Abstract
Background: A high score for controlling nutritional status (CONUT) due to poor nutritional status has been associated with adverse outcomes in patients with chronic heart failure. However, because little is known about the effect of CONUT score on mortality rates after transcatheter mitral valve repair, we evaluated nutrition screening tools for prognosis prediction in patients undergoing transcatheter mitral valve repair using the MitraClipTM
system.
Methods and Results: We retrospectively analyzed 148 patients with severe mitral regurgitation (MR) who underwent MitraClipTM
implantation between April 2018 and April 2021. The preprocedural CONUT scores were assessed at the time of hospitalization, the primary outcome was all-cause death, and the analysis was of the mortality and incidence rates of cardiac events 1 year post-operation. Functional MR was of ischemic origin in the majority of patients (69.6%), with a mean left ventricular ejection fraction of 48.9±15.8%. Kaplan-Meier curves indicated that all-cause death was significantly worse in the high-CONUT score group than in the low-CONUT score group. Cox hazard analysis showed a significant association between all-cause death and CONUT score, as well as MitraScore.
Conclusions: Preprocedural CONUT score, as well as MitraScore, in patients undergoing transcatheter edge-to-edge mitral valve repair may predict an increased risk of all-cause death. This knowledge should allow the heart team to accurately assess the clinical implications and prognostic benefits of the procedure in individual patients.
Transcatheter edge-to-edge mitral valve repair (TEER) with the MitraClipTM
(Abbott Vascular, Santa Clara, CA, USA) System (Abbott Vascular) has become the first-line interventional treatment for patients with severe functional mitral regurgitation (MR) after optimal medical therapy,1–6 and MitraScore,7–9 as a prediction tool for death in patients undergoing TEER, was recently reported as opposed to the EuroSCORE II and Society of Thoracic Surgeons score. Despite recent developments in medical and surgical therapy for patients with heart failure (HF), mortality rates remain high, and could be related to altered intestinal function caused by malnutrition in HF patients,10,11 which is considered a source of inflammation.12–14 The controlling nutritional status (CONUT) score is a risk evaluation tool for assessing nutritional status,15–18 and a high CONUT score is associated with adverse outcomes in patients with chronic HF.18,19 However, for patients in whom the MitraClip yields mechanical improvement of MR, little is known about the association between nutritional status and death. Therefore, in this study we aimed to identify nutritional screening tools that can predict death in patients undergoing TEER with the MitraClip system.
Methods
Patient Population
This retrospective cohort study of 148 consecutive patients who underwent MitraClip was conducted between April 2018 and April 2021 implantation. Indication for TEER using the MitraClip included presence of moderate-to-severe (3+) or severe (4+) MR in symptomatic patents, with a high risk for surgery. All patients were evaluated by a multidisciplinary heart team comprising an interventional cardiologist, echocardiographer, cardiac surgeon, HF specialist, and anesthesiologist. Patients with adverse clinical comorbidities such as endstage cancer that limited their lifespan to <6 months were excluded. Patients were also excluded if the morphology of the mitral valve for MitraClip implantation was unfeasible or impossible according to the EVEREST criteria (i.e., short or calcified posterior leaflet reducing the possibility of successful grasping by the clip or the beginnings of mitral stenosis).20–23
All patients provided informed consent prior to the procedure and the study protocol was approved by the Institutional Review Board of St. Marianna University School of Medicine (approval no. 5959).
Transthoracic and Transesophageal Echocardiographic Measurements
We performed transthoracic and transesophageal echocardiography (TTE) to quantify the MR parameters, as well as to assess morphologic suitability for MitraClipTM
implantation. According to the American Society of Echocardiography guidelines,24,25 MR severity was defined as none or trace (0/4+), mild (1+/4+), moderate (2+/4+), moderate-to-severe (3+/4+), and severe (4+/4+).
MitraClip Implantation Procedure
All clips were implanted via the femoral vein under general anesthesia, using fluoroscopy and TTE. Hemostasis of the femoral vein was achieved by Z-suture and compression of the vein for 8 h.
Predictors of All-Cause Death
The CONUT score was calculated from serum albumin (Alb) level, total lymphocyte count, and total cholesterol level, as described previously.10,26,27 Additionally, we determined the geriatric nutritional risk index (GNRI; see Supplementary Table 1). The preprocedural CONUT score was calculated at the time of hospitalization: a low CONUT score was defined as 0–4 and high CONUT score as 5–12. The patients were divided into 4 groups based on their preprocedural CONUT score: 0–1 normal, 2–4 mildly high, 5–8 moderately high, and 9–12 markedly high with the highest Youden’s J value of 0.55 in receiver-operating characteristic (ROC) analysis, according to previous reports.10,26,27 The MitraScore7 has 8 independent predictors: age ≥75 years, anemia, estimated glomerular filtration rate <60 mL/min/1.73 m2, left ventricular ejection fraction (LVEF) <40%, peripheral artery disease, chronic obstructive pulmonary disease, high diuretic dose (≥80 mg of furosemide/day or use of ≥2 diuretic agents excluding antialdosteronic drugs), and no therapy with renin-angiotensin system inhibitors. The MitraScore is derived by assigning 1 point to each independent predictor, as shown in the Central Illustration. The sum of the weighted integers (range 0–8 points) estimates the risk of follow-up death.
Clinical Follow-up
Patients were evaluated at baseline, during the procedure, and at discharge, as well as at 30 days and 1 year after the procedure. Cardiovascular events included hospitalization for HF and death due to cardiovascular complications. HF was defined as the appearance of signs such as dyspnea and orthopnea with New York Heart Association (NYHA) functional class II–IV, requiring hospitalization. We evaluated clinical follow-up data using medical records.
