2023 年 5 巻 4 号 p. 144-151
2023 年 5 巻 4 号 p. 144-151
Background: Rivaroxaban, a direct oral anticoagulant, is used as a first-line treatment to prevent venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE). However, whether 21 days is optimal for the initial treatment duration has not been investigated.
Methods and Results: In this subanalysis of the prospective multicenter observational J’xactly study, which included 1,039 Japanese patients with acute symptomatic/asymptomatic DVT/PE who were prescribed rivaroxaban, the VTE recurrence rate and incidence of bleeding complications were assessed in 667 patients who underwent intensive rivaroxaban treatment (15 mg, twice daily) for a short (1–8 days), intermediate (9–16), or standard (17–24) duration. The short treatment duration group showed a tendency for increased VTE recurrence/aggravation compared with the standard treatment duration group (6.10% vs. 2.60% per patient-year). The intermediate treatment duration group showed a higher incidence of bleeding events than the standard treatment duration group (9.34% vs. 2.16% per patient-year), without major differences in patient characteristics between the groups.
Conclusions: In this subanalysis of the real-world observational J’xactly study of VTE treatment and prevention in Japanese patients with acute symptomatic/asymptomatic DVT/PE, the standard initial intensive rivaroxaban treatment duration (17–24 days) appeared to be safe and effective, providing important insights into the clinical outcomes of the initial rivaroxaban treatment duration in this population.
Venous thromboembolism (VTE), including deep vein thrombosis (DVT) and pulmonary embolism (PE), is a leading cause of morbidity, mortality, and hospitalization worldwide,1 with its global burden compounded by high recurrence rates.2
Parenteral anticoagulants and vitamin K antagonists have been the cornerstone of VTE treatment to prevent sequelae, including thrombus extension and embolization, recurrent VTE, and death.2 However, direct oral anticoagulants (DOACs), which do not require routine coagulation monitoring, are increasingly used as first-line treatment.3,4 The duration of VTE treatment generally comprises 3 periods: acute/initial, long-term (from the end of acute treatment to 3–6 months), and extended (beyond 3–6 months). To determine the choice of anticoagulant and the duration of therapy, the benefits of anticoagulation should be weighed against the risk of bleeding.
The efficacy and safety of monotherapy with the DOAC rivaroxaban, a Factor Xa inhibitor, in patients with acute VTE have been demonstrated in large-scale multinational clinical trials5,6 and real-world studies.7,8 Based on the results of the EINSTEIN DVT and EINSTEIN-PE trials,5,6,9 in Western countries the recommended rivaroxaban dosage for the initial treatment of acute VTE is 15 mg twice daily (b.i.d.), followed by 20 mg once daily for continued treatment and to prevent recurrence. However, the pharmacokinetics of rivaroxaban differ between Western and Japanese populations. Thus, in Japan, 15 mg b.i.d. for initial consolidation and 15 mg once daily for subsequent maintenance therapy are recommended based on the results of the J-EINSTEIN DVT and EINSTEIN-PE trials.10 The recommended initial intensive treatment duration is 21 days in both Western countries and Japan.
The multicenter Japanese registry of rivaroXAban effectiveness and safety for preventing reCurrence in patients with deep vein Thrombosis and puLmonarY embolism (J’xactly) study investigated the effectiveness and safety of rivaroxaban in 1,039 Japanese patients with VTE in the real-world setting.7,11 Following the diagnosis of DVT or PE, the standard rivaroxaban regimen was 15 mg b.i.d. for 21 days and 15 mg once daily thereafter. Based on the discretion of physicians, approximately 65% of patients were treated with rivaroxaban 15 mg b.i.d. for the initial treatment of VTE. Among these patients, approximately 80% were treated with rivaroxaban 15 mg b.i.d. for less than 21 days.7
We aimed to examine whether the initial intensive treatment duration of 21 days is optimal for acute VTE in terms of risk-benefit weighting in Japanese patients, where this has not yet been investigated in large clinical trials. This subanalysis of the J’xactly study assessed the VTE recurrence rate and incidence of bleeding complications in patients who underwent initial intensive rivaroxaban treatment for acute VTE according to a short, intermediate, or standard treatment duration.
The details of the study design, data collection process, and baseline characteristics of the study population have been reported previously.7,11 Briefly, the J’xactly study was a multicenter prospective observational cohort study in which patients with acute symptomatic/asymptomatic DVT, PE, or both, and who were prescribed oral rivaroxaban for the treatment of acute VTE and prevention of VTE recurrence at centers in Japan, were enrolled from December 2016 to April 2018 and followed up for at least 18 months and up to 3 years after enrollment.
The study was conducted in compliance with the principles of the Declaration of Helsinki and with all applicable legal and regulatory requirements in Japan. The study protocol and related documentation were reviewed and approved by the Institutional Review Board of Nihon University Itabashi Hospital (RK-160913-4); all participating institutions also provided ethics approval. In addition, an independent data and safety monitoring committee reviewed all the study data. The study was registered at the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (ID: UMIN000025072).
