Abstract
Background: Neither the efficacy nor safety of elobixibat has been investigated in the treatment of chronic constipation in patients with heart failure (HF).
Methods and Results: In this prospective, single-center, single-arm study elobixibat (10 mg/day) was administered for 12 weeks to 18 HF patients with chronic constipation defined according to the Rome IV criteria. Spontaneous bowel movement (SBM), stool consistency as measured by the Bristol Stool Form Scale, and degree of straining during defecation were recorded. In addition, biomarkers, blood pressure (BP) measured by ambulatory monitoring, and adverse events were assessed. Although there was no significant difference, the frequency of SBM increased by 2.0/week from baseline to Week 12. Both the degree of straining during defecation and low-density lipoprotein cholesterol (LDL-C) levels were significantly decreased at Week 12 (straining, −0.79 [95% confidence interval (CI), −1.40 to −0.17]; LDL-C, −10.4 mg/dL [95% CI, −17.9 to −2.9]). Although not significant, the difference in BP before and after defecation tended to decrease from baseline by approximately 10 mmHg at Week 12. Serious adverse events were not observed.
Conclusions: Elobixibat reduced the degree of straining during defecation, and improved the lipid profile in HF patients with chronic constipation.
Patients with heart failure (HF) tend to become constipated due to restrictions of water intake, promotion of water excretion by diuretics, impaired mobility and environmental changes associated with hospitalization.1 Previous studies have reported that 25–42% of patients with HF had the complication of constipation.2–4
It has been suggested that straining, a symptom of constipation, may have a major effect on the cardiovascular system. Severe straining can cause great changes in blood pressure (BP),5 leading to an increase in cardiac load, and a relationship between the changes in BP associated with straining during defecation and cardiovascular events has been reported.1,6 Others have also reported that patients with constipation are at high risk of cardiovascular events.7–9 Therefore, controlling constipation is considered an important intervention in patients with HF.
Traditionally, medications such as bulk-forming laxatives, stimulant laxatives, osmotic laxatives, and intestinal secretagogues have been used for the treatment of chronic constipation. In Japan, magnesium oxide, an osmotic laxative, and stimulant laxatives are often used. However, the use of osmotic laxatives in patients with HF can cause dehydration or bradycardia with overdosing.10 Furthermore, magnesium oxide can cause hypermagnesemia in patients with HF who often have renal dysfunction as a complication. Long-term, continuous use of stimulant laxatives results in resistance and can lead to intractable constipation.11 Thus, constipation is often difficult to treat in patients with HF.
Elobixibat has a different mechanism of action from other laxatives: it inhibits bile acid transporters (the ileal bile acid transporter and apical sodium-dependent bile acid transporter) expressed on epithelial cells in the terminal ileum, thus preventing bile acid reabsorption and increasing the amount of bile acids in the large intestinal lumen.12 Elobixibat also increases fluid and electrolyte secretion in the large intestinal lumen together with induction of high-amplitude propagated contractions through activation of the transmembrane G protein-coupled receptor 5 by the bile acids that are present in the large intestine.13,14 In a Japanese phase 3 study in patients with chronic constipation, elobixibat significantly improved constipation parameters, such spontaneous bowel movements (SBM), complete spontaneous bowel movements (CSBM), stool consistency, and the severity of constipation, compared with the placebo group.15 However, the efficacy and safety of elobixibat have not yet been investigated in HF patients with chronic constipation, so we evaluated those in the present study as well as performing an exploratory study of the effects of elobixibat on cardiorenal-related biomarkers, lipid profiles, and BP.
Methods
Study Design
This was an open-label, single-center, single-arm, controlled before-and-after prospective study. Patients were recruited from the outpatients who were treated for HF at Kumamoto University Hospital between May 2020 and March 2022. The study protocol and informed consent form were approved by the Kumamoto University Certified Clinical Research Review Board (certification No.: CRB7200002). The study was registered in the Japan Registry of Clinical Trials (jRCT) (jRCTs071190055). Written consent was given by all study patients prior to participation in the study, which was conducted in accordance with the Declaration of Helsinki.
