2023 年 5 巻 8 号 p. 338-347
2023 年 5 巻 8 号 p. 338-347
Background: The utility of telomere G-tail length to predict coronary artery disease (CAD) remains controversial. CAD results from coronary artery narrowing due to cholesterol and lipid accumulation, augmented by inflammatory cells and other factors. This study explored the significance of telomere G-tail length in suspected CAD patients.
Methods and Results: In all, 95 patients with suspected CAD or ≥1 cardiac risk factor underwent coronary computed tomography angiography (CCTA). We measured leukocyte telomere length and G-tail length using a hybrid protection method, and diagnosed the presence of CAD using CCTA. Associations between G-tail length and the presence of CAD, the number of stenosed coronary arteries, and brachial-ankle pulse wave velocity (baPWV) were analyzed. No significant difference was observed in G-tail length when comparing groups with or without CAD or statin treatment. However, in the non-statin group, G-tail length was significantly shorter in patients with 3-vessel disease compared with 1-vessel disease. Dividing the group using a baPWV of 1,300 cm/s, telomere G-tail length was significantly shorter in the high-risk (baPWV ≥1,300 cm/s) group.
Conclusions: The clinical utility of telomere G-tail length as a CAD risk indicator seems limited. There was a trend for longer telomere G‐tail length in the statin‐treated group. Moreover, telomere G-tail length was reduced in patients at high-risk of cardiovascular events, aligning with the trend of a shortening in telomere G-tail length with CAD severity.
The potential use of telomere G-tail length as a novel predictor of cardiovascular events has been suggested.1 Telomeres are double-stranded DNA consisting of 6 repeats of TTAGGG, and the constituent telomeric DNA is deleted with each replication.2,3 In the final stage of replication, the single-stranded projecting end of this TTAGGG sequence is called the telomere G-tail. Telomere G-tails are key structures that protect telomere DNA from DNA damage.4,5 It has been reported that in diseases such as myocardial infarction and chronic heart failure, telomere length is markedly shortened,6–8 and this shortening is associated with hypertension (HTN), atherosclerosis, and coronary calcification.1,9,10 Because telomere G-tail length, rather than total telomere length, has been reported to function as a trigger for cell viability and senescence, we decided to use the telomere G-tail length in this study.11,12
Although many studies have examined the relationship between telomere G-tail length and cardiovascular disease, few epidemiological studies have validated telomere G-tail length in subjects at risk of some form of heart disease, as in actual clinical practice. Non-invasive means of assessing the severity of atherosclerosis are available, such as pulse wave velocity (PWV), ankle-brachial pressure index (ABI), and coronary artery calcification (CAC). Brachial-ankle PWV (baPWV) is non-invasive, easy to measure, and frequently used to assess the risk of developing cardiovascular disease. CAC indicates the presence of coronary atherosclerosis, with the amount of CAC considered to represent the severity of coronary atherosclerosis.13 Similarly, the number of diseased coronary vessels has been reported to be associated with poor clinical outcomes.14 Many Japanese patients have already received statin therapy as primary prevention, and show a unique association between lipid profile and the development of coronary artery disease (CAD).15 Statin treatment affects telomere G-tail length. At the molecular level, statins may inhibit telomere length shortening by interacting with the telomere/telomerase system and by reducing telomere damage caused by oxidation.16–18
Therefore, the aim of this study was to clarify the relationship between telomere G-tail length and CAD by using established indices reflecting atherosclerosis in Japanese subjects with suspected CAD in real clinical practice.
This study was a single-center, hospital-based, cross-sectional study conducted using the coronary computed tomography angiography (CCTA) database of Fukuoka University from August 2017 to February 2018. Of 100 consecutive patients, 95 patients were enrolled and 5 were excluded due to a lack of data. We included patients who were clinically suspected of having CAD based on abnormal electrocardiogram (ECG) findings and/or chest symptoms, or with ≥1 cardiovascular risk factor, and age ≥20 years. ECG findings suggestive of ischemia included ST changes, T wave changes, and QRS wave changes. Patients with acute coronary syndrome, a history of known CAD, severe renal insufficiency (estimated glomerular filtration rate [eGFR] ≤30 mL/min/1.73 m2), or missing data were excluded.
