2024 年 6 巻 3 号 p. 37-45
2024 年 6 巻 3 号 p. 37-45
Background: Catheter ablation (CA) of atrial fibrillation (AF) triggers, including non-pulmonary vein (PV) foci, contributes to improved procedural outcomes. However, the clinical significance of an AF trigger ablation during second CA procedures for nonparoxysmal AF is unknown.
Methods and Results: We enrolled 94 patients with nonparoxysmal AF undergoing a second CA. Intracardiac cardioversion during AF using high-dose isoproterenol was performed to determine the presence or absence of AF triggers. PV re-isolations were performed if PV potentials recurred, and if AF triggers appeared from any non-PV sites, additional ablation was added to those sites. We investigated the incidence of atrial arrhythmia recurrence (AAR) >3 months post-CA. Of the 94 enrolled patients, AF triggers were identified in 65 (69.1%), and of those with AF triggers, successful elimination of the triggers was achieved in 47 patients (72.3%). Multivariate analysis revealed that no observed AF triggers were a significant predictor of AAR (hazard ratio [HR] 1.97, 95% confidence interval [CI] 1.21–3.46, P=0.019). In a subanalysis of the patients with AF triggers, multivariate analysis showed that unsuccessful trigger ablation was significantly associated with AAR (HR 5.84, 95% CI 2.79–12.22, P<0.01).
Conclusions: Having no observed AF triggers during a second CA session significantly increased the risk of AAR, as did unsuccessful CA of AF triggers.
Pulmonary vein isolation (PVI) is an essential catheter ablation (CA) procedure not only for paroxysmal atrial fibrillation (PAF) but also non-PAF.1 However, the therapeutic effect PVI in cases of non-PAF is often insufficient.2 AF triggers are important for the initiation of AF, whereas substrates are necessary for the maintenance of non-PAF. Previous studies demonstrated that the efficacy of successful elimination of AF triggers was not only by PVI but also the addition of non-PV AF trigger ablation, in non-PAF patients.3,4 Inoue et al advocated that investigating the presence or absence of AF triggers and their origin is useful for deciding the strategy of CA to improve the outcome and avoid unnecessary substrate modification in non-PAF patients with AF triggers.5 However, in clinical practice we often experience atrial arrhythmia recurrence (AAR) after AF trigger-based ablation in patients with non-PAF. Electrical reconnections between the PVs and left atrium (LA), new-onset AF triggers, and the presence of arrhythmogenic substrates could be considered among the reasons for AAR. A second CA session is performed in some cases after the first failed CA, but the efficacy of AF trigger-based ablation during the second CA session is unknown. We retrospectively analyzed non-PAF patients receiving a second CA session to eliminate AF triggers and clarified the clinical significance of AF trigger ablation on the outcomes of the second CA session.
We performed the initial CA of non-PAF to restore sinus rhythm in 282 patients from January 2013 to November 2017. It involved PVI with and without non-PV AF trigger ablation based on the presence of AF triggers and their sites. Of these patients, 135 (47.9%) experienced AAR, defined as atrial tachyarrhythmia recurrence >3 months after the initial CA. In total, 94 patients underwent a second CA session because of AAR and were enrolled in this retrospective study. Non-PAF was defined as persistent and long-standing AF according to the guidelines:6 that is, by the duration of AF prior to the first CA session.
In the present study, the DR-FLASH score was calculated by adding 1 point each for the presence of diabetes mellitus, renal dysfunction (estimated glomerular filtration rate <90 mL/min/1.73 m2), persistent form of AF, left atrial diameter >45 mm, aged >65 years, female sex, and hypertension before the first and second CA sessions, respectively, to estimate the degree of LA remodeling.
Preparation for CAAll antiarrhythmic drugs (AADs) were stopped at least 5 half-lives before CA except for amiodarone, which was stopped >1 month prior. All patients received anticoagulation therapy, such as warfarin or direct oral anticoagulants (DOACs) for >1 month before CA. Anticoagulant administration was uninterrupted on the day of CA, and heparin bridging was not performed. Transesophageal echocardiography or enhanced computed tomography to exclude any left atrial appendage thrombi was conducted within 48 h before CA in all patients. If patients were in sustained AF before CA, we performed pharmacological or electrical cardioversion ≥1 month beforehand. In the case of patients in which AF did not terminate or recurred, CA was performed during AF.
