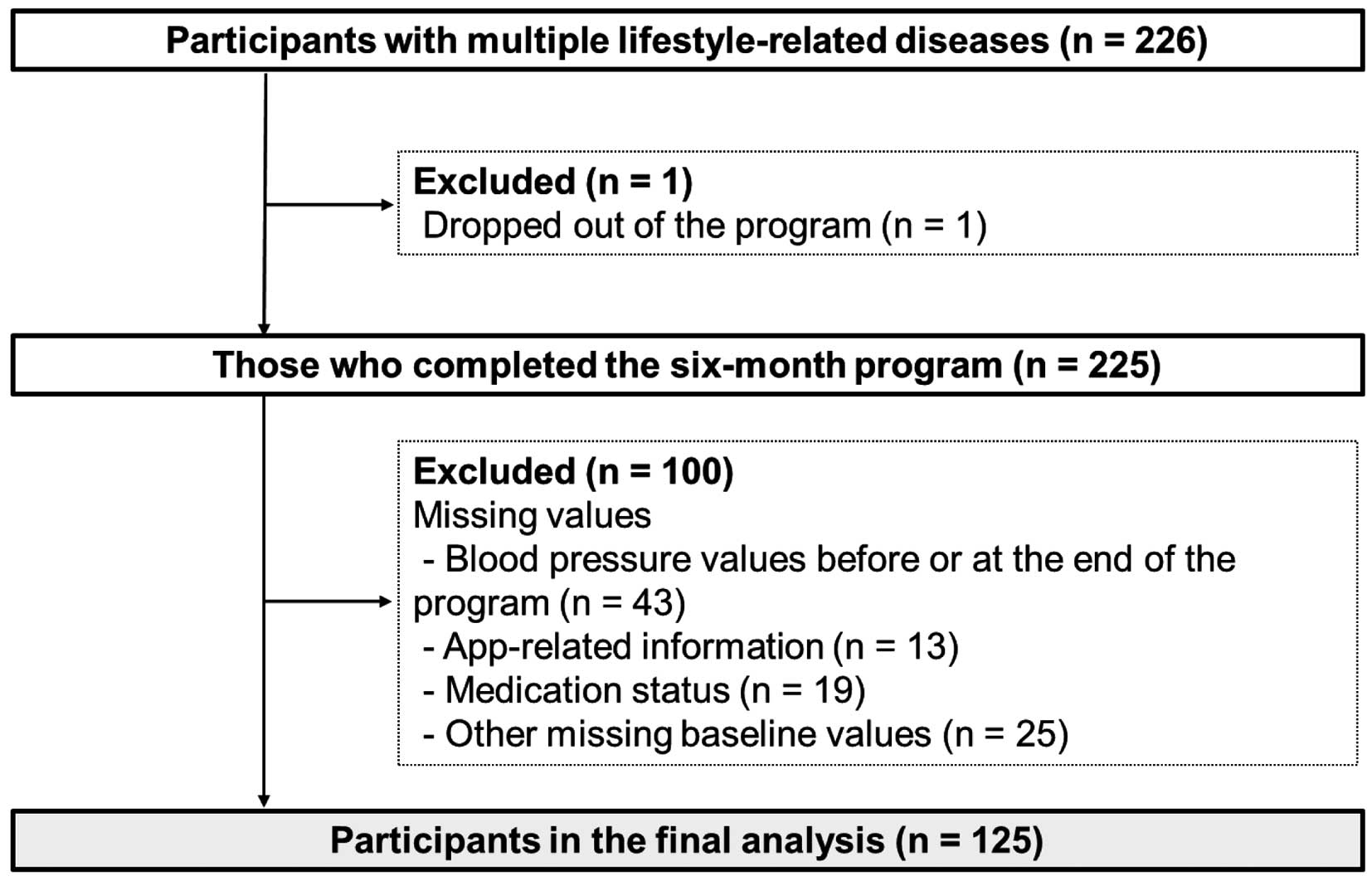
Participant flow in the present study.