Study ParticipantsBriefly, the study inclusion criteria were a diagnosis of acute symptomatic or asymptomatic DVT, PE, or both, and the use of rivaroxaban for the treatment and prevention of VTE. The key exclusion criteria were contraindications to rivaroxaban; the presence of chronic thromboembolic pulmonary hypertension (CTEPH), except for CTEPH plus acute PE or DVT; and active bleeding. All patients provided written informed consent for study participation.
Eligible patients were consecutively enrolled in the study within 3 weeks of starting the rivaroxaban prescription. Data were collected until the end of the follow-up period (November 2019), regardless of whether rivaroxaban was continued, discontinued, or terminated according to the patient’s preference or at the physician’s discretion.
In all, 1,039 patients were enrolled in the study from December 2016 to April 2018. In this subanalysis, patients with VTE who were treated with an initial intensive regimen of rivaroxaban (n=667; 15 mg b.i.d. for up to 24 days) were analyzed. These patients were stratified into 3 groups according to the duration of intensive treatment: short (1–8 days), intermediate (9–16 days), or standard (17–24 days).
Outcome AssessmentsAs described previously,7,11 the primary endpoint was the recurrence or aggravation of symptomatic VTE during the follow-up period. VTE was defined according to established diagnostic criteria.12,13
The primary safety endpoint was major bleeding, as defined by the International Society on Thrombosis and Haemostasis criteria,14 that occurred during the treatment period and up to 2 days after rivaroxaban discontinuation. Secondary endpoints were recurrence or aggravation of symptomatic DVT and PE, death from any cause, death related to VTE and cardiovascular disease (CVD), a vascular event (acute coronary syndrome [ACS] or ischemic stroke), and non-major bleeding. Net clinical adverse events (NACEs) were also evaluated as a composite outcome, in which each component (recurrence or aggravation of symptomatic VTE or major bleeding) was weighted equally. An independent, blinded clinical events committee adjudicated the outcomes.
Statistical AnalysisSubgroup analyses were performed to compare the standard treatment duration with the short and intermediate treatment durations. Subgroups were compared using Student’s t-test, the Wilcoxon rank-sum test, or Fisher’s exact test, as appropriate. Continuous variables are reported as the mean±SD and categorical variables are reported as the number and percentage of patients. Cumulative event incidences and their 95% confidence intervals (CIs) were determined using the Kaplan-Meier method. The results of Cox proportional hazards modeling for between-group differences in clinical outcomes are expressed as hazard ratios (HRs) and 95% CIs. All statistical analyses were performed using SAS version 9.4 for Windows (SAS Institute, Inc., Cary, NC, USA). P<0.05 was considered statistically significant.
Of 1,039 patients with acute DVT, PE, or both enrolled at 152 centers in Japan, 667 underwent treatment with an initial intensive rivaroxaban dosing regimen of 15 mg b.i.d. and were included in the analysis (Figure 1). Among the patients who received an initial intensive dosing regimen of rivaroxaban, the most frequent treatment duration was the standard duration (17–24 days; n=482 [76.4%]), followed by the intermediate duration (9–16 days; n=97 [15.4%]) and the short duration (1–8 days; n=52; [8.2%]). The baseline mean age was comparable between the standard (65.8±15.0 years), intermediate (66.4±15.4 years), and short (67.4±17.8 years) treatment duration groups, and the 3 groups comprised comparable proportions of female patients (54.4%, 56.7%, and 59.6%, respectively; Table 1). There were no major differences in patient characteristics between the 3 groups. In all groups, 29–36% of patients were outpatients. The frequency of DVT or PE and the type of DVT or PE did not differ significantly between the 3 groups, except for symptomatic PE. Significantly more patients in the intermediate treatment duration group had symptomatic PE than in the standard treatment duration group (35.1% vs. 26.6%, respectively; P<0.028). Of the comorbidities, significantly more patients in the intermediate than standard treatment duration group had experienced previous stroke (13.4% vs. 6.2%, respectively; P<0.019).