CA ProcedureCA was performed under conscious sedation with dexmedetomidine and propofol. Two long sheaths (SL0; AF Division, St. Jude Medical, MN, USA) were inserted percutaneously into the LA through the right femoral vein with a transseptal puncture using fluoroscopic guidance. After femoral vein puncture, a single 150 IU/kg bolus of heparin was administered. An activated clotting time between 300 and 350 s was maintained with continuous infusion of heparin during the procedure. Multipolar circular catheters (Inquiry TM A-focus TM II; St. Jude Medical or Lasso, Biosense Webster, CA, USA) were used to assess the PV potentials. Regardless of the presence or absence of PV reconnections, to determine the location of the AF triggers, a high dose of isoproterenol (ISP) was intravenously injected initially at 5 µg/min, and then the dose was gradually increased to a maximum of 20 µg/min over a total administration time of 6 min. If AF was induced, internal electrical cardioversion was performed and we observed for the presence or absence of AF triggers. If AF did not occur using ISP, rapid atrial pacing from the right atrium (RA) was performed to induce AF after the ISP infusion. After pacing-induced AF was sustained for ≥2 min, internal electrical cardioversion was undertaken to restore sinus rhythm, and the presence or absence of AF triggers was observed. Those procedures were repeated at least twice to assess AF reproducibility. In patients with non-PV AF triggers, we repeated the induction protocols ≥3 times, assuming that non-PV AF triggers occurred from different sites, and we attempted to detect those sites using 4 or 5 multipolar catheters. A 20-pole catheter (BeeAT, Japan Lifeline, Tokyo, Japan) was located with 10 poles on the lateral wall of the RA and another 10 poles inserted into the coronary sinus. We manipulated the multipolar circular mapping catheters and a 5-spline mapping catheter (PentaRay, Biosense Webster) or 18-electrode mapping catheter (AdvisorTM HD Grid, St. Jude Medical) to change the location of the catheters according to the intracardiac activation sequence at the timing of AF initiation. We repeated the internal electrical cardioversion and attempted to search for AF triggers by mapping. In each subject, the operators performed voltage mapping of the LA using the PentaRay or AdvisorTM HD Grid catheter during sinus rhythm prior to CA. The mitral annulus and PVs were not included in the calculations of the LA surface area.
When PV reconnections were observed, we re-isolated the PVs under electrophysiological guidance using the multipolar circular mapping catheters. When AF triggers were observed from the superior vena cava (SVC) or LA posterior wall, we performed isolation of the respective trigger site. If AF triggers appeared from any non-PV sites other than the SVC and LA posterior wall, we added a focal ablation to that site. In patients without AF triggers, linear ablation, such as an LA roof line and/or LA bottom line ablation, SVC isolation, or both were added. In some patients with AF triggers, a linear ablation, SVC isolation, or both were performed according to physician discretion. If atrial tachycardia (AT) occurred spontaneously, we created block lines across the isthmus of the AT. The endpoint of the procedure was non-inducibility of AF by the elimination of all PV potentials and non-PV AF triggers or bidirectional conduction block of the linear ablation lines or elimination of SVC potentials. The CA procedures according to the presence or absence of AF triggers and PV reconnections are shown in Figure 1. All ablation procedures were performed using an irrigated-tip ablation catheter (FlexAbilityTM or TactiCathTM, Abbott, MN, USA, or Thermocool®, Biosense Webster) with the guidance of a 3D cardiac mapping system (EnSite PrecisionTM, Abbott or CARTO3, Biosense Webster).
