2023 年 5 巻 4 号 p. 105-113
2023 年 5 巻 4 号 p. 105-113
Gender-affirming hormone treatment generally by cross-sex hormones is an important strategy for transgender people to achieve the physical features affirming their experienced gender. Estrogens and androgens are administrated, usually for a long time, to transgender women and transgender men who would like to physically achieve feminization and masculinization, respectively. Several harmful adverse events have been reported in the literature following the administration of gender-affirming hormones, including worsening of lipid profiles and cardiovascular events (CVE) such as venous thromboembolism, stroke, and myocardial infarction, but it remains unknown whether the administration of cross-sex hormones to transgender people increases the subsequent risk of CVE and death. Based on the findings of the present narrative review of the recent literature, including meta-analyses and relatively large-scale cohort studies, it is likely that estrogen administration increases the risk of CVE in transgender women, but it remains inconclusive as to whether androgen administration increases the risk of CVE in transgender men. Thus, definitive evidence guaranteeing the long-term safety of cross-sex hormone treatment on the cardiovascular system is insufficient because of lack of evidence from well-organized, high-quality, and large-scale studies. In this situation, as well as considering the proper use of cross-sex hormones, pretreatment screening, regular medical monitoring, and appropriate intervention for risk factors of CVE are necessary to maintain and improve the health of transgender people.
According to the definition of the International Classification of Diseases 11th Revision,1 gender incongruence (Chapter 17) is a condition related to sexual health characterized by a marked and persistent incongruence between an individual’s experienced gender and the assigned sex. Transgender women are transgender people whose experienced gender is female although their assigned sex at birth is male; and transgender men are transgender people whose experienced gender is male although their assigned sex at birth is female.
Cross-sex hormone treatment (CHT) has been used by some transgender people.2–4 In general, estrogens and androgens are administrated to transgender women and transgender men who would like to physically achieve feminization and masculinization, respectively, to affirm their gender. However, there are no approved drugs for CHT and no definitive protocols because of a lack of well-designed clinical trials to determine its efficacy and safety, especially with regard to long-term use, although some clinical guidelines provide hormone regimens for transgender people.2,3 Several harmful adverse events of cross-sex hormones have been previously reported, but there are no definitive conclusions because of insufficient evidence as to whether the administration of CHT to transgender people increases the subsequent risk for cardiovascular events (CVE) and death. In this article, we narratively review the relationship between CHT and CVE in transgender people primarily using recently published articles, including literature that evaluated Japanese transgender people.
Estrogen is a cross-sex hormone used in feminizing hormone therapy. Physical changes that may occur in transgender women during the first 3–12 months of estrogen therapy include a redistribution of the fat mass, an increase in the growth of breast tissue, and decreases in facial and body hair (usually mild), oiliness of the skin, spontaneous erections, prostate gland and testicle volume, and sexual desire.2,5 Alternatively, some transgender women use progestogens to gain improvements in breast development, mood, or sexual desire.2 Anti-androgens, such as cyproterone acetate and spironolactone, which directly suppress androgen synthesis or action, are sometimes used by transgender women during gender affirmation.6 Although these drugs are considered to be valuable for transgender women,2 their effects have not been fully elucidated.
Changes in Risk Factors for Cardiovascular Diseases (CVD) by GAHT for Transgender WomenAccording to data from the Behavioral Risk Factor Surveillance System, an epidemiologic health-related data survey in the US, transgender people have higher odds of CVE.7–9 There are various reasons for the increased incidence of CVE, and the atherogenic lipid profile, insulin resistance, and high blood pressure can be used as surrogate parameters to predict the subsequent risk of CVE, as discussed below.