Patients
Eligible patients were those who met all of the criteria shown in Supplementary Table. The major criteria were: ≥20 years of age; chronic constipation meeting the Rome IV criteria for functional constipation; HF defined as Class II–III of the New York Heart Association (NYHA) functional classification and no change in HF medications within the 12 weeks prior to informed consent, not been hospitalized for the treatment of cardiovascular disease within the 6 months before informed consent; and capable of independently recording SBM.
Exclusion criteria are also shown in Supplementary Table. The major criteria were hypersensitivity to elobixibat; diagnosed or suspected of having intestinal obstructive disease associated with a tumor or hernia; any serious liver disorder; possible biliary atresia or decreased bile secretion; and suspected organic constipation.
Procedures
A run-in period of 4 weeks was established for all patients to eliminate the effect of prior treatment, with the last week defined as baseline. Drugs for chronic constipation that the patients had been using were continued during the study period. Additions or changes to drugs for chronic constipation were not allowed, except for the use of rescue medications (suppositories and enemas: e.g., sodium bicarbonate suppositories, bisacodyl suppositories, and glycerin enemas). Dose changes also were not allowed, except for reductions for safety reasons. The patients were instructed to record bowel movements every day from the first visit. After the run-in period, the investigators again assessed eligibility and prescribed elobixibat 10 mg (2 tablets of 5 mg) once daily before a meal for 12 weeks. Elobixibat could be dose-adjusted or interrupted according to symptoms, but the maximum daily dose was 15 mg. The patients used self-recording of bowel movements to document the following properties at each bowel movement: stool consistency (Bristol Stool Form Scale [BSFS]), the presence or absence of a sensation of incomplete bowel evacuation, and the degree of straining (5-point scale: (1) no straining, (2) mild straining, (3) moderate straining, (4) severe straining, or (5) very severe straining). The investigators reviewed the bowel movement record at each visit. Blood tests were performed at baseline, and weeks 4, 8, and 12. Before administration of elobixibat and at the Week 12 visit, BP was measured continuously for ≥24 h, using ambulatory BP monitoring (ABPM).
Endpoints
Primary Endpoint The primary endpoint was a change in SBM from baseline to Week 12. SBM was defined as the frequency of bowel movements without use of suppositories, enemas, or stool extraction.
Secondary Endpoints Secondary endpoints included the parameters of constipation, biomarkers, BP measured by ABPM, and safety.
Endpoints for constipation were the change in SBM from baseline to weeks 4 and 8, change from baseline in the degree of straining during defecation, the time course of BSFS from baseline to Week 12, and change in CSBM (defined as SBM without a sensation of incomplete bowel evacuation) from baseline. The changes from baseline in plasma levels of B-type natriuretic peptide (BNP), renin activity (PRA), and aldosterone (ALD), and serum levels of low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), and total cholesterol (T-Chol), and the estimated glomerular filtration rate (eGFR) were assessed. In addition, BP measured by ABPM at baseline and Week 12 was compared. Safety endpoints were the incidence of adverse events (defined as any disease or dysfunction, death, or infection suspected to be attributable to the conduct of the study in any untoward or unintended medical condition or symptom that occurred in patients), discontinuation rate, and treatment interruption rate.
Statistical Analysis
Analysis data sets were prepared and statistical analyses were performed using SAS version 9.4 program for Windows (SAS Institute Inc., Cary, NC, USA). R version 4.2.2 (R Core Team, 2022, https://www.R-project.org/) was used for validation of some statistical analyses. Adverse events that occurred were coded using the Medical Dictionary for Regulatory Activities/Japanese version (MedDRA/J Version) 25.1. Continuous variables are expressed as mean±standard deviation.
Efficacy was analyzed in the following 2 populations: all study patients who received at least 1 dose of elobixibat and had at least 1 efficacy datum [full analysis set [FAS] (1)] and the study patients in FAS (1) with <6 SBMs during the 2 weeks before the prescription of the study drug, the same as the criterion for SBM specified in a phase 3 study of elobixibat [FAS (2)]. The primary analysis was performed in the FAS (2) that met the criterion for SBM specified in the Japanese phase 3 study, as the primary endpoint of this study was SBM. For the primary endpoint, data were imputed using the last observation carried forward method.