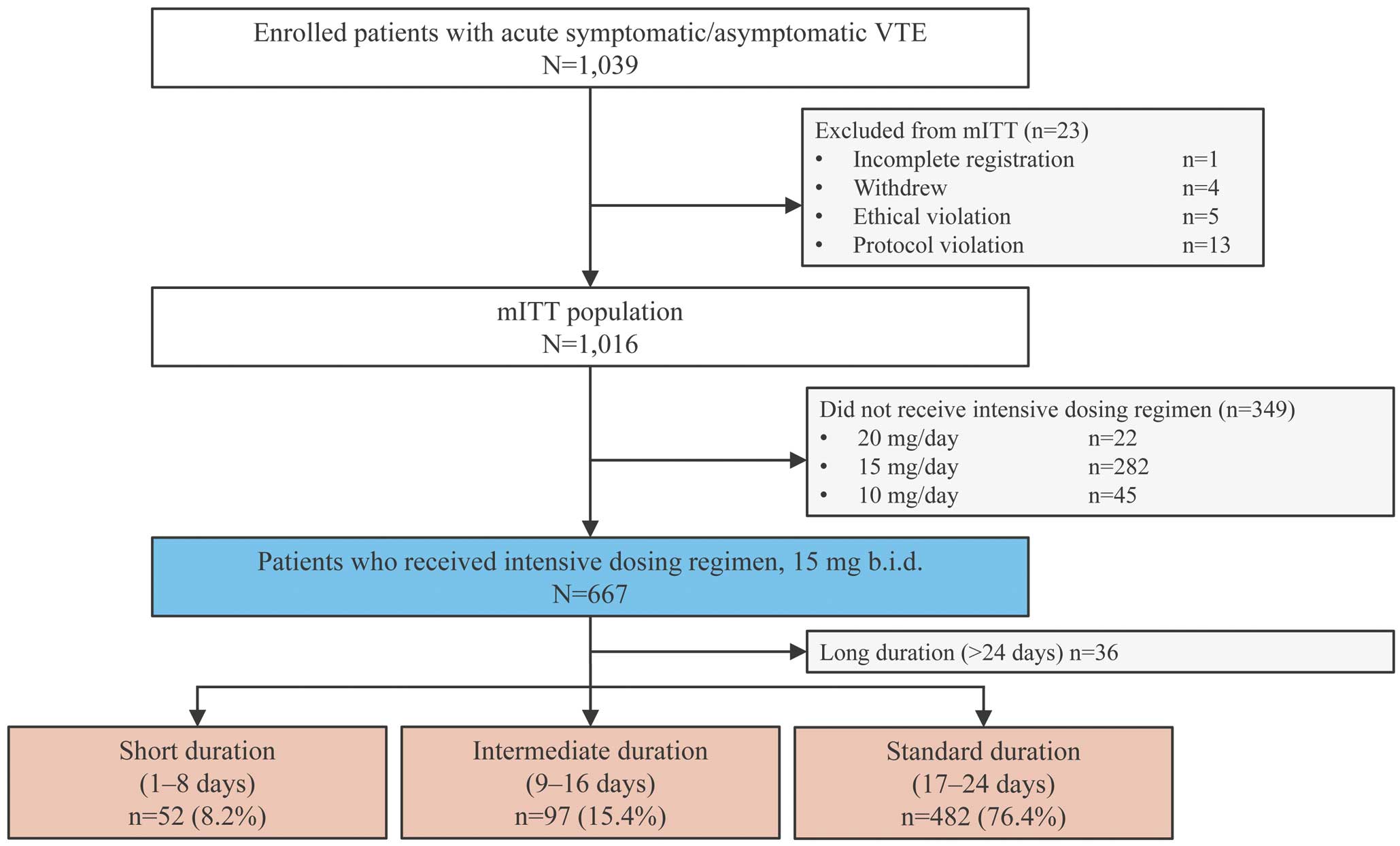
Flow diagram of patient disposition. Venous thromboembolism (VTE) includes deep vein thrombosis, pulmonary embolism, or both. mITT, modified intention-to-treat.
| Duration of initial intensive treatment | P valueA | ||||
|---|---|---|---|---|---|
| Short (n=52) |
Intermediate (n=97) |
Standard (n=482) |
Short vs. standard |
Intermediate vs. standard |
|
| Characteristics | |||||
| Mean age (years) | 67.4±17.8 | 66.4±15.4 | 65.8±15.0 | 0.250 | 0.608 |
| Age ≥75 years | 20 (38.5) | 34 (35.1) | 154 (32.0) | 0.635 | 0.541 |
| Female sex | 31 (59.6) | 55 (56.7) | 262 (54.4) | 0.558 | 0.738 |
| Outpatient | 15 (28.8) | 31 (32.0) | 174 (36.1) | 0.360 | 0.486 |
| Presentation | |||||
| DVT | 44 (84.6) | 87 (89.7) | 435 (90.2) | ||
| Proximal | 14 (26.9) | 33 (34.0) | 134 (27.8) | 0.473 | 0.406 |
| Distal | 30 (57.7) | 54 (55.7) | 301 (62.4) | ||
| Symptomatic DVT | 28 (53.8) | 60 (61.9) | 309 (64.1) | 0.304 | 0.700 |
| PE | 27 (51.9) | 49 (50.5) | 248 (51.5) | ||
| Cardiac arrest or collapse | 0 (0.0) | 0 (0.0) | 4 (0.8) | 0.511 | 0.145 |
| Massive | 0 (0.0) | 5 (5.2) | 10 (2.1) | ||
| Submassive | 9 (17.3) | 20 (20.6) | 77 (16.0) | ||
| Non-massive | 18 (34.6) | 21 (21.6) | 144 (29.9) | ||
| Unknown | 0 (0.0) | 3 (3.1) | 13 (2.7) | ||
| Symptomatic PE | 18 (34.6) | 34 (35.1) | 128 (26.6) | 0.158 | 0.028 |
| Risk factor | |||||
| Inactivity | 15 (28.8) | 41 (42.3) | 161 (33.4) | 0.539 | 0.103 |
| Injury | 9 (17.3) | 20 (20.6) | 35 (7.3) | 0.028 | 0.001 |
| Surgery | 17 (32.7) | 20 (20.6) | 107 (22.2) | 0.118 | 0.789 |
| Active cancer | 9 (17.3) | 13 (13.4) | 92 (19.1) | 0.854 | 0.247 |
| Thrombophilia | 1 (1.9) | 4 (4.1) | 22 (4.6) | 0.716 | 1.000 |
| Previous VTE | 5 (9.6) | 4 (4.1) | 40 (8.3) | 0.791 | 0.207 |
| CrCl (mL/min) | 76.0±34.3 | 84.3±41.8 | 82.4±36.9 | 0.153 | 0.892 |
| <30 mL/min | 0 (0) | 1 (1.0) | 3 (0.6) | 0.268 | 0.752 |
| ≥30 to <50 mL/min | 13 (25.0) | 18 (18.6) | 70 (14.5) | ||
| ≥50 to <80 mL/min | 18 (34.6) | 34 (35.1) | 183 (38.0) | ||
| ≥80 mL/min | 20 (38.5) | 42 (43.3) | 208 (43.2) | ||
| Body weight (kg) | 59.8±10.9 | 62.4±18.2 | 62.5±14.5 | 0.399 | 0.344 |
| <50 kg | 10 (19.2) | 25 (25.8) | 85 (17.6) | 0.833 | 0.172 |
| BMI (kg/m2) | 24.0±4.1 | 24.2±5.1 | 24.1±4.2 | 0.756 | 0.796 |
| D-dimer (μg/mL) | 10.0 [3.4–20.1] | 10.0 [3.8–15.9] | 8.4 [4.7–18.3] | 0.909 | 0.820 |
| Pulse rate (beats/min) | 84.9±13.3 | 86.4±19.0 | 84.9±18.0 | 0.582 | 0.492 |
| Medical history | |||||