Lipid Profiles A prospective study of transgender women treated with estrogen found favorable changes in lipid parameters,10 with increased high-density lipoprotein cholesterol (HDL-C) and decreased low-density lipoprotein cholesterol (LDL-C) concentrations.2 However, it has also been reported that increased body weight, blood pressure, and markers of insulin resistance attenuate these favorable changes in lipid profile.2,10
Regarding CVE risk markers, the European Network for the Investigation of Gender Incongruence (ENIGI) study demonstrated, in a large population, favorable changes in lipid profiles in transgender women, even at the mid-term follow-up after 2 and 3 years.11 In addition, significant (P<0.05) decreases in triglycerides (TG), total cholesterol, and LDL-C were observed at the 2-year follow-up after the start of GAHT in transgender women.12 No significant changes in general and hard CVD risk based on lipid profile were observed over time, where hard CVE was defined as coronary death, myocardial infarction (MI), fatal and non-fatal stroke and general CVE additionally included coronary insufficiency, angina pectoris, transient ischemic attack, intermittent claudication, and congestive heart failure.12 In a meta-analysis, only serum TG was higher at ≥24 months, without changes in other parameters.13 Thus, there is limited evidence to determine the effects of estrogen on lipid metabolism in transgender women.14
According to another meta-analysis, there were no significant differences in serum LDL-C, HDL-C, and total cholesterol between baseline and any time point after the start of GAHT in transgender women (Table 1).15 Serum TG concentrations were significantly higher than baseline only at ≥24 months.15 Further analysis showed that the increase in TG was seen with oral estrogen therapy, and not with transdermal estrogen therapy, which led to a decrease in TG levels (see below).15 Using cross-sectional studies that compared lipid values in the transgender population with those in the control group (natal males), the meta-analysis found that LDL-C concentrations were significantly lower in the transgender population, but there no significant differences in serum TG, HDL-C, or total cholesterol concentrations between the 2 groups.15
| Estimate | 95% CI | I2 | No. studies | |
|---|---|---|---|---|
| Transgender women with GAHT | ||||
| 3–6 months | ||||
| Triglyceride (mg/dL) | 3.8 | −11.6, 19.3 | 63.5 | 6 |
| LDL-C (mg/dL) | −3.1 | −11.6, 5.4 | 0 | 3 |
| HDL-C (mg/dL) | 1.2 | −5.7, 8.1 | 70.0 | 5 |
| Total cholesterol (mg/dL) | −1.1 | −9.4, 7.2 | 0 | 4 |
| 12 months | ||||
| Triglyceride (mg/dL) | 13.6 | −6.4, 33.6 | 89.5 | 10 |
| LDL-C (mg/dL) | −5.7 | −19.5, 8.1 | 85.1 | 7 |
| HDL-C (mg/dL) | 0.3 | −4.3, 5.0 | 77.4 | 8 |
| Total cholesterol (mg/dL) | −7.9 | −19.9, 4.1 | 82.5 | 10 |
| ≥24 months | ||||
| Triglyceride (mg/dL) | 31.9 | 3.9, 59.9 | 94.4 | 6 |
| LDL-C (mg/dL) | 6.6 | −9.7, 22.9 | 83.9 | 5 |
| HDL-C (mg/dL) | 0.4 | −7.7, 8.5 | 93.5 | 5 |
| Total cholesterol (mg/dL) | 9.5 | −5.8, 24.9 | 87.4 | 6 |
| Transgender men with androgen | ||||
| 3–6 months | ||||
| Triglyceride (mg/dL) | 9 | 2.5, 15.5 | 7.7 | 4 |
| LDL-C (mg/dL) | 7.1 | −3.2, 17.3 | 0 | 3 |
| HDL-C (mg/dL) | −6.5 | −11.9, −1.0 | 0 | 3 |
| Total cholesterol (mg/dL) | 10.6 | −4.2, 25.4 | 73.6 | 4 |
| 12 months | ||||
| Triglyceride (mg/dL) | 14.7 | −2.7, 32.1 | 75.4 | 9 |
| LDL-C (mg/dL) | 11.3 | 5.5, 17.1 | 0 | 8 |
| HDL-C (mg/dL) | −8.1 | −10.6, −5.7 | 0 | 8 |
| Total cholesterol (mg/dL) | 12.0 | −3.7, 27.8 | 88.0 | 9 |
| ≥24 months | ||||
| Triglyceride (mg/dL) | 21.4 | 0.1, 42.6 | 80.0 | 3 |
| LDL-C (mg/dL) | 17.8 | 3.5, 32.1 | 84.1 | 3 |
| HDL-C (mg/dL) | −8.5 | −13.0, −3.9 | 70.7 | 3 |
| Total cholesterol (mg/dL) | 14.6 | −5.1, 34.3 | 90.2 | 3 |
Reproduce with permission from Maraka S, et al.15 GAHT, gender-affirming hormone therapy; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Among transgender women, various effects of estrogen therapy on lipid profiles (favorable, unfavorable, and no change) have been reported.7 In contrast to the reported favorable effects of estrogen therapy on lipids,7,15 2 separate meta-analyses showed that estrogen therapy was associated with an increase in TG without significant changes in total cholesterol or other lipoprotein fractions.7,15 It is speculated that the increase in TG was driven by the use of oral estrogens, because sensitivity analyses showed an increase in TG of 28.2 mg/dL with oral estradiol, compared with a decrease of 4.8 mg/dL with transdermal estradiol.7,15 The contrasting effects of oral and transdermal estradiol on TG have been previously reported in postmenopausal cisgender women.7,16
Collectively, the available data indicate that estrogen therapy does not appear to have adverse effects on blood lipids and lipoproteins in transgender women, except for possible increases in TG and decreases in HDL-C, depending on the type of anti-androgen coadministered with estradiol.
Coagulation Markers In transgender women, GAHT resulted in procoagulant changes, including increased levels of Factors IX and XI and decreased levels of protein C,11,17 which likely contribute to the increased rates of venous thromboembolism (VTE) observed in these individuals.11,18–20
Insulin Resistance A systematic review of estrogen therapy in transgender women reported that 5 of 8 studies included showed increased insulin resistance, with 3 finding no effect.11,21 Another study showed that insulin sensitivity and incretin responses after an oral glucose tolerance test decreased with estrogen treatment.11 Finally, transgender women have been reported to be more insulin resistant than cisgender men (mean [±SD] 0.078±0.025 vs. 0.142±0.064 mL/μU; P=0.011).