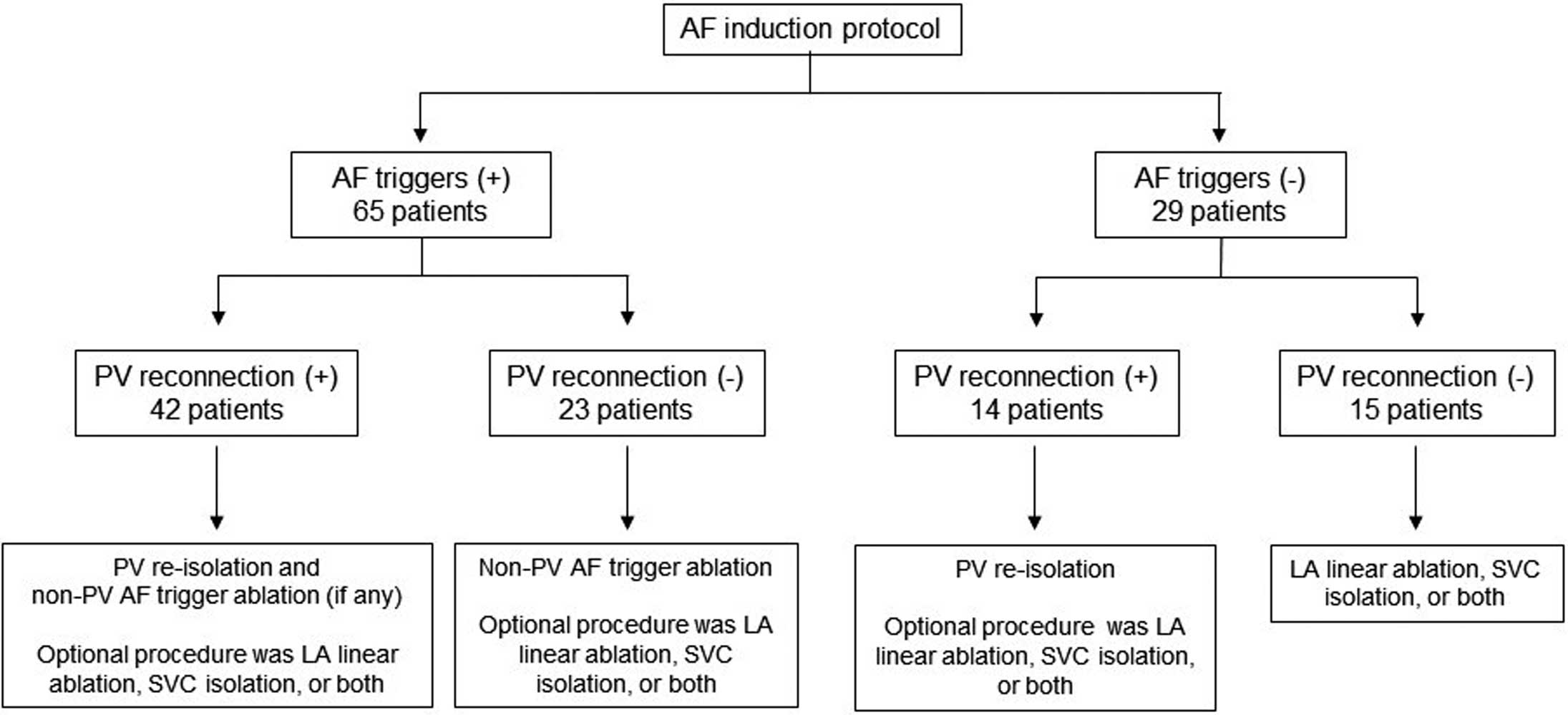
Catheter ablation procedures according to the presence or absence of atrial fibrillation (AF) triggers and pulmonary vein (PV) reconnections. LA, left atrium. SVC, supra vena cava.
Follow-up After CA
All patients were scheduled to visit the outpatient clinic at 1, 2, 3, 6, and 12 months after CA, during which 12-lead ECG and 24-hour Holter ECG were performed. Thereafter, both ECGs were performed annually in the outpatient clinic or whenever any symptoms related to an arrhythmia occurred. Anticoagulant therapy was discontinued if patients with a CHADS2 score <3 or did not experience any recurrence of atrial tachyarrhythmias for ≥3 months after the CA. AADs were given for 3 months to all patients after CA. The AADs were chosen by each physician.
In this study, we investigated the incidence of AAR defined as any recurrence of atrial arrhythmias documented by ECG ≥3 months after CA. The follow-up period was defined as the time until AAR or as the end of the study period (July 31, 2022) for patients who did not experience AAR.
Statistical AnalysisData were analyzed using EZR on R-commander version 1.24 software (Saitama Medical Center, Jichi Medical University, Saitama, Japan), and expressed as the mean±standard deviation for normally distributed variables and as the median (quartile: 25–75%) for continuous variables with a non-normal distribution. We compared the clinical characteristics of the patients with and without AF triggers by univariate analysis (Fisher’s exact test, Mann-Whitney test). Multivariate analysis was performed to evaluate the clinical factors related to the occurrence of AF triggers. Kaplan-Meier analysis using a log-rank test was performed to determine the probability of freedom from AAR. We compared the patients with and without AAR by univariate analysis (Fisher’s exact test, Mann-Whitney test), and multivariate analysis was performed to evaluate the independent predictive factors of AAR. Both the univariate and multivariate analyses were conducted using a Cox proportional hazards model. As a subanalysis, we conducted univariate and multivariate analyses using a Cox proportional hazard model to detect the clinical factors for predicting AAR among the patients who had AF triggers during the second CA session. All parameters with a significance of P<0.10 in the univariate analysis were entered into the multivariate model. In this study, P<0.05 was considered statistically significant.
Patient Consent and Ethics ApprovalThis study was performed in accordance with the Code of Federal Regulations and the Declaration of Helsinki. It was approved by the Ethics Committee of Toho University Omori Medical Center (approval no. M22191). Comprehensive agreement was given by all patients and informed consent was obtained in the opt-out form on the website of Toho University Omori Medical Center.