| Previous stroke | 3 (5.8) | 13 (13.4) | 30 (6.2) | 1.000 | 0.019 |
| CAD | 3 (5.8) | 3 (3.1) | 17 (3.5) | 0.431 | 1.000 |
| Hypertension | 18 (34.6) | 34 (35.1) | 180 (37.3) | 0.764 | 0.730 |
| Diabetes | 6 (11.5) | 15 (15.5) | 59 (12.2) | 1.000 | 0.405 |
| Heart failure | 2 (3.8) | 4 (4.1) | 15 (3.1) | 0.677 | 0.541 |
| Atrial fibrillation | 3 (5.8) | 5 (5.2) | 13 (2.7) | 0.197 | 0.203 |
| Chronic heart and lung disease | 3 (5.8) | 8 (8.2) | 17 (3.5) | 0.431 | 0.051 |
| Concomitant medications | |||||
| Antiplatelet agents | 5 (9.6) | 9 (9.3) | 36 (7.5) | 0.582 | 0.535 |
| NSAIDs | 13 (25.0) | 17 (17.5) | 76 (15.8) | 0.115 | 0.651 |
| Estrogen preparations | 1 (1.9) | 4 (4.1) | 10 (2.1) | 1.000 | 0.269 |
| Anticancer agents | 4 (7.7) | 6 (6.2) | 45 (9.3) | 1.000 | 0.432 |
| Treatment duration (days) | 228 [59.5–574.5] | 223 [99–576] | 369.5 [141–638] | 0.021 | 0.023 |
Unless indicated otherwise, data are given as the mean±SD, median [interquartile range], or n (%). AComparisons of patients with deep vein thrombosis (DVT) only and pulmonary embolism (PE) with or without DVT using Student’s t-test, the Wilcoxon rank-sum test, or Fisher’s exact test, as appropriate. Initial intensive treatment duration was categorized as short (1–8 days), intermediate (9–16 days), and standard (17–24 days). BMI, body mass index; CAD, coronary artery disease; CrCl, creatinine clearance; NSAIDs, non-steroidal anti-inflammatory drugs.
For the primary effectiveness outcome, the Kaplan-Meier analysis indicated that the rate of symptomatic VTE recurrence tended to be higher in the short than standard treatment duration group; however, no significant between-group difference in the cumulative incidence of recurrence or aggravation of symptomatic VTE was observed (6.10% vs. 2.60% per patient-year, respectively; Figure 2). Clinical outcomes are presented in Table 2. There was no significant difference between the standard and intermediate treatment duration groups in the cumulative incidence of recurrence or aggravation of symptomatic VTE (2.60% vs. 3.21% per patient-year, respectively; Figure 2; Table 2). The number of patients in the short, intermediate, and standard treatment duration groups with recurrence or aggravation of symptomatic VTE from the start of initial intensive treatment was 2/53 (3.8%), 0/97 (0%), and 5/482 (1.0%), respectively, at 3 months; 2/53 (3.8%), 0/97 (0%), and 6/482 (1.2%), respectively, at 6 months; and 3/52 (5.8%), 4/97 (4.1%), and 13/482 (2.7%), respectively, at 12 months.