Body Fat and Lean Mass It has been reported that adolescent transgender women on estradiol for at least 3 months have lower body fat than cisgender women (mean [±SD] 31±7% vs. 35±8%; P=0.033) but higher body fat than cisgender men (28±6% vs. 20±10%; P=0.001).22 In addition, transgender women had a higher lean mass than cisgender women (66±6% vs. 62±7%; P=0.032) and a lower lean mass than cisgender men (69±5% vs. 77±9%; P=0.001).22
Vascular Health and Function A recent retrospective cohort study indicated that among transgender women, a higher blood concentration of testosterone was associated with higher odds of having hypertension.7 The investigators also found that transgender women who had received a progestin prescription had lower odds of having hypertension.7
Endothelial function has been reported to be enhanced and arterial stiffness decreased in transgender women receiving estrogen.7 Flow-mediated dilatation in the brachial artery was reported to be higher in transgender women treated with estrogen than in age-matched cisgender men, but similar to that in cisgender women.7
Arterial stiffness in Japanese transgender women (22 untreated and 129 treated with estrogen alone or plus progestin) was evaluated using a volume-plethysmographic apparatus equipped with a multi-element applanation tonometry sensor.23 In that study, systolic blood pressure in estrogen-treated transgender women was only significantly lower than that in untreated transgender women, but brachial-ankle pulse wave velocity was significantly decreased in estrogen-treated transgender women compared with both untreated transgender women and transgender women treated with estrogen plus progestin.23 The carotid augmentation index was significantly lower in transgender women treated with oral estrogen than that in transgender women treated with parenteral estrogen or oral estrogen plus progestin.23 These data indicate that estrogen treatment is likely to have some beneficial effects on vascular function in transgender women; however, progestin administered with estrogen may have adverse effects on arterial stiffness.
Tobacco Use Associated With “Minority Stress” Minority Stress Theory has historically served as the leading theory to explain broad-ranging transgender health disparities.7 Minority stressors, such as gender non-affirmation, discrimination, and victimization experienced by transgender people, contribute to their poor mental and physical health outcomes, including CVD.7,24 Psychological and physiological stress responses are associated with large artery stiffening and endothelial dysfunction.7
Elevated rates of smoking in transgender people have been consistently linked to victimization and minority stress.7 Adjusting for demographic covariates and enacted and felt stigma, a change in the legal document gender marker was associated with lower odds of tobacco use among transgender women.25 However, there were no significant differences in tobacco use according to hormone treatment status.25
No conclusions have been reached as yet, but clinicians should strongly encourage the cessation of tobacco use in transgender women to avoid increased risks of CVD.
Development of CVE Following GAHT in Transgender WomenIt has been speculated that estrogen treatment in transgender women is associated with thromboembolic disease, coronary artery disease, macroprolactinoma, breast cancer, cerebrovascular disease, and cholelithiasis.2
A cohort study revealed that 11 of 214 transgender women experienced venous thrombosis and/or pulmonary embolism during GAHT.26 Five of specifically venous thrombosis and pulmonary embolism occurred during the first year of treatment, whereas another 3 occurred during sex reassignment surgery. Transgender women experienced more MI than the control group of cisgender women (P<0.001), but a similar proportion to the group of control cisgender men.26 The prevalence of CVD was higher in transgender women than in the control group of cisgender men (P<0.03).26 Another multinational study reported only 10 cases of VTE from a cohort of 1,073 transgender women.2,18
Transgender women had higher odds of MI compared with cisgender women (OR 2.56) but not cisgender men.8,27 Another study using the same data found that gender non-conforming individuals reported the highest prevalence of coronary artery disease or MI (17.8%) compared with transgender men (6.6%), transgender women (8.0%), cisgender men (9.0%), and cisgender women (4.8%).8,28
