2024 年 6 巻 3 号 p. 80-85
2024 年 6 巻 3 号 p. 80-85
Background: The relationship between the prognosis of patients with both chronic thromboembolic pulmonary hypertension (CTEPH) and a mental disorder (MD) remains unclear.
Methods and Results: The study group comprised 157 patients with CTEPH who underwent right heart catheterization and were subdivided into 2 groups according to the presence of MDs: MD and non-MD. The patients with MDs were defined as those who had visited a psychiatrist and were under psychotropic drug treatment. The primary outcome was a composite of all-cause death and worsening of PH. The median follow-up period was 1,164 days. The incidence of the primary composite outcome was higher in the MD group than in the non-MD group (24.0% vs. 6.8%), whereas the all-cause mortality rate was comparable between groups (12.0% vs. 6.1%). The mean pulmonary arterial pressure, cardiac index, and pulmonary vascular resistance at baseline were all similar between groups. The Cox proportional hazards model indicated that MD was an independent risk factor for the primary composite outcome (hazard ratio, 2.990; 95% confidence interval, 1.034–8.642).
Conclusions: In the present study, concomitant CTEPH and MD was significantly associated with a poor prognosis and such patients should be carefully followed.
Chronic thromboembolic pulmonary hypertension (CTEPH) is a rare disease characterized by pulmonary vascular remodeling and increased right ventricular afterload resulting from chronic thromboembolism in the pulmonary arteries. Despite there being some treatment options, such as the preferred pulmonary endarterectomy (PEA) and balloon pulmonary angioplasty (BPA), patients with poor hemodynamics have an unfavorable prognosis.1–4
Mental disorders (MDs), such as major depression and schizophrenia, worsen the prognosis of cardiac diseases, such as myocardial infarction and heart failure (HF),5–9 and concomitant MDs and the use of psychotropic drugs increase the incidence of acute pulmonary embolism (PE) and sudden cardiac death.10,11 Furthermore, a history of MD is a risk factor for poor prognosis in patients with acute PE.12
The incidence of CTEPH is potentially higher in patients with psychiatric disorders than in those without psychiatric disorders,13 but few studies have reported on the relationship between concomitant MD and the prognosis of patients with CTEPH.14 Although patients with MDs have a higher mortality rate after undergoing PEA, the exacerbation of right-sided HF or PH is unclear.14 Therefore, we aimed to evaluate the clinical outcomes in CTEPH patients with and without MDs.
We conducted a retrospective single-center observational study that enrolled 157 consecutive patients with confirmed CTEPH who underwent right heart catheterization (RHC) at the Nagoya University Hospital, Japan, between November 2006 and August 2021. All patients provided written informed consent, and the study design was approved by the Human Research Ethics Committee of Nagoya University Hospital (No. 2016-0438). All research on human subjects was conducted in accordance with the tenets of the 1964 Declaration of Helsinki.
CTEPH diagnosis was based on 2 criteria: (1) mean pulmonary arterial pressure (mPAP) ≥25 mmHg and pulmonary artery wedge pressure (PAWP) ≤15 mmHg at rest and (2) chronic occlusion or stenosis by residual thrombus after >3 months of anticoagulation therapy.15 Patients with a MD were defined as (1) continuously visiting a psychiatrist and (2) under continuous psychotropic drug treatment.14
Right Heart CatheterizationA 6-Fr thermodilution catheter (Nipro Corporation, Osaka, Japan) was inserted into the right jugular vein to measure PAP, PAWP, right ventricular pressure, cardiac output (CO), mixed venous oxygen saturation (SvO2), and arterial oxygen saturation. Pulmonary vascular resistance (PVR) and cardiac index were calculated as follows: PVR = (mPAP − PAWP) / CO and cardiac index = (CO / body surface area), respectively.
Follow-up and EndpointThe primary composite outcome was defined as all-cause death or worsening of PH, which met both of the following criteria: (1) exacerbation of PH symptoms, including worsening World Health Organization-functional classification (WHO-FC), or worsening of right HF, such as leg edema, congested liver, jugular vein distension, pleural effusion, and elevated B-type natriuretic peptide (BNP) level from baseline; and (2) addition of selective pulmonary vasodilators to the previous treatment or the initiation of continuous catecholamine infusion. If all pulmonary vasodilators were already initiated in the background therapy, worsening PH was defined only as meeting the 1st criterion The observation period was defined as the first day of RHC to the first primary composite outcome event or the end of follow-up. The secondary outcome comprised the hemodynamic data of patients who were able to undergo follow-up catheterization and symptoms based on their WHO-FC after treatment completion.