Evaluation of CAD Using CCTAAll patients underwent scanning with CCTA, and CAD was defined as a narrowing of >50% in the coronary artery lumen.19 All segments were assessed according to the 15-segment American Heart Association coronary artery model.20 Subjects were classified into 4 groups according to the number of stenosed vessels as follows: 0, 1, 2, and 3 stenosed vessels. CCTA was assessed by trained cardiologists using a Ziostation (Ziosoft Inc., Tokyo, Japan), a computed tomography workstation. These cardiologists were blinded to the clinical characteristics and the results of blood tests. Overall, the severity of CAD was assessed according to the number of stenosed vessels and the CAC score.21 The CAC score was assessed using the Agatston score.22 The severity of CAD was also assessed according to the Gensini score.21
Evaluation of CAD Risk FactorsPatient characteristics are presented in Table 1, including cardiovascular risk factors. Patients who had a current systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg or were receiving anti-hypertensive therapy were considered to have HTN. Patients with low-density lipoprotein cholesterol (LDL-C) ≥140 mg/dL, triglycerides (TG) ≥150 mg/dL, and/or high-density lipoprotein cholesterol (HDL-C) <40 mg/dL, or who were being treated with lipid-lowering therapy were defined as having dyslipidemia (DL). HbA1c was evaluated according to the National Glycohemoglobin Standardization Program. Patients were considered to have diabetes if they satisfied any of the diagnostic criteria as defined by the Japan Diabetes Society.23 Chronic kidney disease was defined as eGFR <60 mL/min/1.73 m2.2,24,25
| (A) | All patients (n=95) |
Non-CAD (n=56) |
CAD (n=39) |
P value Non-CAD vs. CAD |
Non-statin (n=71) |
Statin (n=24) |
P value Non-statin vs. statin |
|---|---|---|---|---|---|---|---|
| Age (years) | 64.8±13.2 | 62.2±13.7 | 68.6±11.8 | 0.019 | 62.9±13.7 | 70.7±10.0 | 0.012 |
| Female sex | 48 (50.5) | 22 (39.3) | 26 (66.7) | 0.0086 | 34 (47.9) | 14 (58.3) | 0.38 |
| HTN | 55 (57.9) | 25 (44.6) | 30 (76.9) | 0.0017 | 37 (52.1) | 18 (75.0) | 0.050 |
| BMI >25 kg/m2 | 37 (38.9) | 25 (44.6) | 12 (30.8) | 0.17 | 29 (40.9) | 8 (33.3) | 0.51 |
| Diabetes | 16 (16.8) | 7 (12.5) | 9 (23.1) | 0.18 | 7 (9.9) | 9 (37.5) | 0.0018 |
| DL | 38 (40.0) | 15 (26.8) | 23 (59.0) | 0.0016 | 17 (23.9) | 21 (87.5) | <0.0001 |
| Smoking | 34 (35.8) | 15 (26.8) | 19 (48.7) | 0.028 | 22 (31.0) | 12 (50.0) | 0.093 |
| LVEF (%) | 64.2±9.2 | 65.7±8.6 | 62±9.7 | 0.052 | 63.8±9.1 | 65.3±9.5 | 0.51 |
| Glucose (mg/dL) | 109.9±28.3 | 108.7±30.4 | 111.8±25.2 | 0.61 | 107.8±28.4 | 116.3±27.5 | 0.21 |
| HbA1c (%) | 5.9±0.9 | 5.8±0.9 | 6.2±0.9 | 0.058 | 5.8±0.9 | 6.3±1.0 | 0.020 |
| eGFR (mL/min/1.73 m2) | 71.0±14.2 | 73.0±14.3 | 68.3±13.6 | 0.11 | 72.9±14.3 | 65.6±12.3 | 0.029 |
| HDL-C (mg/dL) | 56.1±15.3 | 59.3±16.3 | 51.3±12.7 | 0.012 | 55.8±15.9 | 56.8±14.0 | 0.79 |
| LDL-C (mg/dL) | 121.8±33.7 | 126.7±29.5 | 114.9±38.3 | 0.095 | 131.3±32.0 | 93.8±20.7 | <0.0001 |
| Total cholesterol (mg/dL) | 206.7±38.8 | 215.2±35.4 | 194.4±40.6 | 0.0094 | 216.3±38.0 | 178.1±24.5 | <0.0001 |
| Triglycerides (mg/dL) | 129.9±71.5 | 132.1±86.0 | 126.7±44.0 | 0.72 | 139.1±76.5 | 102.8±45.3 | 0.031 |
| Medication | |||||||
| ARB | 24 (25.3) | 11 (19.6) | 13 (33.3) | 0.13 | 14 (19.7) | 10 (41.7) | 0.032 |
| ACE-I | 3 (3.2) | 0 | 3 (7.7) | 0.035 | 2 (2.8) | 1 (4.2) | 0.74 |
| CCB | 38 (40.0) | 19 (33.9) | 19 (48.7) | 0.15 | 24 (33.8) | 14 (58.3) | 0.034 |
| BB | 6 (6.3) | 0 | 6 (15.4) | 0.0024 | 2 (2.8) | 4 (16.7) | 0.016 |
| Statin | 24 (25.3) | 7 (12.5) | 17 (43.6) | 0.0006 | |||
| Oral antidiabetic | 21 (22.1) | 9 (16.07) | 12 (30.8) | 0.090 | 12 (16.9) | 9 (37.5) | 0.036 |
| Leukocytes (×103/μL) | 5.91±1.91 | 5.94±2.18 | 5.86±1.45 | 0.84 | 5.97±2.09 | 5.71±1.22 | 0.58 |
| Log telomere G-tail length | 9.36±0.24 | 9.34±0.24 | 9.39±0.24 | 0.36 | 9.34±0.24 | 9.43±0.23 | 0.093 |
| CAD | 22 (31.0) | 17 (70.8) | 0.0004 | ||||
| No. diseased vessels | |||||||
| 0 | 56 | 0 | 49 | 7 | |||
| 1 | 0 | 14 | 8 | 6 | |||
| 2 | 0 | 11 | 7 | 4 | |||
| 3 | 0 | 14 | 7 | 7 | |||
| CAC score | 0 [0–99.32] |
0 | 99.3 [14.6–335.1] |
0.0002 | 0 [0–33.6] |
62.7 [0.3–280.3] |
0.0064 |
| Gensini score | 5 [0–14.25] |
0 [0–2.5] |
16 [10.5–29] |
<0.0001 | 2.5 [0–10.5] |
13 [3.4–25] |
0.22 |
| (B) | Non-statin group (n=71) | ||||||
| All patients (n=71) |
Non-CAD (n=49) |