Statistical Analysis
Categorical variables are presented as percentages and counts and were compared between groups using the chi-square test or Fisher’s exact test. Continuous variables are presented as mean±standard deviation. The incidences of death and cardiovascular events were compared between groups using Fisher’s exact test. To determine the independent predictors of death, a logistic regression model was constructed on a patient-level basis. Additionally, a multivariate logistic regression model was used to predict death by incorporating the preprocedural CONUT score and MitraScore. Statistical significance was set at P<0.05 for all tests. All statistical tests were performed using IBM SPSS Statistics for Windows (Version 22.0; IBM Corp., Armonk, NY, USA).
Results
Baseline Characteristics
In total, 148 patients (mean aged, 78±9 years; males, 62.8%; NYHA class III/IV, 79%) with severe MR were included. Functional MR was of ischemic origin in 69.6% of the patients, and the mean LVEF was 48.9±15.8%.
Baseline demographics and comorbidities according to the CONUT score classification are summarized in Table 1. Patients in the high-CONUT score group were older (80.8±8.3 vs. 76.5±8.7 years, P<0.01), mostly male, with lower BMI (20.9±3.0 vs. 22.2±4.4, P<0.04), hemoglobin (10.4±1.4 vs. 12.4±1.6 g/dL, P<0.01), and total protein (6.2±0.9 vs. 6.9±0.6, P<0.01) levels. The group also had higher proportions of patients with congestive HF within 1 year (82% vs. 56%, P<0.01) and renal dysfunction (86% vs. 73%, P=0.05), higher C-reactive protein (CRP) levels (1.76±3.6 vs. 0.32±0.6 mg/dL, P<0.01); and had lower GNRI, compared with the low-CONUT score group (Table 1). Patients in the low-CONUT score group had lower MR jet area and less MR regurgitant fraction than the high-CONUT score group.
Table 1.
Baseline Patient Characteristics
| Characteristic |
Overall
(n=148) |
CONUT <4
(n=97) |
CONUT ≥5
(n=51) |
P value |
| Age, years |
78.0±8.6 |
76.5±8.7 |
80.8±8.2 |
<0.01 |
| Male |
93 (62.8) |
56 (57.7) |
37 (72.6) |
0.07 |
| BMI, kg/m2 |
21.7±4.0 |
22.2±4.4 |
20.9±3.0 |
0.04 |
| Congestive HF within 1 year |
96 (65) |
54 (56) |
42 (82) |
<0.01 |
| NYHA class III/IV |
117 (79) |
73 (75) |
44 (86) |
0.14 |
| Diabetes |
50 (33.8) |
29 (29.9) |
21 (41.2) |
0.18 |
| Systolic blood pressure |
157.9±10.7 |
157.3±11.4 |
159.1±9.2 |
0.35 |
| Hypertension |
110 (74.3) |
72 (74.2) |
38 (74.5) |
0.97 |
| Dyslipidemia |
78 (52.7) |
51 (52.6) |
27 (52.9) |
0.97 |
| Liver cirrhosis |
2 (1.4) |
1 (1.0) |
1 (2.0) |
0.68 |
| COPD |
16 (10.8) |
12 (12.4) |
4 (7.8) |
0.40 |
| Prior stroke |
13 (8.9) |
8 (8.4) |
5 (9.8) |
0.78 |
| CKD |
113 (77.4) |
70 (72.9) |
43 (86) |
0.05 |
| CAD |
39 (26.7) |
26 (27.1) |
13 (26) |
0.89 |
| Cardiac valve replacement |
3 (2.0) |
3 (3.1) |
0 (0) |
0.08 |
| Other chest surgery |
8 (5.4) |
6 (6.2) |
2 (3.9) |
0.57 |
| β-blocker |
118 (79.7) |
79 (81.4) |
39 (76.5) |
0.48 |
| ACEi or ARB |
99 (66.9) |
65 (67.0) |
34 (66.7) |
0.96 |
| Loop diuretic |
117 (79.1) |
74 (76.3) |
43 (84.3) |
0.26 |
| Tolvaptan diuretic |
54 (36.5) |
26 (26.8) |
28 (54.9) |
<0.01 |
| Antiplatelet |
70 (47.3) |
48 (49.5) |
22 (43.1) |
0.47 |
| Anticoagulant |
95 (64.2) |
63 (64.9) |
32 (62.7) |
0.79 |
| Prior CABG |
14 (9.5) |
12 (12.4) |
2 (3.9) |
0.05 |
| Prior PCI |
40 (27.0) |
25 (25.8) |
15 (29.4) |
0.63 |
| Cardiac rhythm device implantation |
29 (19.6) |
19 (19.6) |
10 (19.6) |
0.99 |
| LVDd |
56.4±10.9 |
56.3±11.8 |
56.3±9.01 |
0.98 |
| LVDs |
43.5±13.9 |
43.2±15.1 |
43.9±11.5 |
0.76 |
| LVEDV |
140.3±66.5 |
139.3±72.4 |
142.1±54.1 |
0.79 |
| LVESV |
78.0±58.4 |
77.1±64.7 |
79.9±45.0 |
0.76 |
| LAVI |
78.3±37.7 |
74.2±32.0 |
85.9±46.0 |
0.07 |
| Af |
97 (65.5) |
59 (60.8) |
38 (74.5) |
0.09 |
| Functional MR |
103 (69.6) |