Statistical AnalysisContinuous variables are summarized as mean±standard deviation and compared using Welch’s t-test. The BNP level assessment is represented as the median (1st–3rd quartiles) and was compared using the Mann-Whitney U test. Categorical variables are expressed as numbers and percentages, and a chi-squared test was used to compare groups. The Kaplan-Meier method was used to assess the cumulative incidence of the primary composite outcome and all-cause death. Differences in the cumulative incidence between groups were compared using the log-rank test. Furthermore, a Cox proportional hazards model was used to evaluate the relationship between MD and the primary composite outcome. Variables with a P value <0.1 from the univariate analysis were fitted in the multivariate model. The Statistical Package for the Social Sciences version 28.0.0.0 (IBM Corp., Armonk, NY, USA) was used for all analyses. Statistical significance was defined as a P<0.05.
Of the 157 patients, 25 (15.9%) had a concomitant MD (MD group). The major diagnoses were schizophrenia, bipolar disorder, and depression (Table 1), and the median number of psychiatric drugs was 4. Table 2 summarizes the baseline clinical characteristics of the MD group. In total, 8 (32%) and 47 (35.6%) patients were on selective pulmonary vasodilators at baseline in the MD and non-MD groups, respectively. The SvO2, percent of vital capacity, percent diffusing capacity of the lung for carbon monoxide, and peak oxygen uptake at baseline were significantly lower in the MD group than in the non-MD group. The hemodynamic parameters at baseline, including the mPAP, PAWP, cardiac index, and PVR, were similar between groups.
Distribution of Mental Disorders and Details of Antipsychotic Agent Use in the Mental Disorder Group
| No. of patients, n (%) |
|
|---|---|
| Mental disorder | |
| Schizophrenia | 8 (32%) |
| Bipolar disorder | 7 (28%) |
| Major depression | 4 (16%) |
| Obsessive-compulsive disorder | 1 (4%) |
| Panic disorder | 1 (4%) |
| Adjustment disorder | 1 (4%) |
| Drug-induced psychosis (stimulants) | 1 (4%) |
| Others | 2 (8%) |
| Antipsychotic agent | |
| Typical antipsychotic | 2 (8%) |
| Atypical antipsychotic | 15 (60%) |
| Lithium | 7 (28%) |
| Mood stabilizer | 5 (20%) |
| Antidepressant | 8 (32%) |
| Benzodiazepine | 20 (80%) |
Baseline Characteristics of the Patients in the MD and Non-MD Groups
| MD group (n=25) |
Non-MD group (n=132) |
P value | |
|---|---|---|---|
| Age (years) | 60.6±8.5 | 63.9±14.8 | 0.136 |
| Male/female, n (%) | 5 (20.0%)/20 (80.0%) | 51 (38.6%)/81 (61.4%) | 0.074 |
| Hypertension, n (%) | 7 (28.0%) | 46 (34.8%) | 0.507 |
| Diabetes, n (%) | 4 (16.7%) | 16 (12.1%) | 0.540 |
| Former smoker, n (%) | 10 (41.7%) | 42 (32.3%) | 0.373 |
| Lung disease | 5 (20.0%) | 18 (13.6%) | 0.267 |
| Body mass index | 25.1±4.1 | 23.8±4.7 | 0.188 |
| WHO classification | |||
| I | 0 | 2 (1.5%) | 0.420 |
| II | 7 (28.0%) | 58 (43.9%) | |
| III | 16 (64.0%) | 65 (49.2%) | |
| IV | 2 (8.0%) | 7 (5.3%) | |