The baseline characteristics of the patients are listed in Table 1. Among the 94 enrolled patients, AF triggers were identified in 65 (69.1%), and 29 patients (30.9%) did not have any AF triggers, despite undergoing the AF induction protocol. Compared with the patients with no observed AF triggers, those with AF triggers were more likely to have diabetes mellitus. Using a low-voltage cutoff of an electrogram amplitude <0.5 mV, the percentage of low-voltage area (LVA: mean LVA as a percentage of the LA surface area) in patients with AF triggers did not differ from that in patients with no observed AF triggers. Multivariate analyses revealed that no clinical factors were associated with the occurrence of AF triggers during the second CA session.
Baseline Characteristics of the Study Patients
| All (n=94) |
AF triggers (+) (n=65) |
AF triggers (−) (n=29) |
P value | |
|---|---|---|---|---|
| Male, n (%) | 83 (88.3) | 56 (86.2) | 26 (89.7) | 0.75** |
| Age (years) | 63.7 (58.3–70.8) | 63.8 (59.0–70.0) | 63.2 (54.0–72.0) | 0.81*** |
| Body mass index (kg/m2) | 24.4±3.2 | 24.4 (22.3–26.2) | 24.4 (22.8–26.3) | 0.98* |
| Hypertension, n (%) | 59 (62.8) | 38 (58.5) | 20 (69.0) | 0.37** |
| Diabetes mellitus, n (%) | 15 (16.0) | 14 (21.5) | 1 (3.4) | 0.032** |
| Ischemic stroke, n (%) | 8 (8.5) | 6 (9.2) | 2 (6.9) | 1.0** |
| Dyslipidemia, n (%) | 48 (51.1) | 35 (53.8) | 13 (44.8) | 0.51** |
| Heart failure, n (%) | 16 (17.0) | 13 (20.0) | 3 (10.3) | 0.38** |
| CHADS2 score | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 0.62*** |
| CHA2DS2-VASc score | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 0.59*** |
| eGFR (mL/min/1.73 m2) | 68.2 (56.7–80.9) | 67.4 (54.2–81.2) | 69.9 (61.1–79.2) | 0.52*** |
| LDL-cholesterol (mg/dL) | 113.1 (93.3–135.0) | 115.2 (94.5–136.5) | 108.4 (93.3–129.0) | 0.31*** |
| HbA1c (%) | 5.8 (5.5–6.1) | 5.8 (5.6–6.1) | 5.6 (5.5–5.8) | 0.13*** |
| BNP (pg/dL) | 112.4 (25.4–134.9) | 120.4 (25.1–128.4) | 94.9 (26.2–145.3) | 0.43*** |
| LAD (mm) | 41.9 (37.4–47.0) | 42.0 (37.9–47.3) | 41.6 (36.9–45.8) | 0.82*** |
| E/e` | 11.6 (9.3–13.6) | 11.9 (9.5–13.5) | 10.9 (8.9–13.4) | 0.49*** |
| LVDd (mm) | 51.2 (47.6–54.3) | 51.9 (47.4–55.2) | 49.7 (46.9–52.2) | 0.098*** |
| LVDs (mm) | 32.4 (28.1–35.6) | 33.1 (28.5–36.6) | 30.8 (27.7–33.5) | 0.11*** |
| LVEF (%) | 65.8 (60.8–74.0) | 65.1 (60.5–73.6) | 67.4 (63.5–74.5) | 0.38*** |
| Duration of persistent AF, n (%) | ||||
| <1year | 53 (56.4) | 39 (60.0) | 13 (44.8) | 0.069** |
| ≥1 year | 41 (43.6) | 26 (40.0) | 16 (55.2) | 0.19** |
| Percentage of low-voltage area in LA (%) | 25.2 (16.2–34.3) | 27.6 (4.0–38.2) | 22.2 (4.4–38.0) | 0.56*** |
Data are expressed as the mean±standard deviation, median (25–75%), or n (%). P values were determined by *unpaired t-test, **Fisher’s exact test, or ***Mann-Whitney test. AF, atrial fibrillation; BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; LA, left atrium; LAD, left atrial diameter; LDL, low-density lipoprotein; LVDd, left ventricular end-diastolic diameter; LVDs, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction.