68 (70.1) |
35 (68.6) |
0.85 |
| TP |
6.7±0.8 |
6.9±0.6 |
6.2±0.9 |
<0.01 |
| Hemoglobin, g/dL |
11.7±1.8 |
12.4±1.6 |
10.4±1.4 |
<0.01 |
| WBC, ×1,000/μg |
5.6±1.9 |
5.5±1.5 |
5.7±2.4 |
0.56 |
| Serum Na, mEq/L |
138.6±3.7 |
138.9±3.1 |
138.1±4.6 |
0.32 |
| Creatinine, mg/dL |
1.7±1.6 |
1.5±1.3 |
2.0±2.0 |
0.10 |
| eGFR, mL/min/1.73 m2 |
41.8±21.0 |
43.0±18.2 |
39.6±25.7 |
0.40 |
| Median NT-proBNP (IQR), pg/mL |
2,123 (1,017–4,674) |
1,570 (733–3,709) |
4,024 (2,057–6,476) |
0.20 |
| CRP, mg/dL |
0.82±2.3 |
0.32±0.59 |
1.76±3.6 |
<0.01 |
| HbA1c, % |
6.1±0.8 |
6.0±0.7 |
6.2±1.0 |
0.36 |
| LVEF, % |
48.9±15.8 |
50.0±16.3 |
46.9±14.8 |
0.27 |
| GNRI |
95.2±12.3 |
100.5±10.3 |
85.0±8.7 |
<0.01 |
| MitraScore |
3.5±1.4 |
3.2±1.4 |
4.1±1.3 |
<0.01 |
| MR jet area |
29.9±14.1 |
27.9±13.5 |
33.7±14.5 |
0.07 |
| MR vena contracta |
5.9±4.6 |
5.6±4.6 |
6.6±4.6 |
0.35 |
| MR ERO PISA |
0.3±0.2 |
0.3±0.2 |
0.3±0.2 |
0.47 |
| MR RV (PISA) |
45.1±23.0 |
45.0±23.1 |
45.2±23.1 |
0.96 |
| MR RV (volumetric) |
26.7±26.8 |
24.9±29.9 |
30.0±20.2 |
0.34 |
| MR RF (volumetric) |
42.0±19.1 |
39.2±21.6 |
46.4±13.5 |
0.08 |
Values are mean±SD [median] or n (%) interquartile range (IQR). ACEi, angiotensin-converting enzyme inhibitor; Af, atrial fibrillation; ARB, angiotensin-receptor blocker; BMI, body mass index; BNP, B-type natriuretic peptide; CABG, coronary artery bypass graft; CAD, coronary artery disease; CKD, chronic kidney disease; CONUT, controlling nutritional status; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; ERO, effective regurgitant orifice; GNRI, Geriatric Nutritional Risk Index; HF, heart failure; LAVI, left atrial volume index; LVDd, left ventricular end-diastolic diameter; LVDs, left ventricular end-systolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; MR, mitral regurgitation; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PISA, proximal isovelocity surface area; RF, regurgitant fraction; RV, regurgitant volume; TP, total protein; WBC, white blood cell.
Clinical Outcomes in High- vs. Low-CONUT Score Groups
The mean follow-up period was 389±291 days; 26 patients died (1 died before hospital discharge; 2 died within 30 days of undergoing the MitraClip procedure). At 30 days after MitraClip implantation, 2 patients required mitral valve surgery or a repeat MitraClip procedure, but no cases of MitraClip embolization or mitral valve stenosis were detected. The high-CONUT score (≥5) group had significantly higher incidences of death, cardiovascular death, and hospitalization for HF after the procedure, compared with the low-CONUT (<4) group (Table 2). Kaplan-Meier curves indicated that all-cause death was significantly worse in the high-CONUT score group than in the low-CONUT score group (P<0.001; Figure 1). When the patients were divided according to preprocedural CONUT score, increasing CONUT score was associated with an incrementally higher risk of all-cause death (Figure 2). ROC analysis showed an area under the curve for all-cause death of 0.81 (95% confidence interval [CI] 0.70–0.88) for CONUT score (Figure 3). Cox hazard analysis showed that the CONUT score, as well as MitraScore, was significantly associated with all-cause death (hazard ratio [HR]: 1.31, 95% CI: 1.12–1.52, P<0.01) (Table 3).
Table 2.
Events After MitraClip Implantation
| Event |
Overall
(n=148) |
CONUT <4
(n=97) |
CONUT ≥5
(n=51) |
P value |
| Death |
26 (18) |
6 (6) |
20 (39) |
<0.01 |
| Cardiovascular death |
14 (9) |
3 (3) |
11 (22) |
<0.01 |
| Myocardial infarction |
0 |
0 |
0 |
|
| Intersititial pneumonia |
2 (1) |
0 |
2 (4) |
0.16 |
| Stroke |
4 (3) |
2 (2) |
2 (4) |
0.55 |
| Hospitalization for HF after procedure |
19 (13) |
8 (8) |
11 (22) |
0.04 |
CONUT, controlling nutritional status; HF, heart failure.