For the primary efficacy endpoint, the change in SBM was assumed to follow a normal distribution, and a paired t test was carried out. To interpret the P value, a statistically significant difference was evaluated in FAS (1) only when a test result in FAS (2) using the closed testing procedure was statistically significant at a 2-sided level of 5%.
For the secondary efficacy endpoints, 95% confidence intervals (CIs) were calculated for the changes from baseline.
Results
Patients
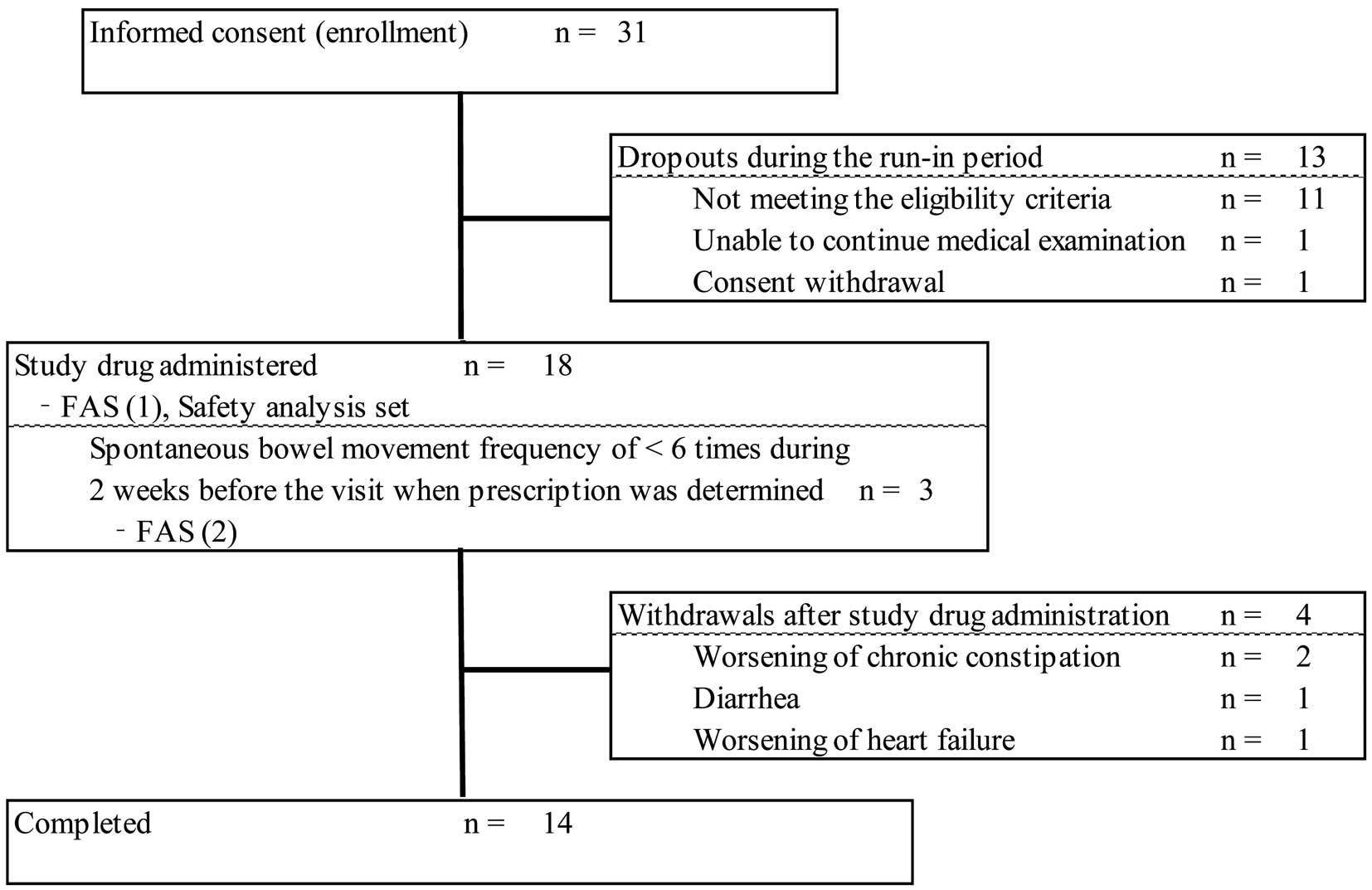
The study flow chart is shown in Figure 1: 31 patients provided informed consent, and all were enrolled in the study. A total of 13 patients dropped out as specified in the protocol during the run-in period: 11 patients did not meet the eligibility criteria that were confirmed after the run-in period, 1 patient could not undergo continual medical examination, and 1 patient withdrew consent. Therefore, 18 patients received elobixibat and were included in the safety analysis set and FAS (1); 3 patients were included in FAS (2). Of the 18 patients treated with elobixibat, treatment was discontinued before Week 12 in 4 due to worsening of chronic constipation (2 patients), diarrhea (1 patient), and worsening of HF (1 patient), and was completed in 14 patients.