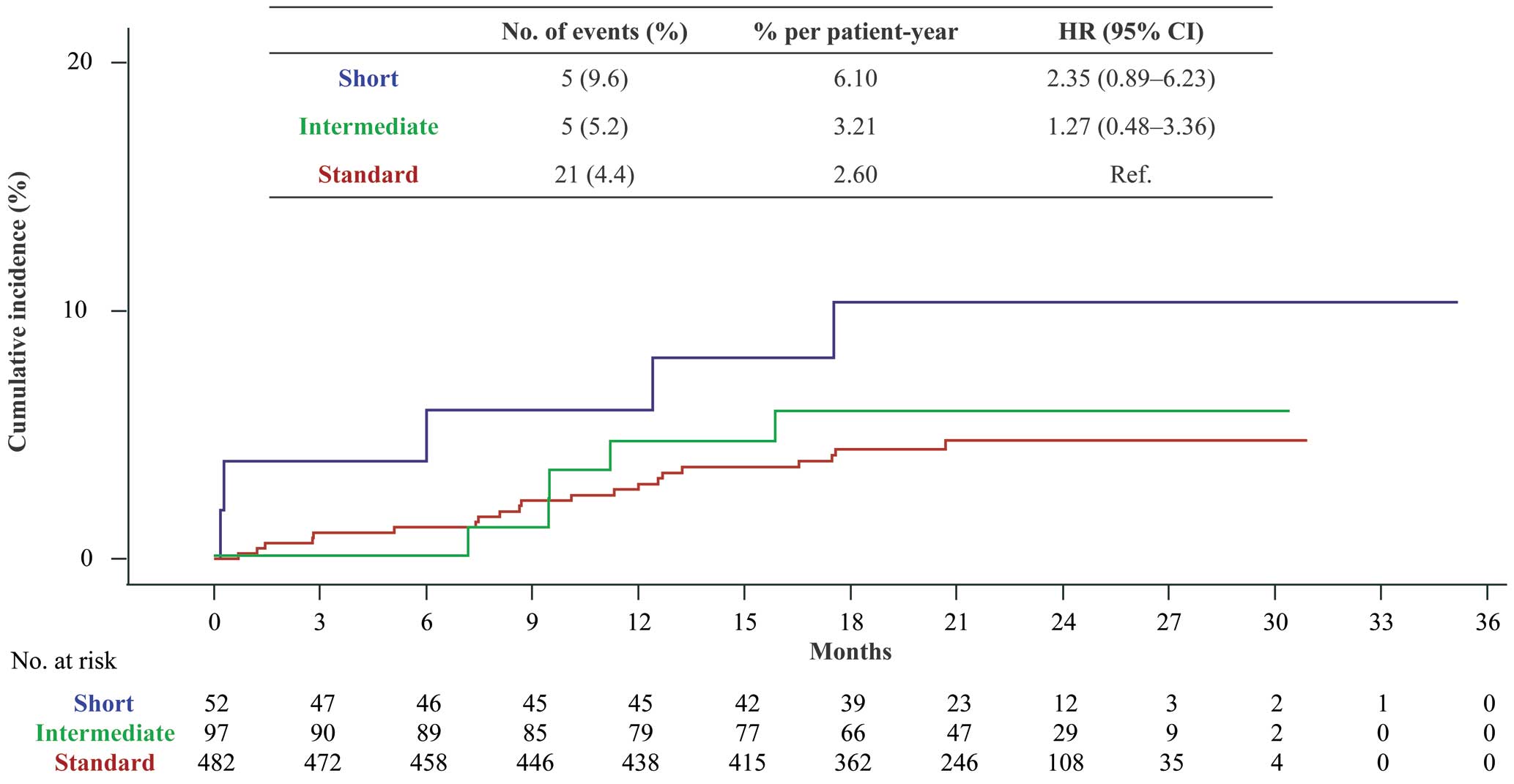
Recurrence or aggravation of symptomatic venous thromboembolism following short, intermediate, or standard rivaroxaban treatment duration. Cumulative incidence, as determined by the Kaplan-Meier method. CI, confidence interval; HR, hazard ratio.