| BNP (pg/mL) | 102.7 (15.7–261) | 66.9 (21.2–270) | 0.954 |
| eGFR (mL/min/1.73 m2) | 61±16 | 63±16 | 0.555 |
| D dimer | 0.71±0.40 | 1.20±5.64 | 0.351 |
| Anticoagulant | |||
| Warfarin | 7 (28.0%) | 57 (43.2%) | 0.031 |
| Direct oral anticoagulant | 17 (68.0%) | 75 (56.8%) | |
| None | 1 (4.0%) | 0 | |
| Taking selective pulmonary vasodilators at baseline | 8 (32.0%) | 47 (35.6%) | 0.729 |
| Echocardiographic data | |||
| LVEF (%) | 66.9±8.2 | 68.4±9.9 | 0.404 |
| TAPSE (mm) | 15.7±3.9 | 17.2±4.2 | 0.091 |
| Hemodynamic data | |||
| PAWP (mmHg) | 9.0±3.3 | 9.4±4.1 | 0.625 |
| mPAP (mmHg) | 39.8±9.7 | 41.6±10.2 | 0.420 |
| Cardiac index | 2.40±0.59 | 2.57±0.56 | 0.182 |
| PVR (wood unit) | 8.27±3.29 | 8.42±4.07 | 0.834 |
| Systolic ABP (mmHg) | 121±20 | 128±25 | 0.178 |
| PaO2 (torr, room air) | 58.1±8.3 | 62.3±9.9 | 0.056 |
| SvO2 (%) | 56.7±9.8 | 61.9±7.6 | 0.018 |
| Spirometry* | |||
| %VC (%) | 92±16 | 100±16 | 0.015 |
| FEV1 (%) | 71±6 | 70±8 | 0.409 |
| %DLCO | 72±12 | 82±17 | 0.001 |
| Exercise tolerance | |||
| Peak V̇O2 (mL/min/kg)** | 11.7±3.3 | 14.2±3.7 | 0.010 |
| V̇E vs. V̇CO2 slope** | 51.5±15.3 | 50.1±13.9 | 0.742 |
| 6MWD (m)*** | 325±125 | 380±93 | 0.052 |
*n=25 (MD group) vs. n=121 (non-MD group). **n=17 (MD group) vs. n=90 (non-MD group). ***n=16 (MD group) vs. n=95 (non-MD group). 6MWD, 6-minute walk distance; ABP, arterial blood pressure; BNP, B-type natriuretic peptide; DLco, diffusing capacity of lung for carbon monoxide; eGFR, estimated glomerular filtration rate; FEV, forced expiratory volume; LVEF, left ventricular ejection fraction; MD, mental disorder; mPAP, mean pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; TAPSE, tricuspid annular plane systolic excursion; VC, vital capacity.
Table 3 summarizes the treatments during the follow-up period in both groups. Invasive treatments, including BPA or PEA, were performed in approximately 80% of the patients and their numbers were comparable in both groups.
Treatment During Follow-up of the MD and Non-MD Groups
| MD group (n=25) |
Non-MD group (n=132) |
P value | |
|---|---|---|---|
| Medication only | 6 (24.9%) | 16 (12.1%) | 0.117 |
| PEA | 5 (20.0%) | 27 (20.5%) | 0.959 |
| BPA | 13 (52.0%) | 82 (62.1%) | 0.342 |
| PEA+BPA | 1 (4.0%) | 7 (5.3%) | 0.786 |
BPA, balloon pulmonary angioplasty; MD, mental disorder; PEA, pulmonary endarterectomy.
Figure A depicts the patient survival curves for the groups. The incidence of the primary composite outcome, including all-cause death and worsening of PH, was significantly higher in the MD group than in the non-MD group (24.0% vs. 6.8%, P<0.001), during a median follow-up period of 1,164 (range, 652–1,912) days. Despite comparable all-cause mortality rates between groups (12.0% vs. 6.1%, P=0.311; Figure B), the incidence of worsening PH was significantly higher in the MD group than in the non-MD group (12.0% vs. 0.8%, P=0.001). Table 4 presents the breakdown of outcomes: there was no difference in mortality rate between groups, but exacerbation of PH was more frequent in the MD group.