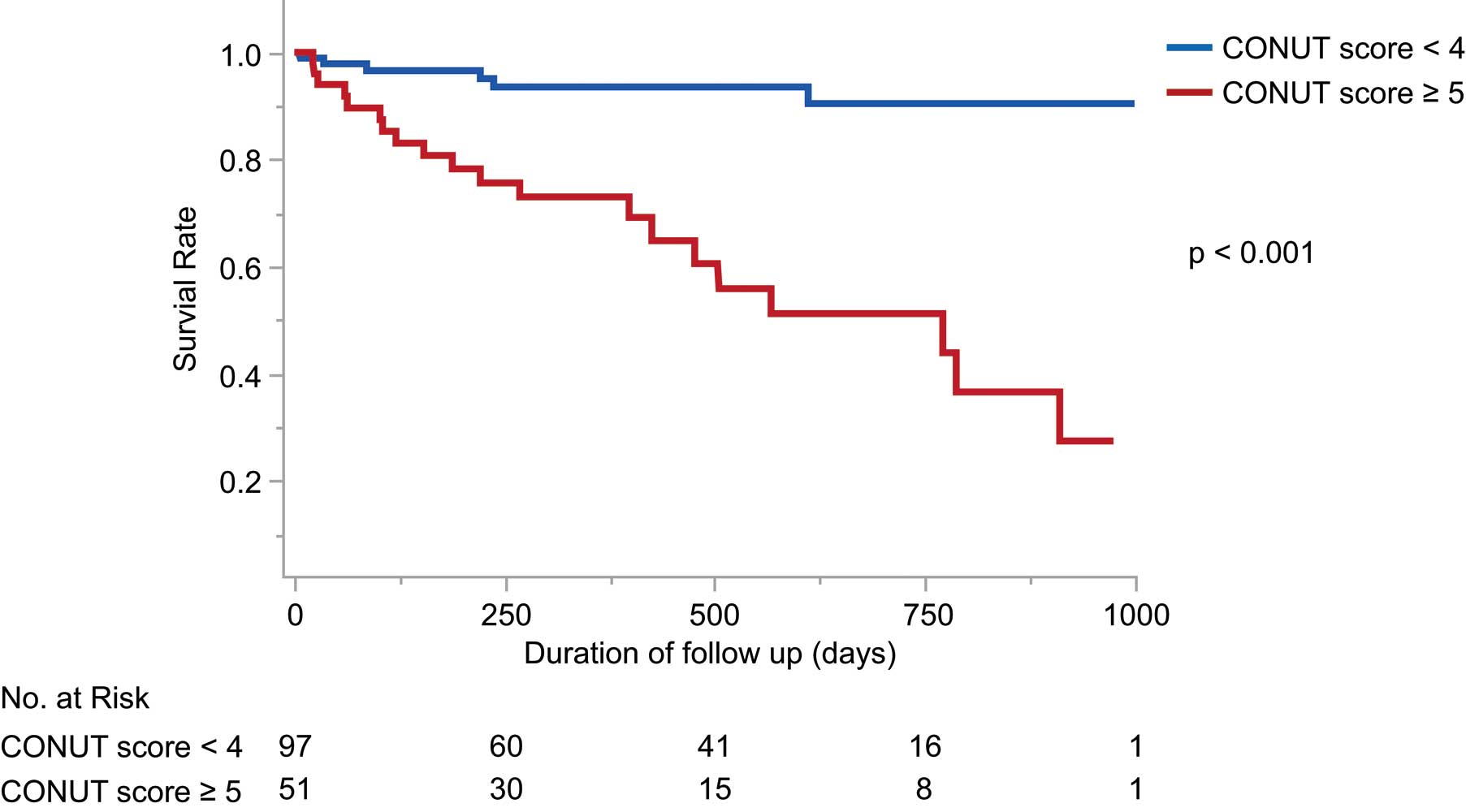
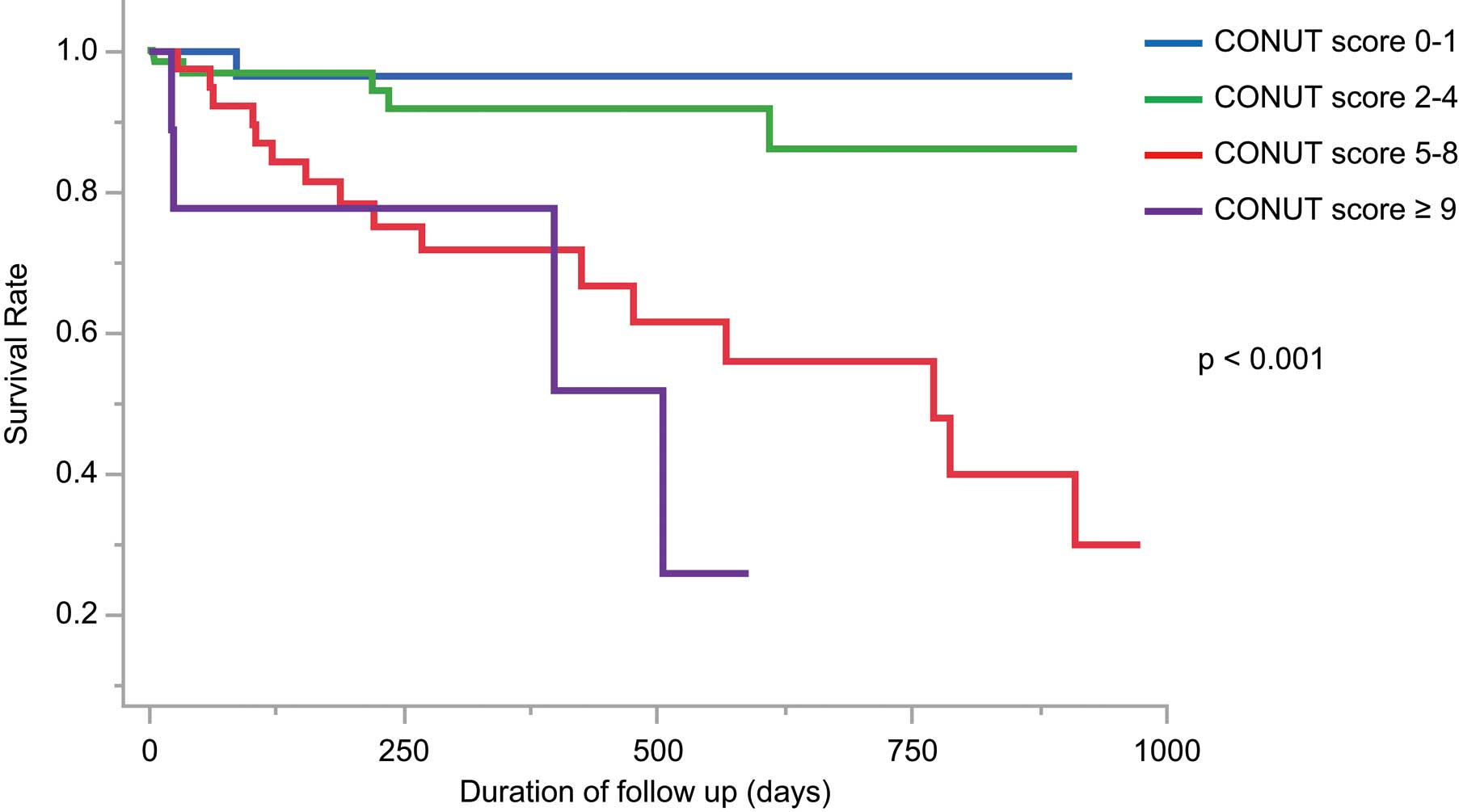

Table 3.
Predictors of All-Cause Death in Univariate and Multivariate Cox Regression Analyses
| |
Univariate analysis |
Multivariate analysis |
| HR |
95% CI |
P value |
Adjusted HR |
95% CI |
P value |
| Age, years |
1.04 |
0.99–1.10 |
0.09 |
|
|
|
| Male |
1.62 |
0.68–4.48 |
0.28 |
|
|
|
| BMI |
0.92 |
0.81–1.04 |
0.19 |
|
|
|
| Congestive HF within 1 year |
3.96 |
1.45–14.1 |
<0.01 |
|
|
|
| NYHA III/IV |
0.76 |
0.30–2.30 |
0.6 |
|
|
|
| GNRI |
0.93 |
0.90–0.97 |
<0.01 |
|
|
|
| Diabetes |
1.80 |
0.82–3.94 |
0.14 |
|
|
|
| Hypertension |
1.15 |
0.46–3.45 |
0.78 |
|
|
|
| Dyslipidemia |
0.74 |
0.34–1.62 |
0.45 |
|
|
|
| Liver cirrhosis |
2.92 |
0.16–14.0 |
0.37 |
|
|
|
| COPD |
2.46 |
0.90–5.80 |
0.08 |
|
|
|
| Prior stroke |
1.66 |
0.48–4.36 |
0.38 |
|
|
|
| CKD |
4.65 |
1.37–29.0 |
<0.01 |
|
|
|
| CAD |
0.50 |
0.18–1.21 |
0.13 |
|
|
|
| Other chest surgery |
0.65 |
0.04–3.10 |
0.66 |
|
|
|
| β-blocker |
1.38 |
0.52–4.74 |
0.54 |
|
|
|
| ACEi or ARB |
0.45 |
0.21–0.98 |
0.045 |
|
|
|
| Loop diuretic |
2.98 |
0.89–18.6 |
0.083 |
|
|
|
| Tolvaptan diuretic |
2.80 |
1.29–6.29 |
<0.01 |
|
|
|
| Antiplatelet |
0.43 |
0.18–0.97 |
0.04 |
|
|
|
| Anticoagulant |
1.20 |
0.55–2.82 |
0.66 |
|
|
|
| Prior CABG |
0.88 |
0.14–2.96 |
0.85 |
|
|
|
| Prior PCI |
0.55 |
0.18–1.35 |
0.2 |
|
|
|
| Cardiac rhythm device implantation |
1.49 |
0.58–3.41 |
0.38 |
|
|
|