| Duration of intensive treatmentA | HR (95% CI) | ||||
|---|---|---|---|---|---|
| Short (n=52) |
Intermediate (n=97) |
Standard (n=482) |
Short vs. standard |
Intermediate vs. standard |
|
| Primary effectiveness outcome | |||||
| Recurrence or aggravation of symptomatic VTE |
6.1 (0.8–11.4) | 3.2 (0.4–6.0) | 2.6 (1.5–3.7) | 2.35 (0.89–6.23) | 1.27 (0.48–3.36) |
| Secondary effectiveness outcome | |||||
| Recurrence or aggravation of symptomatic PE |
2.4 (0.0–5.6) | 1.9 (0.0–4.1) | 1.1 (0.4–1.8) | 2.13 (0.46–9.84) | 1.75 (0.47–6.48) |
| Recurrence or aggravation of symptomatic DVT |
3.5 (0.0–7.5) | 1.3 (0.0–3.0) | 1.8 (0.9–2.8) | 1.91 (0.55–6.58) | 0.71 (0.16–3.09) |
| Acute coronary syndrome | – | – | 0.1 (0.0–0.4) | – | – |
| Ischemic stroke | – | 0.6 (0.0–1.9) | 0.6 (0.1–1.1) | – | 1.02 (0.12–8.75) |
| Death from any cause | 3.4 (0.0–7.2) | 5.0 (1.5–8.5) | 3.9 (2.5–5.2) | 0.88 (0.27–2.88) | 1.33 (0.61–2.88) |
| Death related to VTE | – | – | 0.2 (0.0–0.6) | – | – |
| Death related to CVD | – | 0.6 (0.0–1.9) | 0.2 (0.0–0.6) | – | 2.55 (0.23–28.11) |
| Principal safety outcome | |||||
| Major bleeding | 2.3 (0.0–6.7) | 9.3 (2.9–15.8) | 2.2 (0.9–3.4) | 1.07 (0.14–8.26) | 4.16 (1.67–10.36) |
| Secondary safety outcome | |||||
| Minor bleeding | 11.6 (1.4–21.8) | 11.1 (3.8–18.3) | 6.7 (4.4–9.0) | 1.70 (0.66–4.37) | 1.59 (0.76–3.32) |
AData show the incidence (% per patient-year) of outcomes with the 95% confidence interval (CI) in parentheses. Initial intensive treatment duration was categorized as short (1–8 days), intermediate (9–16 days), and standard (17–24 days). CVD, cardiovascular disease; DVT, deep vein thrombosis; HR, hazard ratio; PE, pulmonary embolism; VTE, venous thromboembolism.
In the safety outcome comparisons, Kaplan-Meier analysis showed no significant difference between the standard and short treatment duration groups (2.16% vs. 2.27% per patient-year, respectively); however, the incidence of major bleeding events was higher in the intermediate than standard treatment duration group (9.34% vs. 2.16% per patient-year, respectively; Figure 3; Table 2). The baseline characteristics of patients with and without major bleeding in the 3 groups are presented in the Supplementary Table. Although the limited number of patients did not allow for a sufficient evaluation, no obvious bleeding risk was found in any of the patients with major bleeding in either of the short and standard treatment duration groups; in the intermediate treatment duration group, those with major bleeding tended to have higher proportion of active cancer and lower body weight and body mass index than those without major bleeding.
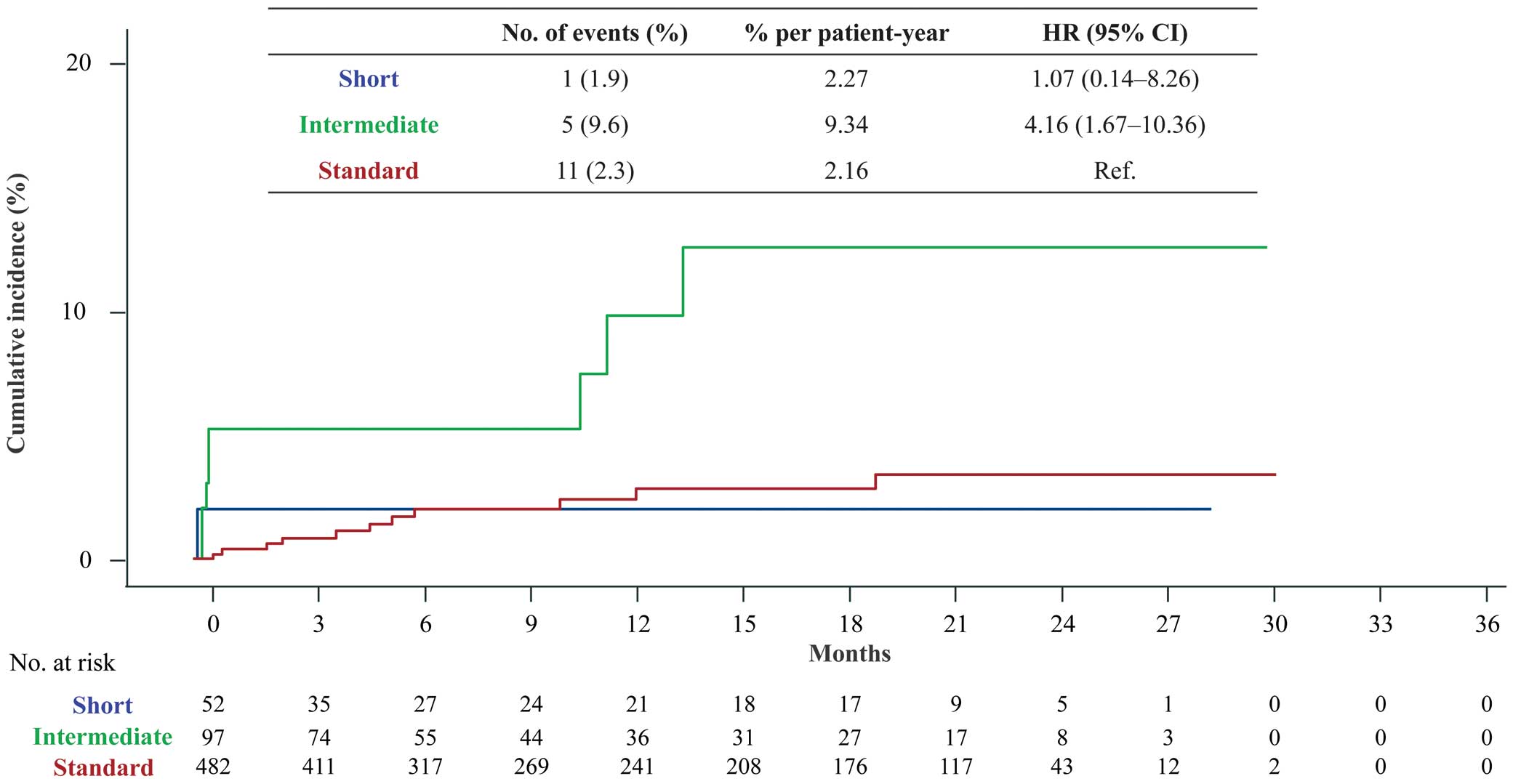
Major bleeding according to the International Society on Thrombosis and Haemostasis criteria following short, intermediate, or standard rivaroxaban treatment duration. CI, confidence interval; HR, hazard ratio.