Details of the AF Triggers and Prevalence of PV Reconnection
In the 65 patients with AF triggers, at least 1 PV reconnection was observed in 42 patients (64.6%). PV AF triggers were detected in 11 patients, non-PV AF triggers in 43, and both PV and non-PV AF triggers in 11. The remaining 23 (35.4%) patients did not exhibit any PV reconnections, and they experienced induced non-PV AF triggers. Non-PV AF triggers were most frequently located on the interatrial septum, including the fossa ovalis (n=16; 24.6%), followed by the LA posterior wall (n=12; 18.5%), and SVC (n=8; 12.3%). We compared the AF trigger sites between the first and second CA sessions in the 65 patients with AF triggers, which revealed that 51 (78.5%) patients experienced new-onset non-PV AF triggers and only 4 (6.2%) patients experienced non-PV AF trigger recurrence. The remaining 10 (15.3%) patients had PV AF triggers due to PV reconnections. There were no significant differences in the percentage of LVAs in the LA of patients with PV AF triggers as compared with those with non-PV AF triggers (12.6% vs. 30.7%, P=0.23).
In the 29 patients with no observed AF triggers, at least 1 PV reconnection was observed in 14 patients (48.3%) and the remaining 15 patients (51.7%) did not exhibit any PV reconnections.
Details of the CA ProcedureOf the 65 patients with AF triggers, CA was successful in 47 (72.3%) patients, but triggers were not completely ablated in the other 18 (27.7%) patients due to an unsuccessful mapping of non-PV AF triggers. The main causes of unsuccessful mapping were AF with a trigger that could not be detected due to multiple origins, followed by AF with an origin that was difficult to determine even though it was reproducible, and finally, nonreproducible AF.
The 29 patients with no observed AF triggers underwent LA linear ablation more frequently than those with AF triggers (LA roof line; 75.9% [22/29 patients] vs. 44.6% [29/65 patients]; LA bottom line; 72.4% [21/29 patients] vs. 35.4% [23/65 patients]). All PVs were successfully re-isolated in the patients with PV reconnections. Bidirectional conduction block of the LA linear ablation lines and elimination of SVC potentials were confirmed in all patients receiving LA linear ablation and SVC isolation. The details of the comparison of the ablation procedures between patients with and without AF triggers are shown in Table 2.
Details of Catheter Ablation Procedure
| Variable | AF trigger (+) (n=65) | AF triggers (−) (n=29) | ||
|---|---|---|---|---|
| PV reconnections (+) (n=42) |
PV reconnections (−) (n=23) |
PV reconnections (+) (n=14) |
PV reconnections (−) (n=15) |
|
| PV re-isolation, n (%) | 42 (100.0) | 0 (0) | 14 (100.0) | 0 (0) |
| Focal ablation of non-PV AF triggers, n (%) |
33 (78.6) | 23 (100.0) | 0 (0) | 0 (0) |
| Linear ablation | ||||
| LA roof line, n (%) | 19 (45.2) | 10 (43.5) | 9 (64.3) | 13 (86.7) |
| LA bottom line, n (%) | 14 (33.3) | 9 (39.1) | 8 (57.1) | 13 (86.7) |
| SVC isolation, n (%) | 12 (28.6) | 8 (34.8) | 4 (28.6) | 4 (26.7) |
AF, atrial fibrillation; LA, left atrium; PV, pulmonary vein; SVC, superior vena cava.
Clinical Outcomes and Predictors of AAR
During a mean follow-up period of 32.2 (range, 6.25–48.8) months, a total of 55 patients (58.5%) experienced AAR, including AF in 49 patients, atrial flutter in 1, and AT in 5. Among the patients with AF triggers, 33 (50.8%) experienced AAR. Among those with no observed AF triggers, 22 (75.9%) developed AAR. The cumulative freedom ratio from AAR was compared between patients with and without AF triggers by a Kaplan-Meier model, and the rates differed significantly (log-rank test, P=0.012, Figure 2). In the univariable analysis, no observed AF triggers was significantly associated with AAR. Multivariate analyses using a Cox proportional hazard model revealed that having no observed AF triggers during a second CA session was an independent risk factor of AAR (hazard ratio [HR] 1.97, 95% confidence interval [CI] 1.21–3.46, P=0.019) (Table 3A).

Kaplan-Meier curve analysis of atrial arrhythmia recurrence (AAR) in the enrolled patients. The comparison of the cumulative non-recurrence ratio of AAR was between patients with and without atrial fibrillation (AF) triggers. The rate differed significantly between groups in the log-rank test.