CAD (n=22) |
P value Non-CAD vs. CAD |
||||
| Age (years) | 62.9±13.7 | 61.1±14.1 | 66.8±12.1 | 0.11 | |||
| Female sex | 34 (47.9) | 19 (38.8) | 15 (68.2) | 0.022 | |||
| HTN | 37 (52.1) | 21 (42.9) | 16 (72.7) | 0.020 | |||
| BMI >25 kg/m2 | 29 (40.8) | 23 (46.9) | 6 (27.3) | 0.12 | |||
| Diabetes | 7 (9.9) | 5 (10.2) | 2 (9.1) | 0.88 | |||
| DL | 17 (23.9) | 9 (18.4) | 8 (36.4) | 0.10 | |||
| Smoking | 22 (31.0) | 14 (28.6) | 8 (36.4) | 0.51 | |||
| Oral antidiabetic | 12 (16.9) | 8 (16.3) | 4 (18.2) | 0.85 | |||
| LVEF (%) | 63.8±9.1 | 65.1±8.5 | 61.2±10.0 | 0.094 | |||
| Glucose (mg/dL) | 107.8±28.4 | 108.6±32.0 | 106.2±19.0 | 0.74 | |||
| HbA1c (%) | 5.8±0.9 | 5.8±0.9 | 5.9±0.7 | 0.65 | |||
| eGFR (ml/min) | 72.9±14.4 | 73.7±14.5 | 71±14.1 | 0.47 | |||
| HDL-C (mg/dL) | 55.8±15.9 | 58.5±2.2 | 49.8±3.3 | 0.031 | |||
| LDL-C (mg/dL) | 131.3±32.0 | 131.2±4.6 | 131.5±6.9 | 0.97 | |||
| Total cholesterol (mg/dL) | 216.3±38.0 | 219.3±35.7 | 209.7±42.9 | 0.33 | |||
| Triglycerides (mg/dL) | 139.1±76.5 | 135.7±88.5 | 146.6±39.3 | 0.31 | |||
| Medication | |||||||
| ARB | 14 (19.7) | 8 (16.3) | 6 (27.3) | 0.28 | |||
| ACE-I | 2 (2.8) | 0 | 2 (9.1) | 0.032 | |||
| CCB | 24 (33.8) | 16 (32.7) | 8 (36.4) | 0.76 | |||
| BB | 2 (2.8) | 0 | 2 (9.1) | 0.032 | |||
| Leukocytes (×103/μL) | 5.97±2.09 | 6.06±2.29 | 5.75±1.58 | 0.56 | |||
| Log telomere G-tail length | 9.34±0.24 | 9.35±0.24 | 9.31±0.24 | 0.59 | |||
| No. diseased vessels | |||||||
| 0 | 49 | 49 | 0 | ||||
| 1 | 8 | 0 | 8 | ||||
| 2 | 7 | 0 | 7 | ||||
| 3 | 7 | 0 | 7 | ||||
| CAC score | 0 [0–33.6] |
0 [0–2.3] |
89.4 [9–354.4] |
0.0001 | |||
| Gensini score | 2.5 [0–10.5] |
0 [0–2.5] |
14.3 [10–24.5] |
<0.0001 | |||
ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BB, β-blocker; BMI, body mass index; CAD, coronary artery disease; CCB, calcium channel blocker; DL, dyslipidemia; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; HTN, hypertension; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction. (A) Normally distributed continuous variables are presented as the mean±SD; non-normally distributed continuous are presented as the median [interquartile range]. Categorical variables are presented as n (%). P values for differences were estimated from Chi-squared tests for categorical variables and from unpaired t-tests for continuous variables. (B) Normally distributed continuous variables are presented as the mean±SD; non-normally distributed continuous are presented as the median [interquartile range]. Categorical variables are presented as n (%). P values for differences were estimated from Chi-squared tests for categorical variables and from unpaired t-tests for continuous variables.
PWV is an index of arterial stiffness and is independently associated with cardiovascular events. PWV is calculated from the difference in rise time of the pulse wave detected at 2 points and the distance between the measurement sites. In the present study, PWV was measured using a volume plethysmography device in accordance with previously described methods.26
Patients were classified into 2 groups according to PWV (i.e., PWV ≥1,300 and <1,300 cm/s) because baPWV ≥1,300 cm/s represents arterial stiffness, which is associated with a risk of cardiovascular disease. Previous Japanese studies reported that coronary-related events were not observed in patients with baPWV <1,300 cm/s.27 Arterial stiffness is also highly associated with the development of albuminuria, with one study showing the threshold of baPWV in relation to the prevalence of albuminuria with respect to arterial stiffness. In that study, the cut-off value for PWV was 1,269 cm/s, and morbid albuminuria increased with every 100 cm/s increase in baPWV.28
Measurement of Leukocyte Total Telomere Length and Telomere G-Tail LengthTotal telomere length was measured using the telomere hybridization protection assay (HPA), and telomere G-tail length was measured using a telomere G-tail HPA.5,29,30 As you pointed out, there was repetition, so I have consolidated it with the previous section. Telomere G-tail length was determined using 0.2 μg denatured genomic (g) DNA or 1 μg non-denatured gDNA. All samples were assessed in triplicate, with gDNA of RKO cells used as a control to correct for interassay variability. The average coefficient of variance for the G-tail was 4.0%.31 The length of the G-tail was represented in logarithmic notation.