For the cumulative incidence of the secondary effectiveness and safety endpoints, including recurrence or aggravation of symptomatic DVT and PE, death from any cause, death related to VTE and CVD, a vascular event (ACS or ischemic stroke), and non-major bleeding, there were no significant differences among the 3 treatment duration groups (Table 2). In terms of NACEs, a composite endpoint of the recurrence or aggravation of symptomatic VTE or major bleeding, there was no significant difference between the standard and short treatment duration groups (3.90% vs. 7.46% per patient-year, respectively; Figure 4). In contrast, the cumulative incidence of NACEs was higher in the intermediate than in the standard treatment duration group (8.02% vs. 3.90% per patient-year, respectively; Figure 4). The Supplementary Figure shows VTE recurrence and major bleeding during initial intensive treatment with rivaroxaban. VTE recurrence was observed in 1 patient in the standard treatment duration group. Major bleeding was observed in 2 patients in the short treatment duration group, 5 patients in the intermediate treatment duration group, and 1 patient in the standard treatment duration group.

Recurrence or aggravation of symptomatic venous thromboembolism or major bleeding according to the International Society on Thrombosis and Haemostasis criteria following short, intermediate, or standard rivaroxaban treatment duration. Cumulative incidence, as determined by the Kaplan-Meier method. CI, confidence interval; HR, hazard ratio.
In this subanalysis of the J’xactly study, 76% of patients underwent initial intensive rivaroxaban treatment for the standard duration (17–24 days), whereas the remaining patients underwent treatment for either intermediate (9–16 days) or short (1–8 days) durations. There were no major differences in patient characteristics among the 3 groups. In terms of clinical outcomes, the incidence of recurrence or aggravation of VTE and major bleeding at 12 months in the standard treatment duration group in the J’xactly study was comparable to that reported in global clinical trials.5,6,9 Thus, the standard duration of intensive treatment with rivaroxaban generally demonstrated effectiveness and safety comparable to that of other settings5,6,9 in this population of patients with acute VTE in real-world clinical practice in Japan. Initial intensive treatment with rivaroxaban for 17–24 days (based on the recommended 21-day duration) may be a potential option for Japanese patients with acute VTE.
The intermediate treatment duration group showed a higher incidence of bleeding events than the standard treatment duration group. In terms of the composite endpoint of recurrence or aggravation of symptomatic VTE or major bleeding, although there was no difference between the intermediate and standard treatment duration groups, among the 3 groups the incidence of events was lowest in the standard treatment duration group. The reason for the increased incidence of bleeding events in the intermediate treatment duration group is unknown, and the possibility of bias cannot be excluded because the number of patients in the intermediate treatment duration group (n=97) was lower than the number of patients in the standard treatment duration group (n=482). Conversely, patients in the intermediate treatment duration group may have been classified in that group because of a bleeding event. It is also possible that the physician decided to shorten the duration of initial intensive therapy because of concerns about bleeding events based on patient characteristics. The incidence of bleeding events may have been higher if the intensive care period had been longer, whereas there was no apparent increase in bleeding events in the standard treatment duration group, which had the longest treatment period in this study. Of the major bleeding events in the present study, most were observed within 16 days after initiation of initial intensive treatment. This suggests that shortening the duration of the initial intensive treatment is not always a favorable strategy when considering the risk of bleeding. That is, easy dose changes should generally be avoided. Moreover, close attention should be paid to bleeding events during initial intensive treatment, especially in patients who are at a high risk of bleeding.