| LVDd |
1.02 |
0.98–1.06 |
0.38 |
|
|
|
| LVDs |
1.02 |
0.99–1.04 |
0.26 |
|
|
|
| LVEDV |
1.00 |
0.99–1.01 |
0.3 |
|
|
|
| LVESV |
1.00 |
0.99–1.01 |
0.32 |
|
|
|
| LAVI |
1.01 |
0.99–1.02 |
0.073 |
|
|
|
| Af |
0.75 |
0.35–1.69 |
0.48 |
|
|
|
| Functional MR |
1.52 |
0.65–4.16 |
0.35 |
|
|
|
| TP |
0.60 |
0.38–0.94 |
0.027 |
|
|
|
| Albumin |
0.28 |
0.14–0.53 |
<0.01 |
|
|
|
| Hemoglobin |
0.70 |
0.55–0.88 |
<0.01 |
|
|
|
| WBC |
1.08 |
0.88–1.28 |
0.43 |
|
|
|
| Na |
0.94 |
0.84–1.05 |
0.28 |
|
|
|
| Cr |
1.10 |
0.94–1.24 |
0.22 |
|
|
|
| eGFR |
0.97 |
0.95–0.99 |
<0.01 |
|
|
|
| NT-proBNP |
1.00 |
0.99–1.00 |
0.23 |
|
|
|
| CRP |
1.13 |
1.05–1.20 |
<0.01 |
|
|
|
| HbA1c |
1.18 |
0.72–1.77 |
0.48 |
|
|
|
| LVEF |
0.99 |
0.96–1.01 |
0.3 |
|
|
|
| MR jet area |
1.00 |
0.97–1.04 |
0.8 |
|
|
|
| MR Vena contracta |
0.96 |
0.76–1.09 |
0.68 |
|
|
|
| MR ERO PISA |
0.55 |
0.03–5.23 |
0.64 |
|
|
|
| MR RV (PISA) |
0.99 |
0.97–1.01 |
0.22 |
|
|
|
| MR RV (volumetric) |
1.01 |
0.99–1.02 |
0.56 |
|
|
|
| MR RF (volumetric) |
1.02 |
0.99–1.05 |
0.31 |
|
|
|
| Residual MR grade |
0.69 |
0.23–1.95 |
0.49 |
|
|
|
| MNA-SF (every 1-point decrease) |
1.09 |
0.98–1.19 |
0.10 |
|
|
|
| MitraScore (every 1-point increase) |
1.82 |
1.37–2.45 |
<0.01 |
1.47 |
1.10–2.00 |
<0.01 |
| CONUT score (every 1-point increase) |
1.40 |
1.23–1.61 |
<0.01 |
1.31 |
1.12–1.52 |
<0.01 |
CI, confidence interval; HR, hazard ratio. Other abbreviations as in Table 1.