A population-based cohort study found that the incidence of CVE in transgender women was 7.1 per 1,000 person-years.29 In transgender women with GAHT, the risk of CVE was 2.4-fold higher compared with cisgender women (hazard ratio [HR] 2.4; 95% confidence interval [CI] 1.3–4.2) and 1.7-fold higher compared with cisgender men (HR 1.7; 95% CI 0.95–2.9).29 In transgender women without GAHT, the risk of CVE was 1.5-fold higher compared with cisgender women (HR 1.5; 95% CI 0.71–3.4) and 1.8-fold higher compared with cisgender men (HR 1.8; 95% CI 0.81–3.8).29 However, Cox regression analysis showed a significantly increased risk of any CVE only among transgender women compared with cisgender women.29
In the US, a Kaiser Permanente study in Georgia and northern and southern California, including 2,842 transgender women and 2,118 transgender men (mean follow-up 4.0 and 3.6 years, respectively) matched to 48,686 cisgender men and 48,775 cisgender women, found that transgender women had a higher incidence of VTE, with >2 year risk differences of 5.1 and 3.2 per 1,000 people relative to cisgender men and cisgender women, respectively (Table 2).19 The overall analyses for ischemic stroke and MI demonstrated similar incidence across groups.19 More pronounced differences for VTE and ischemic stroke were observed among transgender women who initiated hormone therapy during follow-up.19
| Event | Transgender cohort | Adjusted HR (95% CI) | ||
|---|---|---|---|---|
| No. acute CVEs |
Incidence rate per 1,000 person-years (95% CI) |
vs. reference men (cisgender men) |
vs. reference women (cisgender women) |
|
| Transgender women | ||||
| Transgender women overall cohort (n=2,842) | ||||
| VTE | 61 | 5.5 (4.3–7.0) | 1.9 (1.4–2.7) | 2.0 (1.4–2.8) |
| Ischemic stroke | 54 | 4.8 (3.7–6.3) | 1.2 (0.9–1.7) | 1.9 (1.3–2.6) |
| MI | 33 | 2.9 (2.1–4.1) | 0.9 (0.6–1.5) | 1.8 (1.1–2.9) |
| Transgender women estrogen initiation cohort (n=853) | ||||
| VTE | 17 | 6.6 (4.1–10.6) | 3.2 (1.5–6.5) | 2.5 (1.2–5.0) |
| At 0–2 years of follow-up | 6 | 4.3 (1.9–9.6) | 1.5 (0.5–5.1) | 1.7 (0.5–5.5) |
| At >2 years of follow-up | 11 | 9.3 (5.2–16.8) | 5.1 (2.1–12.6) | 3.2 (1.3–7.6) |
| Ischemic stroke | 17 | 6.6 (4.1–10.6) | 2.3 (1.2–4.3) | 2.9 (1.5–5.5) |
| At 0–6 years of follow-up | 9 | 3.8 (2.0–7.3) | 1.3 (0.6–2.9) | 2.3 (1.0–5.4) |
| At >6 years of follow-up | 8 | 36.2 (18.1–72.4) | 9.9 (3.0–33.1) | 4.1 (1.5–11.4) |
| MI in the cohort overall | 4 | 1.5 (0.6–4.1) | 1.0 (0.3–3.2) | 2.4 (0.6–9.4) |
| Transgender men | ||||
| Transgender men overall cohort (n=2,118) | ||||
| VTE | 23 | 3.1 (2.0–4.6) | 1.6 (0.9–2.9) | 1.1 (0.6–2.1) |
| Ischemic stroke | 16 | 2.1 (1.3–3.5) | 1.1 (0.6–2.0) | 1.3 (0.7–2.5) |
| MI | 9 | 1.2 (0.6–2.3) | 0.7 (0.3–1.8) | 1.3 (0.5–3.9) |
| Testosterone initiation cohort (n=585) | ||||
| VTE | 4 | 3.3 (1.3–8.9) | 2.7 (0.6–12.1) | 1.5 (0.4–5.6) |
| Ischemic stroke | 2 | 1.7 (0.4–6.7) | Not calculated | Not calculated |
| MI | 0 | – | – | – |
Reproduce with permission from Getahun D, et al.19 CI, confidence interval; CVE, cardiovascular events; GAHT, gender-affirming hormone therapy; HR, hazard ratio; MI, myocardial infarction; VTE, venous thromboembolism.
Nota et al reported the results of a large cohort study (n=2,517 transgender women with GAHT; median age 30 years).20 The mean and median follow-up durations were 9.07 and 5.95 years, respectively. Incidence ratios for VTE and stroke were significantly higher for transgender women than for reference people (Table 3).
| Event | No. events |
Incidence rate (/100,000 person-years) |
Expected no. events |
Standardized incidence ratio (95% CI) |
Expected no. events |
Standardized incidence ratio (95% CI) |
|---|---|---|---|---|---|---|
| Transgender women with GAHT (n=2,517) | Reference: cisgender women | Reference: cisgender men | ||||
| VTE | 73 | 320 | 13.22 | 5.52 (4.36–6.90)* | 16.04 | 4.55 (3.59–5.69)* |
| Stroke | 29 | 127 | 12.01 | 2.42 (1.65–3.42)* | 16.08 | 1.80 (1.23–2.56)* |
| MI | 30 | 131 | 11.38 | 2.64 (1.81–3.72)* | 38.03 | 0.79 (0.54–1.11) |
| Transgender men with androgens (n=1,358) | Reference: cisgender men | Reference: cisgender women | ||||
| VTE | 2 | 18 | 5.56 | 0.36 (0.06–1.19) | 4.84 | 0.41 (0.07–1.37) |
| Stroke | 6 | 55 | 4.10 | 1.46 (0.59–3.04) | 3.49 | 1.72 (0.70–3.58) |
| MI | 11 | 100 | 10.99 | 1.00 (0.53–1.74) | 2.98 | 3.69 (1.94–6.42)* |
Reproduce with permission from Nota NM, et al.20 *Significant finding. Abbreviations as in Table 2.