The clinical characteristics of participants are presented in Table 1. The mean age of participants was 55.3 years and there were 17 (13.6%) women. Many of the participants were taking antihypertensive medication. The median (IQR) app usage time for the early, late, and all stages of the program was 502.1 (241.0–888.3), 342.5 (164.4–784.8), and 896.0 (448.7–1,793.4) min, respectively.
| Age (years) | 55.3±6.2 |
| Sex | |
| Male | 108 (86.4) |
| Female | 17 (13.6) |
| Obesity | 87 (69.6) |
| Vascular event | |
| Coronary artery disease | 13 (10.4) |
| Stroke | 6 (4.8) |
| Medication | |
| Antihypertensive agent | |
| 0 | 11 (8.8) |
| 1 | 56 (44.8) |
| ≥2 | 58 (46.4) |
| Oral antidiabetic agent | |
| 0 | 21 (16.8) |
| 1 | 44 (35.2) |
| ≥2 | 60 (48.0) |
| Insulin injection | 11 (8.8) |
| Cholesterol-lowering agent | |
| 0 | 38 (30.4) |
| 1 | 79 (63.2) |
| ≥2 | 8 (6.4) |
| SBP (mmHg) | 131.9±12.7 |
| DBP (mmHg) | 83.6±8.9 |
| HbA1c (%; n=118) | 7.1±1.0 |
| L/H ratio (%; n=108) | 2.3±0.7 |
| Creatinine (mg/dL; n=112) | 0.9±0.2 |
Date are shown as the mean±SD or n (%). DBP, diastolic blood pressure; L/H ratio, low-density lipoprotein cholesterol/high-density lipoprotein cholesterol ratio; SBP, systolic blood pressure.
Differences between baseline and postintervention outcomes are presented in Table 2. SBP and DBP were significantly lower after the intervention than at baseline (SBP, 128.0±12.3 vs. 131.9±12.7 mmHg [P<0.001]; DBP, 81.2±9.3 vs. 83.6±8.9 mmHg [P=0.003]). In addition, significant improvements were observed in all lifelog data, except for the number of steps.
| Baseline | After the intervention |
Mean difference (95% CI) |
P value | |
|---|---|---|---|---|
| SBP (mmHg; n=125) | 131.9±12.7 | 128.0±12.3 | 3.8 (1.9, 5.7) | <0.001 |
| DBP (mmHg; n=125) | 83.6±8.9 | 81.2±9.3 | 2.4 (0.8, 3.9) | 0.003 |
| Body weight (kg; n=119) | 82.2±16.6 | 80.6±16.7 | 1.7 (1.2, 2.1) | <0.001 |
| BMI (kg/m2; n=116) | 28.3±4.8 | 27.8±5.0 | 0.5 (0.4, 0.7) | <0.001 |
| No. steps/day (n=119) | 8,636.2±4,437.9 | 9,365.4±3,998.1 | −729.2 (−1,482.2, 23.7) | 0.058 |
| Salt intake (g/day; n=90) | 11.7±3.4 | 10.8±2.8 | 0.9 (0.2, 1.6) | 0.009 |
Unless indicated otherwise, data are given as the mean±SD. BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Table 3 and the Supplementary Table present results of multivariate linear regression analyses of changes in SBP and DBP. No relationship was observed between app usage time and changes in SBP or DBP in any of the models. Multicollinearity was not detected in either model. No relationship was observed between app usage time and blood pressure change (Supplementary Table).
| SBP change | DBP change | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | P value | β | P value | β | P value | β | P value | β | P value | β | P value | |
| App usage time | ||||||||||||
| Early stage | 0.01 | 0.265 | – | – | – | – | 0.09 | 0.338 | – | – | – | – |
| Late stage | – | – | 0.16 | 0.056 | – | – | – | – | 0.14 | 0.104 | – | – |
| All stages | – | – | – | 0.16 | 0.065 | – | – | – | – | 0.15 | 0.106 | |
The models were adjusted for age, sex, obesity, systolic blood pressure (SBP) or diastolic blood pressure (DBP) at baseline, the number of antihypertensive medications, and program duration.