Discussion
In the present study we assessed the preprocedural nutritional status using the CONUT score in patients undergoing TEER, and our main findings were that (1) all-cause death was significantly worse in the high-CONUT score (≥5) group than in the low-CONUT score (<4) group (P<0.001), and (2) the preprocedural CONUT score, as well as the MitraScore, was significantly associated with all-cause death in patients undergoing TEER.
Malnutrition is very common among patients undergoing TEER. In this study, according to the CONUT score, most patients had some degree of risk for malnutrition at the time of their TEER procedure. There are reports on the association between each CONUT score component and clinical outcomes.10,26,27 In particular, a reduction in the lymphocyte count, which may be associated with physical stress, malnutrition, and chronic inflammation, predicted adverse outcomes in patients undergoing TEER using the MitraClip procedure. This information should allow the heart team to more accurately assess the clinical implications and prognostic benefits of the TEER procedure on a patient-by-patient basis. Hypoalbuminemia is also a well-known prognostic predictor for patients with HF, although Alb levels are influenced by reduced renal function and show a shortened half-life due to severe conditions. In fact, the bivariate Cox analysis of Alb and CONUT score with death as the outcome showed that the CONUT score remained a significant predictor of prognosis (Alb HR: 0.67 P=0.53, CONUT score: HR=1.51, P=0.002). Because the CONUT score incorporates total cholesterol, we conducted an additional analysis to explore its association with statin use. That analysis revealed that statin use was not associated with the CONUT score (P=0.930) (Supplementary Figure). We consider that these underlying mechanisms were closely linked to not only nutrition status but also acute exacerbation of HF due to severe MR; it has been hypothesized that the preprocedural CONUT score, and a complex of immune conditions, metabolism, and protein profile are associated with adverse outcomes for patients undergoing TEER using with the MitraClip.28 In this study, 4 patients were excluded because of the morphology of their mitral valve made MitraClip implantation unfeasible or impossible according to the EVEREST criteria. In such high-risk cases, death may occur from complications associated with the procedure, even if the patient is in good general condition and well nourished. In fact, our Cox hazard analysis showed that the CONUT score was significantly associated with all-cause death in patients including the 4 who were excluded by the Everest criteria (HR: 1.30, 95% CI: 1.10–1.59, P<0.01). When considering the preprocedural score, the STS risk score is a known valuable screening tool for predicting death after cardiac surgery. However, our Cox hazard analysis revealed a significant association between all-cause death and the CONUT score as compared with the STS score as well as MitraScore (Supplementary Table 2). Of note, all-cause death were not associated with transfusion administration, elevated CRP level, use of assisted circulation, postoperative acute kidney injury and preoperative CONUT score (Supplementary Table 3).
The CONUT score was originally designed to predict “acute worsening” in surgical patients and subsequently adapted for chronic HF, which may affect its applicability in patients undergoing TEER using the MitraClip procedure.28 In other studies,11,26 the association between the CONUT score at the time of hospitalization and adverse outcomes was reported in patients with acute decompensated HF, and the CONUT score on admission expressed a malignant cycle in patients with HF, whereby HF caused malnutrition through fluid retention and malnutrition, leading to inflammation and neurohormonal activation. Recently, Kalbacher et al reported that being underweight (body mass index <20 kg/m2) was associated with procedural failure, bleeding, and transfusion in hospital deaths after MitraClip implantation.29 As one of the purposes of the MitraClip procedure is to slow down the progression of HF, a multidisciplinary approach to assessing risk is crucial.30 To complicate matters further, 10–15% of HF patients develop cardiac cachexia, characterized by loss of body weight due to deterioration of muscle and adipose tissue.10,27 Implantation of the MitraClip in the early stages of HF may help improve patients’ functional capacity.
Using the CONUT score to assess the fitness of patients before the TEER procedure, we can implement an appropriate nutritional intervention to improve the likelihood of better outcomes for elderly patients with MR.31
Study Limitations
First, the sample size was very small, and the study was conducted at a single center in Japan. Second, nutritional status was assessed only on admission. Additional clinical data obtained using the CONUT score may be useful. The Mini Nutritional Assessment was not included as a nutritional tool in the evaluation conducted in this study. Third, the 95% CIs of the odds ratios for the CONUT score were wide, probably due to the small sample size. Further studies should be conducted in a large population to determine the applicable nutritional assessment score.