The characteristics of the patients [FAS (1) and FAS (2)] are shown in Table 1. The mean age was 72.2±8.4 years (range, 57–83 years), and 67% were male. NYHA classes I, II, and III comprised 6%, 89%, and 6%, respectively. The mean duration of HF was 6.72±4.59 years. Drugs for constipation were used in 61% of patients before enrolment. One patient in NYHA class I did not meet the inclusion criterion but was mistakenly enrolled in the study.
Table 1.
Baseline Demographic Characteristics
| |
FAS (1)
(n=18) |
FAS (2)
(n=3) |
| Age, years (range) |
72.2±8.4 (57–83) |
78.0±4.6 (74–83) |
| Male, n (%) |
12 (67) |
2 (67) |
| Heart Failure classification [n (%)] |
| HFrEF |
4 (22) |
1 (33) |
| HFpEF |
12 (67) |
2 (67) |
| HFmrEF |
2 (11) |
0 (0) |
| HFrecEF |
0 (0) |
0 (0) |
| NYHA functional classification, n (%) |
| I |
1 (6) |
0 (0) |
| II |
16 (89) |
3 (100) |
| III |
1 (6) |
0 (0) |
| Duration of HF, years |
6.72±4.59 |
3.67±3.40 |
| Etiology of HF, cases |
| Myocardial infarction |
4 |
1 |
| Atrial fibrillation |
3 |
0 |
| Aortic valve stenosis |
2 |
0 |
| Aortic valve incompetence |
1 |
1 |
| Silent myocardial ischemia |
1 |
1 |
| Angina pectoris |
1 |
0 |
| 2nd degree atrioventricular block |
1 |
0 |
| Cardiac amyloidosis |
1 |
0 |
| Hypertensive heart disease |
2 |
0 |
| Cardiac sarcoidosis |
1 |
0 |
| Mitral valve incompetence |
1 |
0 |
| Pulmonary hypertension |
1 |
0 |
| Hypertrophic cardiomyopathy |
1 |
0 |
| Non-compaction cardiomyopathy |
1 |
0 |
| Post mitral valve replacement |
1 |
0 |
| Post pacemaker implantation |
1 |
0 |
| Height, cm |
159.18±7.64 |
158.85±0.78 |
| Weight, kg |
60.59±13.43 |
74.80* |
| BMI, kg/m2 |
25.39±5.57 |
29.44* |
| Duration of chronic constipation, years |
8.07±10.97 |
–** |
| Drugs for the treatment of chronic constipation before enrolment, n (%) |
| No |
7 (39) |
2 (67) |
| Yes |
11 (61) |
1 (33) |
| Magnesium oxide (cases) |
9 |
1 |
| Lubiprostone (cases) |
3 |
0 |
| Stimulant laxatives (cases) |
2 |
0 |
| Use of concomitant medications to be administered with caution, n (%) |
| None |
18 (100) |
3 (100) |
| Concomitant use of drugs for HF, n (%) |
| β-blockers |
9 (50) |
1 (33) |
| ACE inhibitor/ARB/ARNI |
10 (56) |
1 (33) |
| MRA |
7 (39) |
1 (33) |
| Diuretic |
10 (56) |
2 (67) |
| SGLT2 inhibitor |
2 (11) |
0 (0) |
*Only the mean value is presented as n=1; **Not applicable because n=0. Data in the table are expressed as mean±standard deviation, unless otherwise specified. ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; ARNI, angiotensin-receptor-neprilysin inhibitor; BMI, body mass index; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HFrecEF, heart failure with recovered ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; SGLT, sodium-glucose cotransporter.