Statistical AnalysisNo statistical sample size calculations were performed. The post hoc results showed that the required sample size for the difference between the statin and non-statin groups was 199, with an α error at the 5% level of significance and a β error at the 20% level of significance. However, due to the high cost of measuring G-tail length, the study was limited to 100 samples.
All calculations were performed using JMP® 14 (SAS Institute Inc., Cary, NC, USA). Continuous data are reported as the mean±SD or as the median with interquartile range (IQR). Categorical and continuous variables were compared between the groups using Chi-squared tests or unpaired t-tests, respectively. Taking into account the issue of multiplicity, we conducted an adjustment for the false discovery rate (FDR) using the Benjamini-Hochberg method. We set the threshold for statistical significance at FDR <0.05. Continuous variables that were not normally distributed and are reported as the median and IQR were compared using the Wilcoxon rank-sum test. The Spearman rank correlation coefficient was used to evaluate associations between the groups. Log-normalization was used for telomere G-tail length, which was not normally distributed. The significance of differences in telomere G-tail length between groups with 1-, 2-, and 3-vessel disease (VD) were determined by 1-way analysis of variance (ANOVA) with Hsu’s multiple comparison with the best (MCB). A multivariate analysis was performed using an ordinal logistic regression analysis for independent variables that were related to the number of stenosed vessels without statin treatment. For the selection of explanatory variables related to arterial stiffness in multiple regression analysis, we used the backward selection method with P>0.3 for the likelihood ratio test as the exclusion criterion. Statistical significance was set at P<0.05.
Table 1A shows the clinical characteristics for all patients (n=95), as well as for those with and without statin treatment and according to the absence (n=56) or presence (n=39) of CAD separately. Of all patients, 25.3% were treated with statins. The CAD group was significantly older, had a higher proportion of women and smokers, and had a significantly higher prevalence of HTN and DL. The use of a statin and CAC scores were significantly higher in the CAD group. Telomere G-tail length was not associated with the presence of CAD (P=0.36; Figure 1A). Table 1A also presents the clinical characteristics of all patients according to statin treatment. Patients who had DL treatment other than a statin were included in the non-statin group. The statin group had significantly lower TC, LDL-C, and TG concentrations. In addition, the presence of diabetes, DL, and HTN, and the percentage of CAD morbidity were higher in the statin than non-statin group. However, there was no significant difference in telomere G-tail length between these 2 groups (P=0.093; Figure 1A). The statin group was significantly older than the non-statin group, so when we compared the G-tail length adjusting for age, the P value was 0.049, indicating a significant difference. Nevertheless, when multiplicity was considered, the FDR was 0.140, which was not statistically significant (Figure 1A). In addition, when comparing within the CAD group based on the presence or absence of statin treatment, there was a significant elongation of telomere G-tail length in the statin-treated group of patients (P=0.024). However, considering multiplicity, the FDR was 0.072, which was not statistically significant (Figure 1B).

Comparison of telomere G-tail length in (A) in all patients according to the presence of coronary artery disease (CAD) and statin treatment and (B) in the CAD group according to statin treatment. The boxes show the interquartile range, with the median value indicated by the horizontal line; whiskers show the range. Data were compared using Student’s t-test. FDR, false discovery rate.
Next, patients on non-statin treatment were further divided into non-CAD (n=49) and CAD (n=22) groups (Table 1B). In the non-statin group, the percentage of patients with HTN was higher and HDL-C concentrations were lower in the CAD than non-CAD group. Figure 2 shows telomere G-tail length in patients without statin treatment according to the number of stenosed vessels. Telomere G-tail length was significantly shorter for patients with 3VD than for those with 1VD and 2VD (9.46 [9.25–9.53] vs. 9.26 [9.14–9.47] and 9.32 [9.02–9.37], respectively; P<0.05). In contrast, the CAC score was not associated with telomere G-tail length (r=−0.056, P=0.64; Figure 3).