No serious adverse events considered to be related to the mHealth-based disease management program were reported.
The present study investigated the effects and safety of an mHealth-based disease management program in people with multiple lifestyle-related diseases at risk of developing vascular disease. The results showed that the mHealth-based disease management program significantly reduced blood pressure in these people and that the program was safe. However, no relationship was found between app usage times, used as a measure of app-related information, and changes in blood pressure.
Pre-Post Comparison of Blood Pressure and Other Lifelog DataThe results of the present study showed that the mHealth-based disease management program for people with multiple lifestyle-related diseases at risk of developing vascular disease reduced SBP by 3.8 mmHg and DBP by 2.4 mmHg. Regarding mHealth-based interventions, Logan et al24 indicated that home blood pressure telemonitoring with automated self-care support reduced uncontrolled systolic hypertension in diabetic patients. Lu et al25 reported the effects of mHealth-based interventions for hypertensive adults in a meta-analysis, particularly the potential antihypertensive effects of interactive, individualized feedback. Lu et al showed that, compared with the control group, mHealth-based interventions were associated with significant changes in SBP and DBP of −3.85 (95% confidence interval [CI] −4.74, −2.96) and −2.19 (95% CI −3.16, −1.23] mmHg, respectively.25 The Mystar program used mHealth-based interventions to incorporate interactive and individualized feedback through telephone calls and chat functions. Because the present study did not have a control group, a direct comparison was not possible; however, antihypertensive effects similar to those reported in the meta-analysis25 appear to have been achieved.
Antihypertensive treatments may be divided into drug therapy and lifestyle modifications.26 Because participants in the present study had multiple lifestyle-related diseases and more than 90% were already taking antihypertensive medication, lifestyle modifications were considered necessary to reduce blood pressure. Kim et al12 reported the effects of an mHealth-based disease management program on a population with other lifestyle-related diseases in addition to hypertension. They used a mHealth-based intervention to encourage health behaviors, such as the cessation of smoking, reducing alcohol consumption, and performing exercise, but did not achieve significant reductions in blood pressure.12 The Mystar program used in the present study is a disease management program that provides comprehensive modification support for multiple lifestyle habits, such as exercise, diet, alcohol consumption, smoking, sleep, and stress, and thus may contribute to lifestyle modifications in individuals at risk of developing vascular disease. Although the postintervention BMI and salt intake of participants were still higher than the control targets of 25 kg/m2 and 6 g/day, respectively,26 the reductions in body weight and salt intake were sufficient to exert antihypertensive effects. One meta-analysis estimated that SBP and DBP decreased by approximately 1.1 and 0.9 mmHg per 1.0-kg decrease in body weight, respectively.27 There is currently no definition of the antihypertensive effects of changes in salt intake. A reduced salt intake has been suggested to play an important role in blood pressure decreases and the incidence of ischemic disease;28 therefore, stricter instructions for salt intake may be warranted. Although we were unable to objectively evaluate diet photographs recorded in the app, nutritional balance guidance and recommendations to increase potassium intake may have contributed to blood pressure decreases.29 Conversely, because most of the participants in the present study were middle-aged working men and their baseline physical activity was high, the increase observed in the number of steps by mHealth-based interventions may not have been adequate. In addition to the amount of physical activity, its intensity is expected to contribute to decreases in blood pressure;30 therefore, more precise guidance is needed.
Safety of the mHealth-Based Disease Management ProgramThe results of the present study showed that the mHealth-based disease management program was safely implemented for people with multiple lifestyle-related diseases at risk of developing vascular disease. The first reason for this may be that participants had to receive approval from their attending physicians in order to participate in the Mystar program, and medical staff and attending physicians were able to collaborate through reports during the course of the program. The second reason was the strict criteria for participation in the program. This was in line with a Japanese study on the safety of telemedicine for patients with lifestyle-related diseases, which excluded patients with severe cardiac or renal diseases.31 Participants in the present study included 13 (10.4%) with a history of coronary artery disease and 6 (4.8%) with a history of stroke. Although these diseases were still reported to have a high recurrence rate,32,33 no recurrences were observed during the Mystar program.