Conclusions
Malnutrition may be related to all-cause death in patients undergoing MitraClip procedure, so preprocedural assessment of nutritional status may provide additional prognostic information, allowing the heart team to more accurately assess both the clinical implications and prognosis before deciding on the best course of action.
Acknowledgments
We thank Editage (www.editage.com) for English language editing.
Disclosures
S.K. and M.I. are consultants at Abbott Medical in Japan. Y.J.A. is a member of Circulation Reports’ Editorial Team. The other authors have no conflicts of interest to declare.
IRB Information
The Ethics Committee of St. Marianna University School of Medicine (No. 5959).
Data Availability
6th
May 2023.
Supplementary Files
Please find supplementary file(s);
https://doi.org/10.1253/circrep.CR-23-0055
References
- 1.
McCauley BD, Herrmann HC, Chen T, Anwaruddin S. MitraClip for secondary mitral regurgitation: Approach to the 2020 ACC/AHA Valvular Heart Disease guidelines. JACC Case Rep 2021; 3: 361–365, doi:10.1016/j.jaccas.2020.12.008.
- 2.
Giustino G, Camaj A, Kapadia SR, Kar S, Abraham WT, Lindenfeld J, et al. Hospitalizations and mortality in patients with secondary mitral regurgitation and heart failure: The COAPT Trial. J Am Coll Cardiol 2022; 80: 1857–1868, doi:10.1016/j.jacc.2022.08.803.
- 3.
Rios S, Li W, Mustehsan MH, Hajra A, Takahashi T, Chengyue J, et al. Impact of frailty on outcomes after transcatheter edge-to-edge repair with MitraClip (from the National Inpatient Sample Database). Am J Cardiol 2022; 179: 58–63, doi:10.1016/j.amjcard.2022.06.019.
- 4.
Scotti A, Munafò A, Adamo M, Taramasso M, Denti P, Sisinni A, et al. Transcatheter edge-to-edge repair in coapt-ineligible patients: Incidence and predictors of 2-year good outcome. Can J Cardiol 2022; 38: 320–329, doi:10.1016/j.cjca.2021.12.003.
- 5.
Wong N, Yeo KK. MitraClip in Asia: Current adoption and regional data. Circ Rep 2019; 1: 397–400, doi:10.1253/circrep.CR-19-0074.
- 6.
Stolz L, Braun D, Higuchi S, Orban M, Doldi PM, Stocker TJ, et al. Transcatheter edge-to-edge mitral valve repair in mitral regurgitation: Current status and future prospects. Expert Rev Med Devices 2023; 20: 99–108, doi:10.1080/17434440.2022.2098013.
- 7.
Raposeiras-Roubin S, Adamo M, Freixa X, Arzamendi D, Benito-González T, Montefusco A, et al. A score to assess mortality after percutaneous mitral valve repair. J Am Coll Cardiol 2022; 79: 562–573, doi:10.1016/j.jacc.2021.11.041.
- 8.
Boekstegers P, Hausleiter J, Schmitz T, Bufe A, Comberg T, Seyfarth M, et al. Intraprocedural residual mitral regurgitation and survival after transcatheter edge-to-edge repair: Prospective German Multicenter Registry (MITRA-PRO). JACC Cardiovasc Interv 2023; 16: 574–585, doi:10.1016/j.jcin.2022.12.015.
- 9.
Shechter A, Vaturi M, Kaewkes D, Koren O, Koseki K, Solanki A, et al. Prognostic value of baseline tricuspid annular plane systolic excursion to pulmonary artery systolic pressure ratio in mitral transcatheter edge-to-edge repair. J Am Soc Echocardiogr 2023; 36: 391–401.e319, doi:10.1016/j.echo.2022.12.026.
- 10.
Nakagomi A, Kohashi K, Morisawa T, Kosugi M, Endoh I, Kusama Y, et al. Nutritional status is associated with inflammation and predicts a poor outcome in patients with chronic heart failure. J Atheroscler Thromb 2016; 23: 713–727, doi:10.5551/jat.31526.
- 11.
Takikawa T, Sumi T, Takahara K, Kawamura Y, Ohguchi S, Oguri M, et al. Prognostic importance of multiple nutrition screening indexes for 1-year mortality in hospitalized acute decompensated heart failure patients. Circ Rep 2019; 1: 87–93, doi:10.1253/circrep.CR-18-0018.
- 12.
Watanabe Y, Horiuchi Y, Nakase M, Setoguchi N, Ishizawa T, Sekiguchi M, et al. Malnutrition, hemodynamics and inflammation in heart failure with reduced, mildly reduced and preserved ejection fraction. Heart Vessels 2022; 37: 1841–1849, doi:10.1007/s00380-022-02090-3.
- 13.
Muto Y, Higuchi R, Yoshihisa A, Hagiya K, Saji M, Takamisawa I, et al. Prevalence, predictors, and mid-term outcomes of non-home discharge after transcatheter aortic valve implantation. Circ Rep 2020; 2: 617–624, doi:10.1253/circrep.CR-20-0085.
- 14.
Li Q, Lu X, Chen W, Huang H, Chen S, Chen W, et al. Malnutrition increases the risk of left ventricular remodeling. J Nutr Health Aging 2022; 26: 1094–1100, doi:10.1007/s12603-022-1862-0.
- 15.