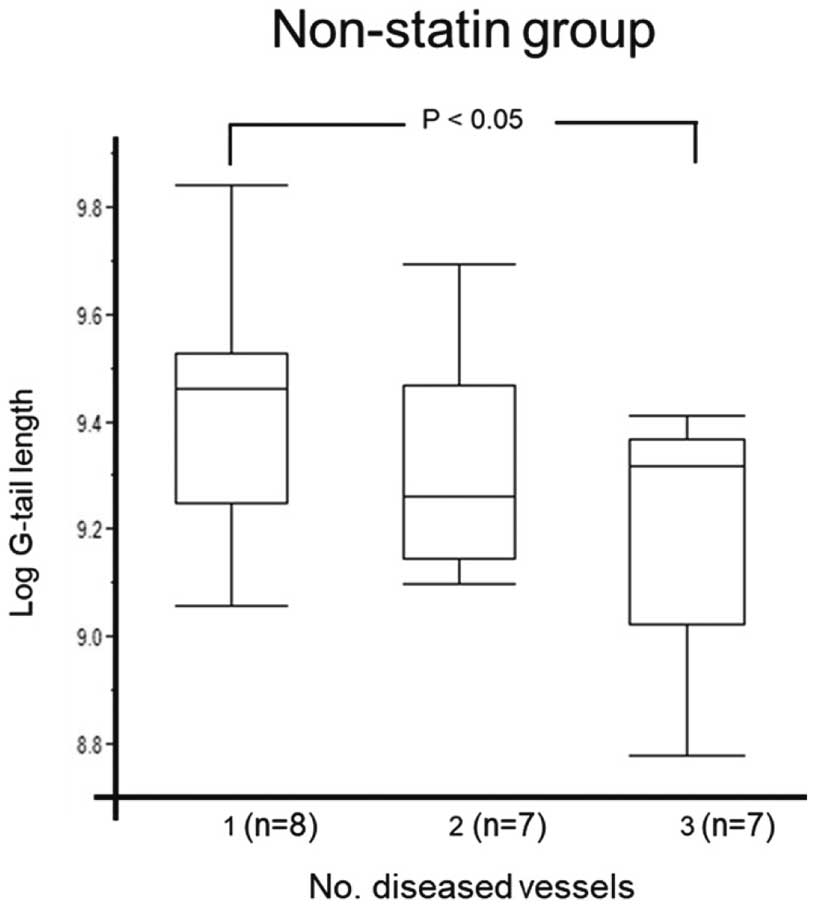
Comparisons of telomere G-tail length according to the number of diseased vessels in the non-statin-treated group. The boxes show the interquartile range, with the median value indicated by the horizontal line; whiskers show the range. Data were compared using 1-way ANOVA with a Hsu’s MCB test.
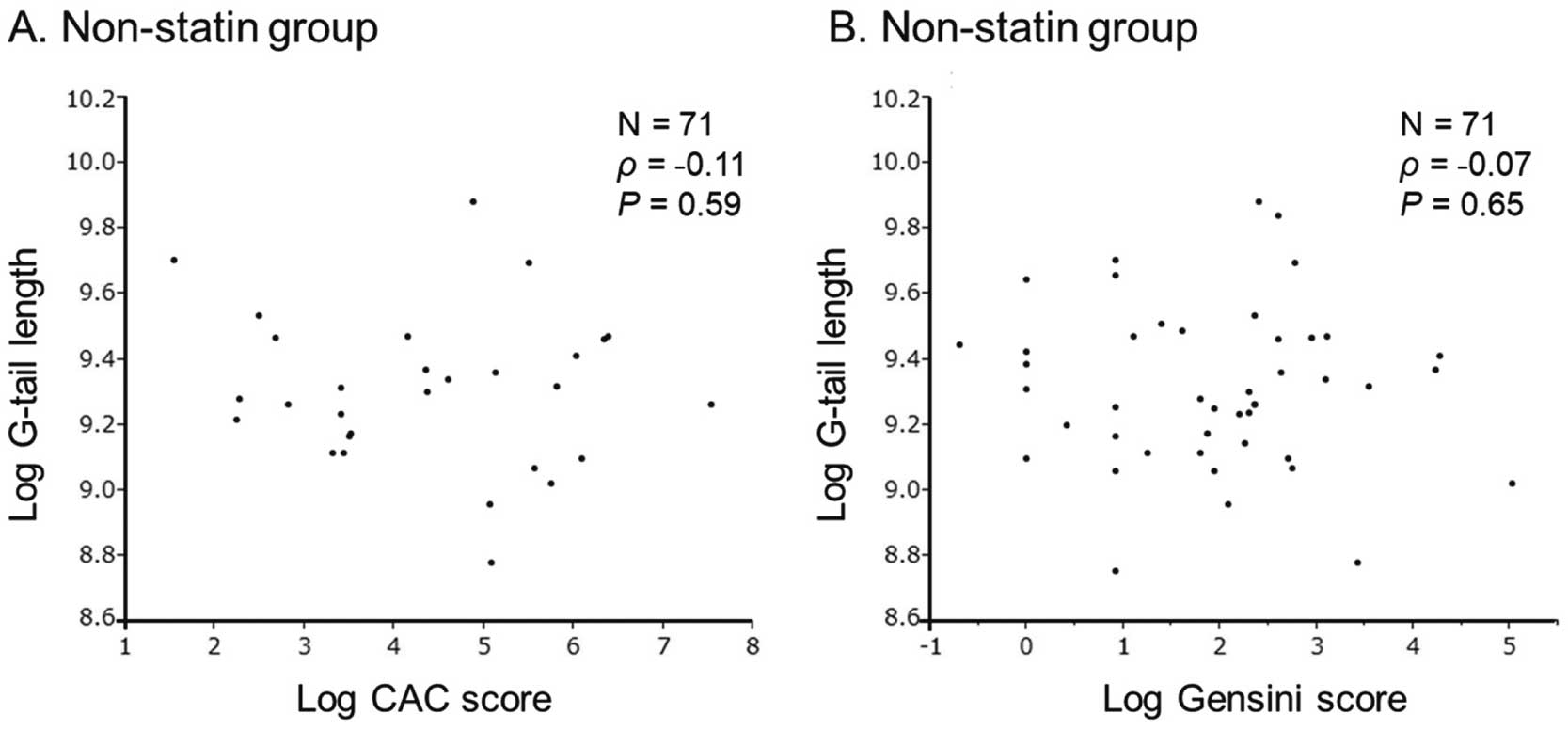
Scatter plots showing the relationships between telomere G-tail length and (A) the coronary artery calcium (CAC) score and (B) the Gensini score in the non-statin-treated group. Data were analyzed using correlation analysis.