Although safety was demonstrated in this way, a time lag in the sharing of information was a concern for the Mystar program, because it was coordinated with the attending physicians through the participants.
App-Related Information and Changes in Blood PressureThe results of the present study showed that none of the app usage times in the early, late, or all stages of the program were associated with changes in blood pressure. In mHealth-based interventions, app-related information, such as app usage and user engagement, has been shown to improve the effectiveness of disease management for lifestyle-related diseases16,34 and health behavior.35,36 Agnihothri et al34 reported that heavier app use was associated with greater reductions in blood pressure. Sepah et al16 revealed that website logins and group participation in the early phase of a digital diabetes prevention program correlated with weight loss at 1 year. Unfortunately, the present study did not find a significant association between app usage time and changes in blood pressure. However, because higher levels of app usage were reported to be associated with increased intervention efficacy,37 some participants in the present study may have improved their own health management through the use of the app, which improved their adherence to treatment.38
In a systematic review, Hamine et al39 investigated tools to increase adherence to mHealth for chronic disease management, which were categorized as SMS, video messages, a telephone plus software or apps, a telephone plus a specific instrument, a wireless or Bluetooth-compatible device, and others. Because the app used in the present study included most of these components, participants actively used the app and increased their adherence to treatment and lifestyle modifications, as demonstrated by the low dropout rate of the program over a 6-month period. We could not examine the details of the most regularly used app functions in this study, so future studies should examine the effects of specific app use and mHealth-based interventions.
Study LimitationsThis study has some limitations that need to be addressed. Because we did not have a control group, we were unable to confirm whether the mHealth-based disease management program actually affected blood pressure and lifelog data. Because we have not been able to follow up on changes in medication status for all participants during this study period, we cannot deny that this may have played a role in reducing blood pressure. Furthermore, participants used devices provided by PREVENT Inc. to measure the number of steps taken and salt intake, but used their own personal devices for blood pressure and body weight measurements, which may have distorted the results obtained. Moreover, although the primary outcome of the present study was blood pressure, some participants may have been instructed to focus on improving diabetes or dyslipidemia management. In addition, because the timing of blood test measurements, such as blood glucose and cholesterol levels after the intervention, varied, we were unable to examine the effects of the intervention on diabetes and dyslipidemia. To overcome this issue, the Mystar program is currently in the process of sending blood collection kits after the intervention. Another limitation is that we were unable to perform a cost-effectiveness analysis of the Mystar program, which is regarded as an important evaluation for both mHealth-based interventions15 and disease management programs;10 therefore, this needs to be incorporated into future studies. Cost-effectiveness for interventions to prevent the development of vascular disease is a controversial issue with regard to whether a population approach or a high-risk approach should be taken.40 The Mystar program is a high-risk approach and an intervention that requires human resources in addition to the mobile app. However, as reported in another study,41 it may have had an effect on medical cost optimization as a result of the disease management program. Furthermore, because the present study used secondary data, the program’s inclusion criteria were specific and did not cover all people who require lifestyle changes. Thus, caution should be used when generalizing the results of the present study.
The present study indicated that the mHealth-based disease management program may reduce blood pressure in people with multiple lifestyle-related diseases at risk of developing vascular disease and that the program was safe. These results will contribute to the development of future health instructions using mHealth-based interventions.
The authors thank the individuals who participated in the Mystar program and the employees of PREVENT Inc. for their assistance.
This research received no external funding.
M.K. has received consulting fees from PREVENT Inc. and is a non-regular staff member of PREVENT Inc. Y.H. is a founder and stockholder of PREVENT Inc. T.T., K.Y., and M.A. are employees of PREVENT Inc.
This study was approved by the Research Ethics Committee of Konan Women’s University (Approval no.: 2021008).
The deidentified participant data will not be shared.
Please find supplementary file(s);
http://dx.doi.org/10.1253/circrep.CR-22-0024