There is an increased risk of VTE associated with estrogens in general. A 20-fold increase actual VTE was reported in a cohort of transgender women.5 This increase may have been associated with the use of the synthetic estrogen ethinyl estradiol. Because ethinyl estradiol is particularly associated with VTE,11 its use by transgender women is not recommended.5,12
Oral routes of administration of estrogens are more thrombogenic than transdermal and parenteral routes of administration due to the first-pass metabolism of estrogen in the liver; the risk of VTE is dose dependent.2 Transdermal or injectable estrogen preparations may offer advantages in older transgender women who may be at higher risk of thromboembolic disease.2,7
No randomized control trials have examined whether GAHT are safe when they are used to help transgender women undergoing gender affirmation.6 Further studies are needed to conclusively determine the effects of GAHT on the development of CVE in transgender women.
The use of synthetic estrogens and conjugated estrogens is undesirable because of an inability to regulate doses by measuring serum concentrations and the risk of VTE. Oral, transdermal, or injectable 17β-estradiol, which is monitored by measuring serum estradiol concentrations, is recommended for use as part of a GAHT plan in transgender women.
Serum estradiol and serum testosterone can be measured to monitor oral, transdermal, and intramuscular 17β-estradiol concentrations to maintain them at levels in premenopausal women (100–200 pg/mL and 50 ng/dL, respectively).2 A key issue is to avoid supraphysiologic doses of estrogen.
Monitoring D-dimer concentrations during treatment is not always recommended.2 In transgender women, estrogen treatment results in procoagulant changes, including increased levels of Factors IX and XI and decreased Levels of protein C,11,17 which likely contribute to the increased rates of VTE observed in these individuals.11,18–20
The risk factors for thrombosis in Japanese people have not been fully elucidated, whereas Factor V Leiden is a well-known risk factor for thrombosis in populations of European ancestry.30 In previous studies on thrombophilia in Japan, most participants had a dysfunction of the activated protein C anticoagulation system, mainly due to the reduced activity of protein S in Japanese populations.31–34 An Austrian group reported that administering gender-affirming hormones to 162 transgender women was not associated with VTE, despite the incidence of thrombophilia being as high as 8.0%.2 However, before initiating hormone treatment, thrombophilia screening is necessary for those transgender people with a personal or family history of VTE.2
Death in Transgender Women With GAHTThe aforementioned studies provided relevant insights into the effects of estrogen on the markers of CVD.10 However, data on the real mortality associated with estrogen treatment remain limited. In a large cohort of transgender women (n=816; mean age 41 years; mean follow-up 10 years) showed no increase in cardiovascular mortality, despite a 32% rate of tobacco use.5
Conversely, a retrospective cohort study of 2,927 transgender women who visited Amsterdam University Medical Centre in the Netherlands between 1972 and 2018 found that 317 (10.8%) transgender women had died over the 46-year period, which was higher than expected compared with both men and women in the general population (Figure A).35 Cause-specific mortality in transgender women was high for CVD, lung cancer, HIV-related disease, and suicide.35 That observational study showed an increased mortality risk in transgender people using hormone treatment, regardless of treatment type. This increased mortality risk did not decrease over time.35