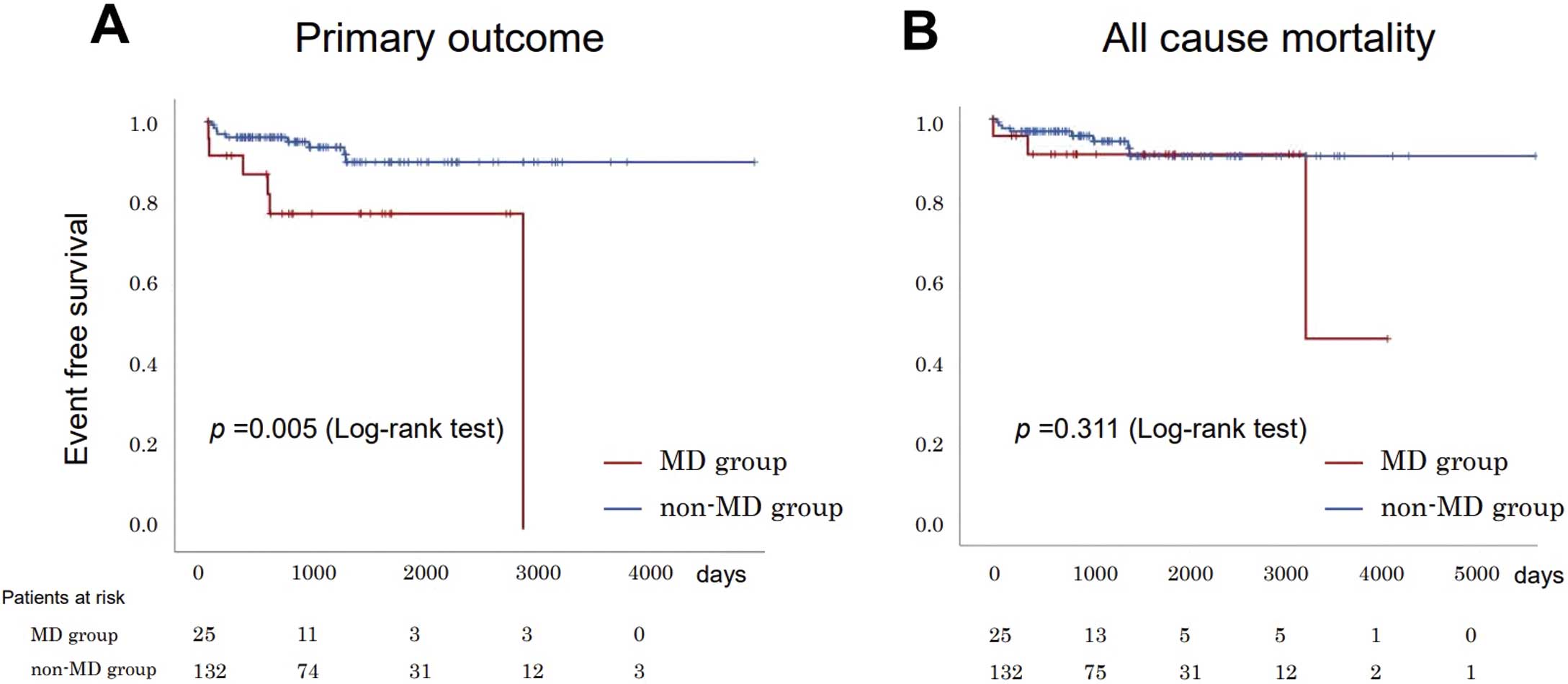
Kaplan-Meier analysis of primary outcome (A) and all-cause death (B). MD, mental disorder.
Clinical Outcomes of the MD and Non-MD Groups
| MD group (n=25) |
Non-MD group (n=132) |
P value | |
|---|---|---|---|
| All-cause death | 3 (12.0%) | 8 (6.1%) | 0.286 |
| Pulmonary hypertension | 0 | 1 | |
| Perioperative period of PEA | 0 | 1 | |
| Suicide | 1 | 0 | |
| Sepsis | 0 | 3 | |
| Unknown | 2 | 3 | |
| Worsening pulmonary hypertension | 3 (12.0%) | 1 (0.8%) | 0.001 |
MD, mental disorder; PEA, pulmonary endarterectomy.
Among those who experienced worsening PH, 1 patient in the MD group was on unplanned selective pulmonary vasodilators; 2 patients in the MD group and 1 in the non-MD group were treated with catecholamines for right-sided HF. The patients who underwent follow-up catheterization demonstrated improved hemodynamics, and there was no significant difference between groups (Supplementary Table 1). We observed no difference in baseline symptoms between groups; although symptoms tended to improve in both groups after treatment, nonetheless, they tended to worsen in the MD group (Supplementary Table 2).