(A) Clinical Factors Related to Late Recurrence of an Atrial Arrhythmia, (B) Clinical Factors of Late Recurrences of an Atrial Arrhythmias in Patients With AF Triggers
| (A) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Male, n (%) | 1.06 (0.64–1.75) | 0.83 | ||
| Age (years) | 0.97 (0.94–1.0) | 0.051 | 0.97 (0.94–1.0) | 0.028 |
| Body mass index (kg/m2) | 0.92 (0.84–1.0) | 0.074 | 0.92 (0.84–1.0) | 0.071 |
| Hypertension, n (%) | 0.74 (0.43–1.29) | 0.27 | ||
| Diabetes mellitus, n (%) | 0.61 (0.29–1.31) | 0.21 | ||
| Ischemic stroke, n (%) | 0.50 (0.16–1.62) | 0.25 | ||
| Dyslipidemia, n (%) | 0.69 (0.41–1.18) | 0.18 | ||
| Heart failure, n (%) | 1.06 (0.55–2.06) | 0.86 | ||
| CHADS2 score | 1.01 (0.72–1.04) | 0.62 | ||
| CHA2DS2-VASc score | 0.99 (0.98–1.01) | 0.54 | ||
| eGFR (mL/min/1.73 m2) | 0.99 (0.98–1.01) | 0.84 | ||
| LDL-cholesterol (mg/dL) | 1.00 (0.99–1.0) | 0.84 | ||
| HbA1c (%) | 0.71 (0.50–1.02) | 0.061 | ||
| BNP (pg/dl) | 0.99 (0.99–1.01) | 0.65 | ||
| LAD (mm) | 0.98 (0.95–1.03) | 0.46 | ||
| E/e` | 0.97 (0.77–1.21) | 0.76 | ||
| LVDd (mm) | 1.01 (0.96–1.05) | 0.82 | ||
| LVDs (mm) | 1.00 (0.97–1.03) | 0.87 | ||
| LVEF (%) | 1.00 (0.98–1.02) | 0.92 | ||
| Duration of persistent AF, n (%) | ||||
| <1year | 0.88 (0.52–1.50) | 0.63 | ||
| ≥1 year | 1.14 (0.67–1.94) | 0.63 | ||
| PV reconnections | 0.76 (0.44–1.30) | 0.32 | ||
| LA linear ablation | 1.63 (0.93–2.87) | 0.09 | 1.45 (0.80–2.63) | 0.21 |
| Absence of AF triggers | 1.99 (1.15–3.47) | 0.016 | 1.97 (1.21–3.46) | 0.019 |
| (B) | Univariate analysis | Multivariate analysis | ||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Male, n (%) | 1.41 (0.49–4.02) | 0.52 | ||
| Age (years) | 0.97 (0.94–1.0) | 0.17 | ||
| Body mass index (kg/m2) | 0.92 (0.84–1.0) | 0.051 | 0.92 (0.83–1.02) | 0.10 |
| Hypertension, n (%) | 0.75 (0.38–1.51) | 0.42 | ||
| Diabetes mellitus, n (%) | 0.68 (0.29–1.58) | 0.37 | ||
| Ischemic stroke, n (%) | 0.52 (0.12–2.19) | 0.37 | ||
| Dyslipidemia, n (%) | 0.79 (0.40–1.58) | 0.51 | ||
| Heart failure, n (%) | 1.10 (0.49–2.43) | 0.82 | ||
| CHADS2 score | 0.91 (0.72–1.14) | 0.62 | ||
| CHA2DS2-VASc score | 1.01 (0.73–1.02) | 0.73 | ||
| eGFR (mL/min/1.73 m2) | 0.99 (0.98–1.01) | 0.48 | ||
| LDL cholesterol (mg/dL) | 1.00 (0.99–1.0) | 0.73 | ||
| HbA1c (%) | 0.68 (0.46–1.01) | 0.053 | 0.66 (0.42–1.03) | 0.070 |
| BNP (pg/dl) | 0.99 (0.99–1.01) | 0.95 | ||
| LAD (mm) | 1.00 (0.96–1.06) | 0.87 | ||
| E/e` | 1.00 (0.97–1.03) | 0.97 | ||
| LVDd (mm) | 1.19 (0.89–1.60) | 0.24 | ||
| LVDs (mm) | 1.02 (0.96–1.06) | 0.64 | ||
| LVEF (%) | 1.01 (0.96–1.05) | 0.78 | ||
| Duration of persistent AF, n (%) | ||||
| <1year | 1.15 (0.56–2.33) | 0.71 | ||
| ≥1 year | 0.87 (0.43–1.78) | 0.71 | ||
| PV reconnections | 0.90 (0.44–1.86) | 0.78 | ||
| LA linear ablation | 1.25 (0.62–2.48) | 0.53 | 1.36 (0.67–2.76) | 0.40 |
| Unsuccessful AF triggers ablation | 5.51 (2.70–11.22) | <0.01 | 5.84 (2.79–12.22) | <0.01 |
CI, confidence interval; HR, hazard ratio. Other abbreviations as in Table 1.