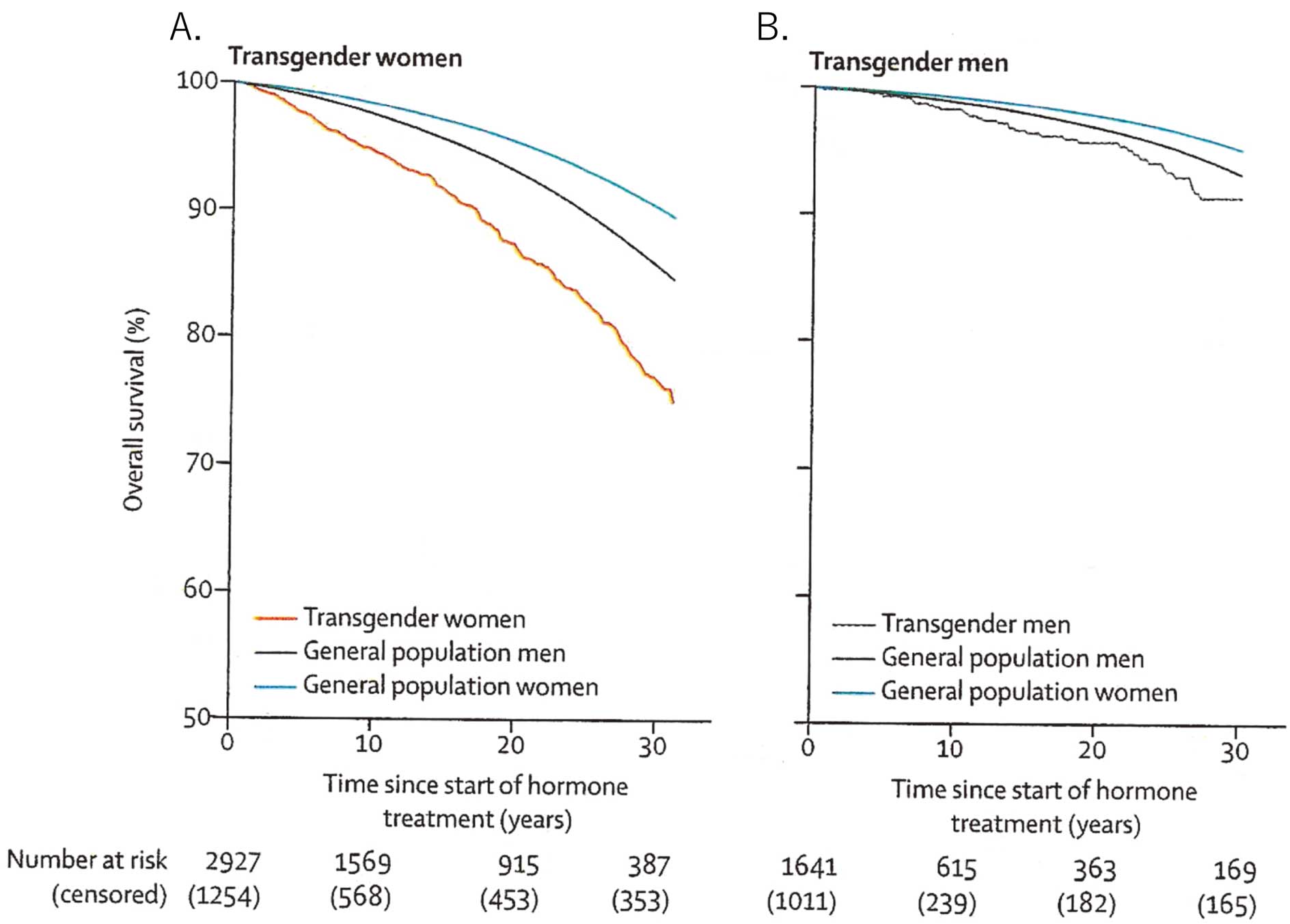
Cumulative survival in (A) transgender women and (B) transgender men during follow-up after the start of hormone therapy. Reproduced with permission from de Blok et al.35
The cause-specific mortality risk of CVD does not indicate a specific effect of hormone treatment, but rather indicates that monitoring, optimization, and, if necessary, treatment of medical morbidities and lifestyle factors remain important in transgender health care.
In the Coronary Drug Program, 5 mg conjugated estrogen therapy in men aged 30–64 years with a history of MI was associated with increased cardiovascular mortality, whereas 2.5 mg conjugated estrogen therapy was associated with a trend towards an increased risk of VTE.36
The increased prevalence of CVD in transgender women raises concerns about the extent to which estrogen preparations can cause harmful events; if so, modifications of GAHT may be necessary. This is an important area for future research on transgender women.
Androgen is a cross-sex hormone used to induce masculinization in women. Cessation of menses, deepening of the voice, growth of facial/body hair, increased muscle mass/strength, and clitoral enlargement develop as desired effects of continuous testosterone administration. The efficacy of androgen treatment in the masculinization of transgender men has also been reported in the Japanese literature.37,38 The phenotype induced by testosterone contributes to reducing gender dysphoria/gender incongruence in transgender men. In a study that asked 336 Japanese transgender men what they most anticipated after starting androgen therapy, the most frequent answer was the cessation of menses (52.7%), followed by a deepened voice (32.4%).39
Conversely, androgens have several undesired effects, such as skin oiliness/acne, scalp hair loss, and fat redistribution.2,3,38 In addition, although it has been speculated that androgen administration may increase the risk of CVE such as DVT, MI and stroke, solid evidence based on the well-organized prospective or randomized controlled trials is lacking.
Changes in Risk Factors for CVD Following Androgen TreatmentThe atherogenic lipid profile, insulin resistance, and high blood pressure are surrogate parameters that can be used predict the subsequent risk of CVE. In a meta-analysis,15 transgender men who were administered testosterone had significantly higher serum TG concentrations at 3–6 and ≥24 months and serum LDL-C concentrations at 12 and ≥24 months compared with baseline (Table 1). In addition, serum concentrations of HDL-C, which has anti-atherogenic properties, decreased significantly across all follow-up time points.15 However, van Velzen et al found no significant differences in the cardiometabolic effects (e.g., on TG, LDL-C, and HDL-C) of different testosterone products, such as testosterone gel, injections of testosterone esters, and testosterone undecanoate.40
In another meta-analysis, Elamin et al reported on the effects of androgens on blood pressure.14 Testosterone administration significantly increased systolic blood pressure (by 1.74 mmHg; P=0.03), but not diastolic blood pressure (1.45-mmHg increase; P=0.16).14 This increase in systolic blood pressure does not appear to be clinically meaningful.