The multivariate Cox proportional hazards model indicated that MD (hazard ratio (HR), 2.990; 95% confidence interval (CI), 1.034–8.642; P=0.043) and WHO-FC III/IV at baseline (HR, 9.222; 95% CI, 1.157–73.527; P=0.036) were significantly associated with the primary composite outcome (Table 5).
Univariate and Multivariate Cox Proportional Hazards Models for Primary Outcomes Including All-Cause Death and Worsening Pulmonary Hypertension
| Univariate models | Multivariate models | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Mental disorder | 3.955 | 1.404–11.139 | 0.009 | 2.990 | 1.034–8.642 | 0.043 |
| Age ≥80 years | 1.501 | 0.192–11.725 | 0.698 | – | – | – |
| Female sex | 0.807 | 0.287–2.270 | 0.685 | – | – | – |
| Hypertension | 2.277 | 0.820–6.319 | 0.114 | – | – | – |
| Diabetes | 1.187 | 0.265–5.308 | 0.823 | – | – | – |
| Former smoker | 1.303 | 0.431–3.937 | 0.639 | – | – | – |
| WHO-FC III/IV (vs. I/II) | 13.268 | 1.723–102.20 | 0.013 | 9.222 | 1.157–73.527 | 0.036 |
| TAPSE <15 mm | 1.038 | 0.354–3.042 | 0.946 | – | – | – |
| mPAP (mmHg) | 1.034 | 0.984–1.086 | 0.182 | – | – | – |
| Cardiac index | 0.440 | 0.164–1.182 | 0.103 | – | – | – |
| PVR (wood unit) | 1.095 | 0.995–1.206 | 0.064 | 1.051 | 0.923–1.196 | 0.445 |
| BNP per 100 pg/mL | 1.101 | 1.007–1.204 | 0.034 | 1.039 | 0.923–1.169 | 0.527 |
| eGFR <60 mL/min/1.73 m2 | 2.219 | 0.785–6.269 | 0.133 | – | – | – |
CI, confidence interval; HR, hazard ratio; WHO-FC, WHO-functional classification. Other abbreviations as in Table 2.
The present study had 2 major findings. First, 16% of the 157 patients with CTEPH had a MD. Second, MD and WHO-FC III/IV at baseline were independent risk factors for poor CTEPH prognosis. Despite the poor prognosis of patients undergoing PEA for CTEPH,14 our novel report demonstrated that MD was significantly associated with the composite outcome of all-cause death and worsening of PH regardless of standard treatment.
Patients with CTEPH can have a high prevalence of MDs. Schizophrenia is prevalent in 7.3% of patients with CTEPH, in 0.7% of patients with pulmonary artery hypertension, and in 0.6% of the general Japanese population.13 In the present study, 5.1% of the patients had schizophrenia, which was a relatively large proportion but similar to the previous finding.
There are several explanations for the high prevalence of MDs in patients with CTEPH. Patients with MDs report a higher prevalence of venous thromboembolism (VTE) and acute PE,10 possibly patients with schizophrenia have lower respiratory function and exercise tolerance (i.e., short 6-min walk distance) compared with healthy controls.16 Impaired exercise performance in patients with MDs, including schizophrenia and other diseases, is attributed to being overweight and metabolic complications, muscular weakness, high smoking rates, emotional status, and the use of antipsychotropic drugs.17,18 In the present study, the patients with MDs had poor respiratory function, such as low percent of vital capacity and low percent diffusing capacity of the lung for carbon monoxide, despite similar rates of lung disease between the groups. Furthermore, exercise tolerance was lower in the MD group compared with the non-MD group. Restrictions on daily activities in patients with MDs can increase the risk of developing VTE, similar to that while taking antipsychotropic drugs.11 Olanzapine, an antipsychotic, has been associated with antiphospholipid syndrome.19 In the present study, 6 patients with MDs were prescribed olanzapine, although they did not have a definitive diagnosis of antiphospholipid syndrome.