Subanalyses of patients with AF triggers showed that the incidence of AAR was higher in the patients with unsuccessful AF trigger ablation than in those with successful AF trigger ablation (94.4% [17/18 patients] vs. 34.0% [16/47 patients], P<0.01). The Kaplan-Meier analysis demonstrated that the cumulative freedom ratio of AAR was significantly lower in patients with unsuccessful AF trigger ablation than in those with successful AF trigger ablation (log-rank test, P<0.01, Figure 3). In the univariable analysis, unsuccessful AF trigger ablation was significantly associated with AAR. In the multivariate analyses using a Cox proportional hazard model, unsuccessful AF trigger ablation was a significant risk factor of AAR (HR 5.84, 95% CI 2.79–12.22, P<0.01) (Table 3B). The patients with unsuccessful AF trigger ablation had many more non-PV AF triggers than those with successful AF trigger ablation (2.0 [2.0–3.0] vs. 1.0 [0.0–2.0], P<0.01). All AF triggers that could not be completely eliminated were generated from non-PV sites.

Kaplan-Meier curve analysis of atrial arrhythmia recurrence (AAR) in the patients with atrial fibrillation (AF) triggers. The comparison of the cumulative non-recurrence ratio of AAR was between the patients with successful and unsuccessful AF trigger ablation. The rate differed significantly between groups in the log-rank test.
In the present study, we studied the effect of AF trigger ablation during a second CA session on the outcomes of non-PAF and found that (1) approximately 70% of the enrolled patients had AF triggers, most of which were generated from non-PV sites; (2) having no observed AF triggers led to worse outcomes than having AF triggers; and (3) when AF triggers occurred, unsuccessful AF trigger ablation had almost 2-fold higher risk of AAR than successful AF trigger ablation.
Adjunctive ablation including non-PV AF trigger ablation and substrate modification in addition to PVI has been considered an important strategy for improving the outcomes of CA of persistent AF. Extensive ablation for substrate modification, such as complex fractionated atrial electrogram-guided ablation or LA linear ablation, in addition to PVI, has been developed and applied in non-PAF patients.7,8 However, some randomized trials failed to prove the beneficial effect of substrate modification strategies as an adjunctive procedure to PVI as compared with PVI alone in those populations.9,10 With regard to other clinical studies of substrate modification, the clinical utility of those procedures was inconsistent among the studies.11,12 However, several studies have demonstrated the efficacy of non-PV AF trigger ablation for improving the outcomes of CA of non-PAF.3,4,13,14 In such cases, the frequency of non-PV AF trigger ablation in addition to PVI has increased as a first-line procedure, but the outcomes of AF trigger ablation during a second CA session for non-PAF have not been fully investigated. In the present study, we found that having no observed AF triggers resulted in almost 2-fold increased risk of AAR than having AF triggers.
We performed PV re-isolation if the PV potential recurred. Studies investigating AF recurrence after PVI show that during the repeat CA session, 63–86% of patients have ≥1 PV reconnection,15 which accorded with the findings from the present study. PV re-isolation, LA linear ablation, and SVC isolation were performed with the same or greater frequency in patients with no observed AF triggers than in those with AF triggers. In particular, LA linear ablation can cause atrial tachyarrhythmia recurrence through reconnections and new substrates. However, in the present study LA linear ablation was not identified as a predictive factor of AAR by multivariate analysis. In addition, among 39 patients with no observed AF triggers, there was no difference in AAR between groups based on the presence or absence of LA linear ablation (log-rank test, P=0.20). Although LA linear ablation was performed more frequently in patients with no observed AF triggers than in those with AF triggers, LA linear ablation itself may not have influenced the higher rate of AAR in patients with no observed AF triggers. The poor outcomes in patients with no observed AF triggers suggest that the CA procedures, including PV re-isolation, LA linear ablation, and SVC isolation, were not sufficient to improve the outcomes in that population.
Among the 65 patients with AF triggers, 49 received only AF trigger ablation, and there was no difference in the rate of AAR between the two groups: those who received AF trigger ablation alone and those who received an adjunctive procedure, including LA linear ablation (log-rank test, P=0.13). Considering that LA linear ablation may especially cause AAR due to reconnections and new-onset substrates, AF trigger ablation alone may be sufficient to improve the outcomes when AF triggers are induced during the second CA session. In our subanalysis of the patients with PV reconnections (n=56), the rate of AAR was significantly higher in the patients with no observed PV AF triggers than in those with PV AF triggers (log-rank test, P=0.043). On the other hand, in the subanalysis of patients with no PV reconnections (n=38), the rate of AAR tended to be higher in the patients with no observed non-PV AF triggers than in those with non-PV AF triggers (85.7% vs. 52.2%, log-rank test, P=0.070). However, this did not reach a significant statistical difference, possibly because of the small sample size. Based on our results, we conclude that the elimination of AF triggers, regardless of their PV or non-PV origin, during a second CA session may be useful to improve outcome.