Because women with polycystic ovary syndrome, in which the ovaries produce an excess amount of androgens, are often insulin resistant, it may be speculated that there could be a close correlation between testosterone administration and insulin resistance. However, a systematic review suggested that testosterone therapy has no negative effect on, and may even be associated with an improvement in, insulin sensitivity.21
Recently, Cocchetti et al reported changes in body mass index (BMI), lipid profile, and blood pressure in 165 transgender men with a prospective 2-year follow-up after testosterone administration as part of the ENIGI study.12 Cocchetti et al found no significant changes in BMI and blood pressure, but did observe significant impairment of the lipid profile.12 Emi et al compared clinical and laboratory data between 48 Japanese transgender men treated with androgen for a mean of 45 months and 63 untreated Japanese transgender men (mean age 27.9 vs. 26.5 years; NS).41 In that study, BMI (22.8 vs. 22.3 kg/m2; NS), glucose (92.3 vs. 97.6 mg/dL; NS), and HbA1c (4.8 vs. 4.8%; NS) were comparable between transgender men with and without androgen treatment, whereas systolic blood pressure (117.4 vs. 110.4 mmHg; P<0.01), diastolic blood pressure (68.6 vs. 64.6 mmHg; P<0.02), and TG (103.0 vs. 69.2 mg/dL; P<0.01) were increased, and HDL-C (60.9 vs. 69.3 mg/dL; P<0.02) was decreased, in transgender men treated with androgen.41
Thus, testosterone administration to transgender men, including Japanese transgender men, impairs the lipid profile but has no or only subtle clinical effects on blood pressure and insulin sensitivity. In female, testosterone reduces nitric oxide availability in endothelial cells, leading to impaired vasodilation and resulting in endothelial dysfunction and mild hypertension.42 Gulanski et al demonstrated that flow-mediated vasodilation, used to evaluate brachial vasodilatory responses, was significantly lower in 11 transgender men receiving testosterone (mean [±SD] 4.5±2.7%) than in 20 cisgender women (8.1±2.9%; P=0.002).43 In another study, Emi et al reported that the brachial-ankle pulse wave velocity was significantly higher in transgender men with (n=48) than without (n=63) androgen treatment (mean [±SD] 1,202.8±138.2 vs. 1,080.0±113.7 cm/s, respectively; P<0.01), although there was no significant difference in carotid augmentation index between the 2 groups.41 Thus, it is likely that long-term exposure to high-dose androgen results in endothelial dysfunction and arterial stiffness.
Recently, an analysis of 13 Japanese transgender men for whom electrocardiograms (ECGs) were available before and after testosterone administration revealed J-point elevation and significantly increased T-wave amplitudes after testosterone administration, leading to a higher prevalence of a male pattern ECG after testosterone (23.1% vs. 92.3%; P<0.001).44 The prevalence of the early repolarization pattern and QRS amplitudes (II, III, V1–V6) also increased significantly after treatment.44 In addition to the impaired lipid profile, these androgen-induced ECG changes may be related to lethal arrhythmias.
Development of CVE Following Androgen TreatmentNumerous studies have investigated the effects of serum testosterone concentrations on CVE in cisgender men, as well as testosterone therapy for cisgender men with testosterone deficiency. An analysis of guidelines for testosterone therapy produced by major international medical societies summarized comments on the effects of testosterone therapy on major adverse CVE, such as MI and cerebrovascular accident.45 All guideline committees either commented that there was insufficient data in the literature that testosterone therapy increases the risk of major adverse CVE or acknowledged the conflicting data.45 Thus, it remains unknown whether testosterone administration is harmful to the cardiovascular system in cisgender men due to insufficient evidence.
Many studies have investigated testosterone therapy for female sexual dysfunction in postmenopausal women.46 The global consensus position statement on the use of testosterone therapy for women endorsed by the International Menopause Society, and others, recommends testosterone treatment for naturally and surgically postmenopausal women with hypoactive sexual desire disorder/dysfunction in doses that approximate physiological testosterone concentrations for premenopausal women.47 Non-oral testosterone therapy in doses that approximate physiological testosterone concentrations for premenopausal women have not shown adverse effects on lipid profiles, blood pressure, blood glucose, HbA1c levels, and the risk of deep vein thrombosis, although long-term safety remains unknown.47 In addition, limited data preclude assessment of the effects of testosterone therapy on MI or death.47 Conversely, in transgender men, testosterone products with concentrations comparable to those in hypogonadal cisgender men have routinely been used to achieve serum testosterone concentrations comparable to those in healthy cisgender men, and are sufficient for masculinization. Ichihara et al investigated the pharmacokinetics of 250 mg testosterone enanthate, intramuscularly, in 10 Japanese transgender men without hysterectomy or oophorectomy.48 The mean Tmax of total testosterone was 1.7 days, the Cmax of total testosterone was 29.4–18.1 ng/mL, and the mean total testosterone decreased gradually to <3.0 ng/mL again on Day 21.48 The total testosterone concentration was ≥10 ng/mL for more than 2 weeks. Thus, serum testosterone concentrations repeatedly change from supra- to infraphysiologic after every intramuscular injection of testosterone enanthate. To estimate the adverse events of testosterone, it is difficult to apply data derived from postmenopausal women receiving low-dose testosterone treatment in an attempt to maintain testosterone concentrations seen in premenopausal women.
Several clinical and epidemiological studies, as well as meta-analyses, have investigated CVE in transgender men treated with testosterone.12,15,20,35,49–52 Because it is highly speculative whether it takes a long time to develop CVE after androgen administration, especially in young subjects, even if it is true, the age of the individuals and the follow-up period of the study should be considered when interpreting the data.