MDs may be considered a potential factor in the development of PH. Despite complex mechanisms underlying MDs, the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) signaling pathway is significantly associated with antidepressant effects.20 Dysregulation of this pathway has been associated with schizophrenia and other human diseases,21 and atypical antipsychotics activate the AKT pathway.21 However, PI3K/AKT signaling leads to pulmonary artery thickening; inhibition of this signaling pathway attenuated pulmonary artery remodeling in a rat model of hypoxia.22 Psychotropic drugs may exacerbate the negative effects of PH by activating the AKT pathway; however, further studies are needed to clarify this mechanism. These factors may have been involved in the high prevalence of MDs in patients with CTEPH in our study.
In general, MDs are associated with an increased risk of cardiovascular disease and poor prognosis.23 Despite the correlation between poor hemodynamics and poor prognosis of CTEPH,1,24 concomitant MD and presenting with WHO-FC III/IV at baseline were associated with poor outcomes in our study, not hemodynamics. Moreover, among the patients who were followed up, the symptoms were less improved in those who had a comorbid MD despite improvement in hemodynamics, regardless of the MD. MDs could also affect improvement in symptoms, even when receiving appropriate HF treatment.25 Therefore, besides hemodynamics, other risk factors may affect the prognosis.
MDs increase the risk of VTE; nonetheless, concomitant MD with acute PE is associated with a poor prognosis.12 Furthermore, antipsychotic medications increase the risk of sudden cardiac death in a dose-dependent manner,26 and MDs may affect cardiac health status. Patients with schizophrenia and normal left ventricular ejection fraction (LVEF) demonstrate concentric cardiac remodeling on cardiac magnetic resonance imaging, compared with matched healthy controls. This remodeling is observed in the left and right ventricles and is independent of the dose or duration of antipsychotic drugs.27 In a study that evaluated native T1 mapping using cardiac magnetic resonance imaging, diffuse T1 time prolongation was observed in individuals with schizophrenia, compared with healthy controls,28 which suggests early diffuse myocardial fibrosis and/or subclinical myocardial inflammation in patients with schizophrenia. We evaluated the LVEF and tricuspid annular plane systolic excursion in the present patients, but certain risk factors may not be assessed using echocardiography.
According to a medication adherence study in patients with CTEPH, adherence varied between 60% and 91%, depending on the drug type.29 Age, sex, and educational level were not associated with adherence; however, concomitant MD was not considered. Patients with schizophrenia report 35% adherence to antipsychotropic drugs,30 and patients with severe MDs, including schizophrenia, report worse adherence.31 Adherence in patients with CTEPH and comorbid MDs is unknown, and thought to be low in our patient group. Thus, concomitant MDs in patients with CTEPH is a potential risk for poor prognosis.
Study LimitationsFirst, this retrospective, single-center study included a relatively small number of patients. Second, the clinical outcomes may depend on the year of enrollment due to updated CTEPH treatment. However, several patients were administered pulmonary vasodilators, PEA, and/or BPA. Third, despite different anticoagulants being administered at baseline, 24 patients changed treatment during the follow-up period, making it difficult to assess the drugs’ effect on prognosis. Fourth, MDs were not defined according to the International Classification of Diseases (11th edition) or the Diagnostic and Statistical Manual of Mental Disorders (5th edition). Most patients were diagnosed at other hospitals, so the full details of their MDs were unclear. The non-MD group may have included patients with borderline psychosis, thus warranting improving the accuracy by screening all cases in cooperation with psychiatrists. Moreover, information on adherence was not obtained for either the MD or non-MD group; Distinguishing between the origin of symptoms is challenging due to the overlap between HF and MD symptoms.25 Future collaboration with psychiatrists may contribute to confirming the identified associations.
In conclusion, patients with CTEPH and a MD showed a higher incidence of a composite outcome of all-cause death or worsening of PH, despite receiving appropriate treatment. Worsening of PH was particularly frequent when patients had comorbid MDs. Patients with CTEPH have several comorbid MDs, which should be recognized and followed up cautiously because it can affect prognosis.
T.M. is a member of Circulation Reports’ Editorial Team.
The authors declare that there are no conflicts of interest.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The present study was approved by the Human Research Ethics Committee of Nagoya University Hospital (No. 2016-0438). All participants provided written informed consent.
Please find supplementary file(s);
https://doi.org/10.1253/circrep.CR-23-0074