It is difficult to standardize the ablation strategy for non-PAF because the pathophysiological characteristics of PAF vary among cases. The mechanism of AF recurrence after the first failed CA in patients with AF triggers during a second CA session may be due to AF triggers. Investigating the presence of AF triggers and their sites may be useful for deciding the ablation strategy during a second CA session, as well as in the first CA session. If AF triggers are induced, prioritizing their elimination may be desirable. ISP is used to assess AF triggers because it has >80% success rate in provoking PV and non-PV AF triggers.16 Although adenosine triphosphate (ATP) is effective in inducing AF, we did not use it to induce AF in this study. It should be noted that the effects of ISP are not the same as the effects of ATP. However, it is well known that ATP rarely induces ectopy that causes AF.17 In addition, the efficacy of the elimination of AF triggers induced by ATP is controversial.18 Therefore, we hypothesize that the main results of this study would not differ with the administration of ATP. In the present cases of non-induction of AF triggers, the elimination of AF triggers was inherently difficult. From that perspective, AF non-inducibility may have led to worse outcomes in patients with no observed AF triggers than in those with AF triggers. Other ablation procedures such as a targeted ganglionic plexus ablation,19 and/or a rotor and driver ablation to modify the substrate factors may contribute to improving the outcomes in patients with no observed AF triggers.20–22 Further studies are required to determine the effective adjunctive procedures to improve outcomes in those patients.
Previous studies report that approximately 30–40% of non-PAF patients experience induced non-PV AF triggers during the first CA session.23–25 In our study, non-PV AF triggers were observed in approximately 60% of the enrolled patients. Considering that the induction rate of AF triggers during the second CA session was equal to or greater than that during the first CA session, we need to try to identify AF triggers using the AF induction protocol to also eliminate them in the second CA session. In approximately 80% of cases of AF triggers, the triggers originated from non-PV sites that were different from those during the first CA session, which meant the main cause of non-PV AF triggers during the second CA session was new-onset non-PV AF triggers. Previous studies suggest that atrial substrate remodeling contributes to AF initiation and maintenance through perpetuation of triggers.26,27 LA remodeling may have progressed after the first CA session. In fact, the DR-FLASH score, which has been proven to be effective in predicting the presence of LVAs,28 increased significantly from before the first CA session (from 3.46±1.32 to 3.65±1.28, P<0.01). The mean duration between the first and second CA sessions was 35 months, and the method of AF induction was the same as that during the first CA session. Unfortunately, we were not able to compare the LVAs in the LA between the first and second CA sessions because some patients in the first CA session did not receive LA voltage mapping. In some patients, a non-PV ablation in the first CA session may have caused LA remodeling, which could cause new-onset non-PV AF triggers.29 In addition, AF recurrence after the first CA session may have increased the LA remodeling. AF triggers, in particular, and non-PV AF triggers have not only an elusive mechanism but also limited treatment methods. We achieved successful elimination of AF triggers, including of non-PV AF triggers, in approximately 75% of the patients with AF triggers, which may have resulted in the better outcomes than in those patients with no observed AF triggers. However, a subanalysis of the 65 patients with AF triggers showed that unsuccessful AF trigger ablation was significantly associated with AAR. In cases of difficulty in eliminating all AF triggers, other, as yet unknown, procedures are needed to improve outcomes.
Study LimitationsFirst, this was a retrospective and single-center study. In addition, the number of enrolled patients was relatively small, which limited the generalizability of our study results. Second, inducing AF triggers under ISP infusion and burst pacing/cardioversion protocol has not been verified or standardized, and may be associated with a risk of inducing non-clinical AF triggers. Additionally, the depth of sedation during the procedure may influence AF trigger induction. Third, a heterogeneous ablation strategy of AF trigger ablation with or without simultaneous LA linear ablation and SVC isolation was performed in patients with AF triggers, which significantly reduces the veracity of our statement about the sole significance of AF triggers. A randomized trial evaluating an AF trigger ablation alone vs. an AF trigger ablation with LA linear ablation and/or SVC isolation is needed to confirm the hypotheses of the current study. Finally, the AAR rate might have been underestimated because asymptomatic atrial tachyarrhythmias might have been undetected by 24-hour ECG as compared with an implantable loop recorder.
Patients with no observed AF triggers during a second CA session had a significantly increased risk of AAR, as did those with unsuccessful ablation of AF triggers.
This research received no grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors have no conflicts to disclose.
The study was approved by the Ethics Committee of Toho University Omori Medical Center (approval no. M22191).
The deidentified participant data will not be shared.