There were no significant associations between telomere G-tail length and traditional CAD risk factors (age, HTN, diabetes, and DL; Figure 4).
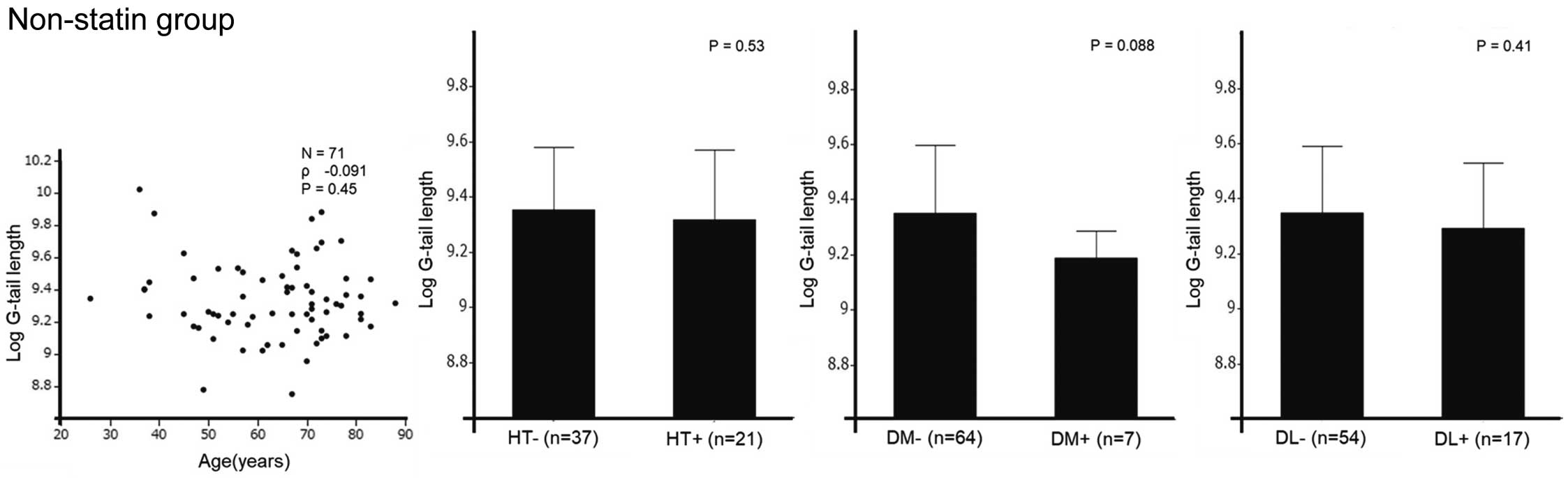
Relationships between telomere G-tail length and coronary artery disease risk factors in patients not on statin treatment. Where appropriate, data are given as the mean±SD. Data were analyzed using correlation analysis and compared using Student’s t-test. DL, dyslipidemia; DM, diabetes; HT, hypertension.
An ordinal logistic regression analysis that corrected for independent variables was performed in the non-statin group (Table 2). Telomere G-tail length, in addition to smoking, was independently associated with the number of diseased vessels in patients without statin treatment.
| Variables | Multivariate | ||
|---|---|---|---|
| AOR | 95% CI | P value | |
| Age | 1.04 | 0.96–1.15 | 0.33 |
| Smoking | 2.76 | 1.05–8.39 | 0.051 |
| Hypertension | 2.58 | 0.80–10.5 | 0.13 |
| Log(telomere G-tail length) | 0.0026 | 0.000011–0.22 | 0.016 |
Multiple Ordinal Regression was used to identify the factors associated with the increase in the number of diseased vessels, and the results are reported as AORs with 95% CIs. AOR, adjusted odds ratio; CI, confidence interval.