Getahun et al evaluated the incidence of CVE during CHT.19 In that study, data were collected for 2,118 transgender men using androgens, 20,780 matched reference cisgender men, and 29,807 matched reference cisgender women from an electric medical record-based cohort study of transgender members of integrated healthcare systems. Two-thirds of subjects were 18–35 years of age at the index date. Acute CVE was observed in 54 of 2,118 transgender men with a mean follow-up of 3.6 years. The incidence rate (number of cases per 1,000 person-years) was 3.1 for VTE, 2.1 for ischemic stroke, and 1.2 for MI. HRs adjusted by BMI, smoking status, blood pressure, and total blood cholesterol level, were not significant for VTE, ischemic stroke, and MI compared with reference cisgender men or reference cisgender women (Table 2).19 Nota et al reported the results of a large cohort study of 1,358 transgender men with hormone therapy (median age 23 years), with a mean and median follow-up duration of 8.10 and 4.10 years, respectively.20 In that study, transgender men had a 3.69-fold higher risk of MI than reference cisgender women (Table 3), but no increased risk of stroke or VTE compared with reference cisgender women and reference cisgender men.20 In the ENIGI study, which prospectively evaluated 165 transgender men (mean age 26.78 years) over a 2-year follow-up after testosterone administration, the cardiovascular risk was calculated according to the Framingham 30-year cardiovascular risk estimate.11 “Hard CVE” was defined as coronary death, MI, fatal and non-fatal stroke. General CVE additionally included coronary insufficiency, angina pectoris, transient ischemic attack, intermittent claudication, and congestive heart failure. Although there were no significant changes in BMI-based calculated risk in transgender men, lipid-based calculated general cardiovascular risks were significantly increased from 5.93% at baseline to 7.42% at 12 months and 8.82% at 24 months (P<0.002).12 Similarly, lipid-based calculated hard cardiovascular risks were significantly increased from 2.79% at baseline to 3.46% at 12 months and 4.16% at 24 months (P<0.006).12 The increase in general and hard cardiovascular risks during the 2-year period after testosterone administration was more significant in the younger (18–22 years) than older (32–59 years) subgroup at baseline.
Based on a systematic review of 29 studies, Maraka et al reported that among transgender men with testosterone administration ranging from 3 months to 41 years, 1 of 771 experienced VTE, 0 of 340 experienced stroke, 1 of 478 experienced MI, and 3 of 651 died.15
In Japan, no studies have investigated the long-term cardiovascular risk of androgen in transgender men. The cardiovascular risk of androgen remains unknown for recent transgender men undergoing treatment under strict medical supervision according to the Diagnostic and Therapeutic Guidelines for Patients with Gender Identity Disorder, 4th revised edition proposed by the Japanese Society of Psychiatry and Neurology.4 Thus, it is difficult to reach firm conclusions because of the low quality of evidence derived from the literature based on small sample sizes, heterogeneous types of hormone treatment, and various methods and durations of follow-up.
Death in Transgender Men Following Androgen TreatmentRecently, de Blok et al reported mortality trends over 5 decades in a large cohort of adult transgender people in the Netherlands.35 That study analyzed data for 1,641 transgender men who visited the Gender Identity Clinic of Amsterdam University Medical Center and started testosterone administration between 1972 and 2018. The median age at the start of hormone treatment was 23 years and the median follow-up period was 5 years. Standardized mortality ratios (SMR) were calculated using general population mortality rates stratified by age, calendar period, and sex. Over the duration of the study, 44 (2.2%) people died, which was higher than expected compared with women in the general population (SMR 1.8; 95% CI 1.3–2.4), but not compared with men in the general population (SMR 1.2; 95% CI 0.9–1.6).35 Cumulative survival curves were shown in Figure B. Cause-specific death was high for non-natural causes of death (SMR 3.3; 95% CI 1.2–6.4), such as suicide, compared with women in the general population women, but not for CVD and cancer.35 The most suicide cases occurred in the first decade (before 1980). The authors commented that monitoring, optimizing, and, if necessary, treating comorbidities such as CVD, tobacco use, and HIV, remain important in transgender health care.35
Denby et al showed that transgender people, prior to the initiation of hormone therapy, had high rates of undiagnosed and untreated comorbidities such as hypertension and dyslipidemia, which increased the risk of CVD.53 In addition, studies conducted in the US indicate that transgender people with CVD are older and have a lower annual income, higher rates of smoking, lower rates of exercise, and higher rates of medical comorbidities.54 As recommended in the clinical guidelines,2,3 pretreatment screening and appropriate regular medical monitoring of weight, blood pressure, physical examinations, evaluation of the risk of CVE, and appropriate interventions are necessary to maintain and improve the health of transgender people.
Definitive evidence to guarantee the long-term safety of GAHT for either transgender women or transgender men is insufficient. However, estrogen administration likely increases the risk for CVE in transgender women, but it remains inconclusive as to whether androgen administration increases the risk of CVE in transgender men. Nevertheless, globally, there are still no approved cross-sex hormones for individuals with gender incongruence because well-designed clinical trials have not been performed. Further studies are needed to prove the tolerability and safety of CHT, especially in the case of long-term use, with proper medical counseling and management.