Ni J, Fang Y, Zhang J, Chen X. Predicting prognosis of heart failure using common malnutrition assessment tools: A systematic review and meta-analysis. Scott Med J 2022; 67: 157–170, doi:10.1177/00369330221122300.
- 16.
Uemura Y, Shibata R, Miyagaki Y, Takemoto K, Ishikawa S, Murohara T, et al. A comparative study of three nutritional risk/screening indices for predicting cardiac events and physical functioning among patients with acute heart failure. Int Heart J 2022; 63: 541–549, doi:10.1536/ihj.21-809.
- 17.
Agnoletti D, Arcaro G, Scaturro G, Turcato E, Grison E, Ferrari E, et al. Controlling nutritional status score predicts 2-year outcomes in elderly patients admitted for acute heart failure. Intern Emerg Med 2023; 18: 1031–1039, doi:10.1007/s11739-023-03230-x.
- 18.
Liang L, Zhao X, Huang L, Tian P, Huang B, Feng J, et al. Prevalence and prognostic importance of malnutrition, as assessed by four different scoring systems, in elder patients with heart failure. Nutr Metab Cardiovasc Dis 2023; 33: 978–986, doi:10.1016/j.numecd.2023.01.004.
- 19.
Kinugasa Y, Sota T, Kamitani H, Nakayama N, Nakamura K, Hirai M, et al. Diagnostic performance of nutritional indicators in patients with heart failure. ESC Heart Fail 2022; 9: 2096–2106, doi:10.1002/ehf2.13886.
- 20.
Glower DD, Kar S, Trento A, Lim DS, Bajwa T, Quesada R, et al. Percutaneous mitral valve repair for mitral regurgitation in high-risk patients: Results of the EVEREST II study. J Am Coll Cardiol 2014; 64: 172–181, doi:10.1016/j.jacc.2013.12.062.
- 21.
Puls M, Tichelbäcker T, Bleckmann A, Hünlich M, von der Ehe K, Beuthner BE, et al. Failure of acute procedural success predicts adverse outcome after percutaneous edge-to-edge mitral valve repair with MitraClip. EuroIntervention 2014; 9: 1407–1417, doi:10.4244/eijv9i12a238.
- 22.
Attizzani GF, Ohno Y, Capodanno D, Cannata S, Dipasqua F, Immé S, et al. Extended use of percutaneous edge-to-edge mitral valve repair beyond EVEREST (Endovascular Valve Edge-to-Edge Repair) criteria: 30-day and 12-month clinical and echocardiographic outcomes from the GRASP (Getting Reduction of Mitral Insufficiency by Percutaneous Clip Implantation) registry. JACC Cardiovasc Interv 2015; 8: 74–82, doi:10.1016/j.jcin.2014.07.024.
- 23.
Lesevic H, Karl M, Braun D, Barthel P, Orban M, Pache J, et al. Long-term outcomes after MitraClip implantation according to the presence or absence of EVEREST inclusion criteria. Am J Cardiol 2017; 119: 1255–1261, doi:10.1016/j.amjcard.2016.12.027.
- 24.
Klein AL, Abbara S, Agler DA, Appleton CP, Asher CR, Hoit B, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: Endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2013; 26: 965–1012.e1015, doi:10.1016/j.echo.2013.06.023.
- 25.
Presume J, Lopes P, Freitas P, Albuquerque F, Reis C, Horta E, et al. Secondary mitral regurgitation: Maintaining coherence with the American Society of Echocardiography grading guidelines, which proportionality concept best predicts prognosis in the real world? Rev Port Cardiol 2022; 41: 1025–1032, doi:10.1016/j.repc.2022.03.005.
- 26.
Takada T, Jujo K, Inagaki K, Abe T, Kishihara M, Shirotani S, et al. Nutritional status during hospitalization is associated with the long-term prognosis of patients with heart failure. ESC Heart Fail 2021; 8: 5372–5382, doi:10.1002/ehf2.13629.
- 27.
Narumi T, Arimoto T, Funayama A, Kadowaki S, Otaki Y, Nishiyama S, et al. Prognostic importance of objective nutritional indexes in patients with chronic heart failure. J Cardiol 2013; 62: 307–313, doi:10.1016/j.jjcc.2013.05.007.
- 28.
Caneiro-Queija B, Raposeiras-Roubin S, Adamo M, Freixa X, Arzamendi D, Benito-González T, et al. Prognostic impact of nutritional status after transcatheter edge-to-edge mitral valve repair: The MIVNUT registry. J Am Heart Assoc 2022; 11: e023121, doi:10.1161/jaha.121.023121.
- 29.
Kalbacher D, Tigges E, Boekstegers P, Puls M, Plicht B, Eggebrecht H, et al. Underweight is associated with inferior short and long-term outcomes after MitraClip implantation: Results from the German TRAnscatheter mitral valve interventions (TRAMI) registry. Am Heart J 2020; 222: 73–82, doi:10.1016/j.ahj.2019.12.022.
- 30.
Kuwata S, Izumo M, Okuno T, Shiokawa N, Sato Y, Koga M, et al. Impact of renal congestion in patients with secondary mitral regurgitation after mitral transcatheter edge-to-edge repair. Circ J 2023, doi:10.1253/circj.CJ-23-0240.
- 31.
Nochioka K, Sakata Y, Takahashi J, Miyata S, Miura M, Takada T, et al; CHART-2 Investigators. Prognostic impact of nutritional status in asymptomatic patients with cardiac diseases: A report from the CHART-2 Study. Circ J 2013; 77: 2318–2326, doi:10.1253/circj.CJ-13-0127.