The baseline characteristics of patients with baPWV ≥1,300 and <1,300 cm/s are presented in Table 3, whereas comparisons of unadjusted telomere G-tail length according to baPWV overall and for the non-statin groups are shown in Figure 5. Significant differences in telomere G-tail length were observed in both the entire patient group and in the non-statin group (P=0.032 [FDR=0.032] and P=0.030 [FDR=0.032], respectively). We selected TG, eGFR, and smoking history as explanatory variables in multiple regression analysis. After adjustment for TG, eGFR, and smoking history, telomere G-tail length was significantly shorter in the baPWV ≥1,300 than <1,300 cm/s group (9.49±0.06 vs. 9.34±0.03, respectively; P=0.015). Regarding the units, additional information has been added in the method section. The data was log-transformed and thus has no units.
| baPWV <1,300 (n=19) |
baPWV ≥1,300 (n=75) |
P value | |
|---|---|---|---|
| Age (years) | 52.8±14.9 | 68.0±11.0 | <0.0001 |
| Female sex | 11 (57.9) | 37 (49.3) | 0.50 |
| HTN | 8 (42.1) | 47 (62.7) | 0.10 |
| BMI >25 kg/m2 | 10 (52.4) | 27 (36.0) | 0.19 |
| Diabetes | 4 (21.1) | 12 (16.0) | 0.60 |
| Hypercholesterolemia | 6 (31.6) | 32 (42.7) | 0.38 |
| Smoking | 6 (31.6) | 28 (37.3) | 0.64 |
| LVEF (%) | 63.1±8.1 | 64.5±9.5 | 0.57 |
| Glucose (mg/dL) | 107.1±31.6 | 110.0±27.7 | 0.60 |
| HbA1c (%) | 5.8±1.0 | 6.0±0.9 | 0.63 |
| eGFR (mL/min/1.73 m2) | 74.8±12.1 | 70.0±14.6 | 0.20 |
| HDL-C (mg/dL) | 54.1±17.4 | 56.4±14.9 | 0.56 |
| LDL-C (mg/dL) | 117.0±29.6 | 123.0±34.9 | 0.49 |
| Total cholesterol (mg/dL) | 203.3±27.9 | 207.4±41.4 | 0.68 |
| Triglycerides (mg/dL) | 142.2±92.3 | 127.3±66.0 | 0.42 |
| Medication | |||
| ARB | 4 (21.1) | 20 (26.7) | 0.62 |
| ACE-I | 0 | 3 (4.0) | 0.38 |
| CCB | 5 (26.3) | 33 (44.0) | 0.16 |
| BB | 2 (10.5) | 4 (5.3) | 0.41 |
| Statin | 4 (21.1) | 20 (26.7) | 0.62 |
| Leukocytes (×103/μL) | 5.88±1.61 | 5.94±1.99 | 0.91 |
| Telomere G-tail length | 9.46±0.23 | 9.33±0.24 | 0.032 |
| No. diseased vessels | |||
| 0 | 12 | 43 | |
| 1 | 4 | 10 | |
| 2 | 3 | 8 | |
| 3 | 0 | 14 | |
| CAC score | 0 [0–27.4] | 9.7 [0–159.1] | 0.63 |
Normally distributed continuous variables are presented as the mean±SD; non-normally distributed continuous are presented as the median [interquartile range]. Categorical variables are presented as n (%). P values for differences were estimated from Chi-squared tests for categorical variables and from unpaired t-tests for continuous variables. baPWV, brachial-ankle pulse wave velocity. Other abbreviations as in Table 1.

Comparison of telomere G-tail length according to brachial-ankle pulse wave velocity (baPWV) <1,300 or ≥1,300 cm/s in (A) all patients and (B) patients not on statin treatment. Data are given as the mean±SD. Data were compared using Student’s t-test. FDR, false discovery rate.
This study focused on the correlation between telomere G-tail length and CAD in a Japanese population with suspected CAD. It also explored the relationship between statin therapy and the severity of CAD. The results showed that telomere G-tail length was not associated with the presence of CAD. Conversely, a trend towards elongation of telomere G-tail length was observed in the statin-treated group. In the non-statin group, telomere G-tail length was significantly shorter in patients with 3VD than in those with 1VD. An independent association was found between telomere G-tail length and number of diseased vessels. Furthermore, patients with a baPWV ≥1,300 cm/s had significantly shorter telomere G-tail length.
Our results could potentially be significant because previous studies have associated shorter telomere G-tail length with an elevated risk of cardiovascular disease in hemodialysis patients, suggesting a correlation with aging and vascular risk factors.4,32 Statin treatment is a well-established, safe, and effective therapy for CAD that also has an anti-atherosclerotic effect. Numerous studies have shown a correlation between statin treatment and telomere length, indicating that telomere length is often longer in patients receiving statin treatment, regardless of age, than those not receiving such treatment.16,33,34 Similarly, the results of the present study indicated that patients being treated with statin have a trend towards longer telomere G-tail length, particularly in patients with CAD (Figure 1). Given the findings of the previous studies and the present study, it could be suggested that, at least in patients with shortened telomere G-tail length such as untreated CAD patients, statin treatment may improve the length of the telomere G-tail. However, a lack of clear statistical significance suggested that the usefulness of telomere G-tail length for assessing CAD may be limited in patients at risk of CAD, as in the present study. In contrast, there was no difference in telomere G-tail length in the entire patient cohort, regardless of the presence of CAD; although there were significantly more elderly patients in the CAD group, that group also contained more statin-treated patients, which may have influenced our findings.35 The individuals in this study were at risk of CAD and some were already receiving statin therapy. Against such a background, telomere G-tail length may not be of much value in assessing CAD.