In terms of VTE recurrence, there was no statistically significant difference by treatment duration in the present study; however, a trend towards an increase in VTE recurrence with a shorter treatment duration was suggested. Particularly in the short treatment duration group, some patients had VTE recurrence in the very early period after initial intensive treatment with rivaroxaban. In the intermediate treatment duration group, the incidence of VTE recurrence was similar to that of the standard group. Although further investigation is needed because of the small number of patients, the results of the present study suggest that 1–8 days of initial intensive treatment with rivaroxaban may be insufficient to prevent VTE recurrence. In particular, at 12 months, the proportion of patients with recurrent VTE tended to higher with a shorter duration of initial intensive treatment. Based on this observation, it is possible that a sufficiently intense DOAC administration after the onset of VTE may be effective in preventing VTE recurrence in the long term.
In previous cohort studies in which patients with VTE were treated with heparin for approximately 1 week followed by warfarin as a conventional therapy, the incidence of recurrence was high within 1 month after onset, and many bleeding events were observed.15,16 With rivaroxaban, it is considered reasonable to limit the dose to 1.5-fold the maintenance dose so as not to increase the risk of bleeding complications during the early onset period, while extending the treatment period to 3 weeks to decrease the recurrence of VTE within 1 month. However, the rivaroxaban dose used in Japan is two-thirds of the dose used in Western countries owing to differences in rivaroxaban metabolism between Japanese and Caucasian ethnicities.17 As a result, the initial intensive rivaroxaban dose (15 mg b.i.d.) is twice as high as the maintenance dose (15 mg once daily) in Japan.10
In this subanalysis of the observational J’xactly study of patients with VTE in real-world clinical practice in Japan, the effectiveness and safety of the standard rivaroxaban regimen were demonstrated. However, further investigation is warranted because the number of patients in the short and intermediate treatment duration groups was low relative to the number of patients in the standard treatment duration group.
This study has several limitations that should be considered. First, this analysis was not designed as a prospective research study; rather, it was a subanalysis of the J’xactly cohort study, and the patient characteristics were not adjusted. Second, the number of patients differed between the 3 groups, limiting between-group comparisons. Third, although the Kaplan-Meier curves suggest that the acute phase had a significant impact on the overall results of the study, this study was not focused on acute outcomes. Finally, the duration of the initial intensive rivaroxaban dosing regimen was determined at the discretion of each physician and was thus not prespecified; hence, some patients may have been allocated to a particular group because of a bleeding event, which may have introduced bias.
The standard initial intensive rivaroxaban treatment duration of 17–24 days (based on the recommended 21-day duration) appeared to be safe and effective in Japanese patients. This analysis of the most extensive real-world observational study of VTE treatment and prevention in Japanese patients with acute symptomatic/asymptomatic DVT, PE, or both provides important insights into the clinical outcomes of the initial rivaroxaban treatment duration in this population.
The authors thank all the patients and investigators at the centers who participated in this study. In addition, the authors thank Serina Nakamoto and other members of Mebix for their assistance with data collection, storage, and analysis. The authors thank Masahiro Takita of Mebix for encouragement and assistance with reporting the study findings. The authors also thank Nila Bhana of Edanz (www.edanz.com) for providing medical writing support, which was funded by Bayer Yakuhin, Ltd.
This study was supported financially by Bayer Yakuhin, Ltd.
M.N., I.F., M.T., and T. Yamazaki have no relationships relevant to the contents of this paper to disclose. N.Y. has received lecture fees from Bayer Yakuhin, Ltd., Pfizer Japan Inc., and Daiichi-Sankyo Co., Ltd. T. Yamashita has received lecture fees, manuscript fees, and research funding from Daiichi-Sankyo Co., Ltd., Bristol-Myers Squibb, and Bayer Yakuhin, Ltd., and lecture fees from Ono Pharmaceutical Co., Ltd., Toa Eiyo, Ltd., Novartis Pharma K.K., Otsuka Pharmaceutical Co., Ltd., and Nippon Boehringer Ingelheim Co., Ltd. T.I. has received lecture fees from Bayer Yakuhin, Ltd., Bristol-Myers Squibb, and Nippon Boehringer Ingelheim Co., Ltd., and research funding from Daiichi-Sankyo Co., Ltd. M.M. has received lecture fees from Bayer Yakuhin, Ltd. Y.O. has received lecture fees, research funding, scholarship funds, and donations from Bayer Yakuhin, Ltd.; lecture fees, scholarship funds, and donations from Daiichi-Sankyo Co., Ltd.; research funding from Bristol-Myers Squibb; scholarship funds and donations from Johnson & Johnson; and is associated with endowed departments sponsored by Boston Scientific Japan, Abbott Medical Japan, Medtronic Japan Co., Ltd., Nihon Kohden Co., Nihon Medi-Physics Co., Ltd., and Japan Lifeline Co., Ltd. A.H. has received lecture fees from Daiichi-Sankyo Co., Ltd. and Bayer Yakuhin, Ltd. A.H. is a member of Circulation Reports’ Editorial Team.
This study was approved by the Nihon University Itabashi Hospital, Clinical Research Judging Committee (RK-160913-4).
The deidentified participant data will not be shared.
Please find supplementary file(s);
https://doi.org/10.1253/circrep.CR-23-0008