Because CAC reflects atherosclerosis and is a global measure of atherosclerosis, it can be used to assess the risk of CAD. In the present study, telomere G-tail length was not clearly related to the CAC score, regardless of statin treatment. Mainous et al reported that there was no relationship between telomere length and CAC in Blacks, who have less coronary calcification than Whites.10 This is noteworthy because the incidence of a high CAC score is generally seen as lower in Japanese compared with Whites.36,37 It is possible that the number of patients in the present study with a high CAC score (>400) was too small and insufficient for validation. In addition, the results of this study indicated that low calcified lesions with a low CAC score in Japanese patients may not be suitable as surrogate markers of coronary calcification.
The association between telomere G-tail length and disease severity was also analyzed in CAD patients. Because the natural history of CAD is influenced by the number of artery branches affected, we stratified disease severity according to the number of affected branches and evaluated it in relation to telomere G-tail length. In the non-statin group, there was a trend towards shorter telomere G-tail length as the number of diseased vessels increased, with a significant difference between the 1VD and 3VD groups. As indicated in Table 2, telomere G-tail length was demonstrated to be a significant factor related to the number of affected branches, independent of the age factor reported in previous studies, suggesting a correlation with severity.4,32 It has been previously reported that vessels with plaque have markedly shorter telomeres and that telomere shortening was closely associated with an increase in the severity of atherosclerosis.38 On this point, our results are not inconsistent with those previous reports.
In the present study, we further evaluated the efficacy of telomere G-tail length by comparing patients with baPWV ≥1,300 and <1,300 cm/s. Statin treatment has been reported to decrease baPWV.39,40 In the present study, significant differences in telomere G-tail length were observed after adjusting for confounding factors that can affect baPWV, such as smoking history, eGFR, and TG. It has been reported that baPWV is considered to reflect the sum of cardiovascular risks, and 1,400 cm/s is equivalent to a moderate risk using the Framingham risk score.41 Some cross-sectional and cohort studies have reported that baPWV is positively associated with cardiovascular disease.42–45 Recently, telomere G-tail length was shown to be an independent predictor of increased baPWV in a general Japanese population.46 The authors of that study argued that a decrease in telomere length influenced the risk of cardiovascular disease at a very early stage when individuals could still take necessary precautions before cardiovascular disease leads to a symptomatic health outcomes. In the present study, we used a baPWV cutoff of 1,300 cm/s, in accordance with previous studies showing that baPWV <1,300 cm/s did not indicate coronary artery-related events; that the lower limit of normal baPWV for women was 1,300 cm/s; and setting a baPWV of 1,400 cm/s as the cut-off value for cardiovascular events.27,47,48 Our previous report that telomere G-tail length was a predictor of cardiovascular events and the results of the present study showing that the group with lower baPWV had significantly longer telomere G-tail length suggest a similar implication regarding cardiovascular events.32
Previous reports have indicated that oxidative stress and chronic inflammation play major roles in telomere shortening.49,50 Cholesterol accumulation in blood vessels has been found to bring about the chronic inflammatory process of atherosclerosis by promoting the uptake of oxidized LDL.51 Conversely, the rate of telomere shortening is highly dependent on oxidative damage to the cell. It is suggested that the shortening of telomeres in endothelial progenitor cells, mediated by increased oxidative DNA damage, may contribute to the pathophysiology of CAD.52 Thus, it is reasonable to assume that a greater number of diseased vessels or a greater severity of the lesions would imply greater chronic inflammation, which would result in shorter telomere length.
This study has several limitations. First, the sample size was relatively small, which limited our ability to determine significance. Both the 2VD and 3VD groups were small. Compared with previous studies, fewer subjects had high-severity stenosis or a high CAC score. A large number of subjects with complex lesions, higher CAC scores, and multivessel disease may be needed to reveal a relationship between telomere G-tail length and the severity of CAD. Second, the patients in this study were at some risk of CAD, and many were being treated with statins. Comparisons with healthy individuals without CAD should also be made. Third, telomere G-tail length has been reported to shorten primarily with aging and in cancer diseases, but, in relation to the present study, there may be other factors associated with conditions such as cardiomyopathies.31 It is difficult to assess the direct effect of time-dependent changes related to CAD risk in a cross-sectional study of telomere G-tail length measurements. A large-scale prospective study, including different populations, will be needed to address these issues.
In conclusion, telomere G-tail length appears to have limited utility as a clinical indicator for patients at risk of CAD. One contributing factor could be the observed tendency for telomere G-tail length to extend with statin treatment. However, those at higher risk of cardiovascular events had significantly shorter telomere G-tail lengths than those at lower risk. Furthermore, a trend towards shortening telomere G-tail length was observed in non-statin-treated individuals corresponding with the severity of CAD. This suggests the possibility that telomere G-tail length may shorten with the progression of coronary atherosclerosis and plaque formation.
The authors express their gratitude to Yukie Nishiyama, Yuko Kamikawa, and Eriko Aoki for their clinical research advice and technical assistance.
The current study did not receive any funding or financial support.
H.T. is a founder and director of the board of MiRTeL Co. LTD and owns stock in MiRTeL Co. LTD. The views expressed in this publication are those of the authors and not those of the acknowledged institution. S.M. is a member of Circulation Reports’ Editorial Team. The remaining authors declare no conflicts of interest.
This study was approved by the Institutional Review Board of Fukuoka University Hospital (Fukuoka University Hospital EC/IRB: #U19-07-015).