Efficacy
Primary Endpoint In FAS (2), the SBM rate was 2.3±1.2/week at baseline and 4.3±2.1/week at Week 12, and the point estimate of the mean change from baseline to Week 12 was 2.0/week (95% CI, −0.5 to 4.5; P=0.07); although there was a tendency for SBM to increase, there was no statistically significant difference. In FAS (1), the SBM rate was 7.0±4.1/week at baseline and 7.7±4.2/week at Week 12, and the point estimate of the mean change from baseline to Week 12 was 0.7/week (95% CI, −0.8 to 2.2) (Figure 2).
Secondary Endpoints
Parameters of Constipation The point estimates of the mean change in SBM from baseline (FAS (2), 2.3±1.2/week; FAS (1), 7.0±4.1/week) were as follows: in FAS (2), 1.3/week (95% CI, −3.8 to 6.5) at Week 4 and 1.3/week (95% CI, −4.9 to 7.6) at Week 8; and in FAS (1), 0.6/week (95% CI, −0.7 to 1.9) at Week 4 and 0.8/week (95% CI, −0.9 to 2.5) at Week 8 (Table 2). The CSBM increased by 1.9/week from baseline (4.3±4.0/week) to Week 12, with no statistically significant difference (Table 2). The point estimates of the mean change in degree of straining during defecation (median of each evaluation interval) from baseline (3.11±0.85) were −0.53 (95% CI, −0.88 to −0.18) at Week 4, −0.57 (95% CI, −0.99 to −0.15) at Week 8, and −0.79 (95% CI, −1.40 to −0.17) at Week 12, showing significant decreases at all time points (Table 2). The mean BSFS score at baseline was 3.92 and within the range of normal stool, and the range of normal stool was maintained until Week 12 of elobixibat treatment (Table 2).
Table 2.
Time Course of Parameters of Constipation
| |
Baseline |
Week 4 |
Week 8 |
Week 12 |
| SBM, /week |
| FAS (1) |
| Mean±SD, n |
7.0±4.1 (n=18) |
7.6±4.3 (n=18) |
8.2±5.4 (n=14) |
8.1±4.6 (n=14) |
| Change (95% CI) |
– |
0.6 (−0.7 to 1.9) |
0.8 (−0.9 to 2.5) |
0.6 (−1.3 to 2.6) |
| FAS (2) |
| Mean±SD, n |
2.3±1.2 (n=3) |
3.7±2.1 (n=3) |
3.7±2.9 (n=3) |
4.3±2.1 (n=3) |
| Change (95% CI) |
– |
1.3 (−3.8 to 6.5) |
1.3 (−4.9 to 7.6) |
2.0 (−0.5 to 4.5) |
| CSBM, /week |
| Mean±SD, n |
4.3±4.0 (n=18) |
5.6±4.9 (n=18) |
6.2±5.8 (n=14) |
6.3±4.9 (n=14) |
| Change (95% CI) |
– |
1.3 (−0.4 to 3.1) |
1.9 (−0.5 to 4.2) |
1.9 (−0.5 to 4.4) |
| Degree of straining during defecation |
| Mean±SD, n |
3.11±0.85 (n=18) |
2.71±0.75 (n=17) |
2.71±0.73 (n=14) |
2.50±1.13 (n=14) |
| Change (95% CI) |
– |
−0.53 (−0.88 to −0.18) |
−0.57 (−0.99 to −0.15) |
−0.79 (−1.40 to −0.17) |
| BSFS |
| Mean±SD, n |
3.92±1.36 (n=18) |
3.85±1.09 (n=17) |
3.75±1.48 (n=14) |
4.21±1.54 (n=14) |
| Change (95% CI) |
– |
0.06 (−0.81 to 0.93) |
0.14 (−0.42 to 0.70) |
0.61 (−0.46 to 1.67) |
For the CSBM, degree of straining during defecation and BSFS, the results in FAS (1) are presented. BSFS, Bristol Stool Form Scale; CI, confidence interval; CSBM, complete spontaneous bowel movement; SBM, spontaneous bowel movement; SD, standard deviation.
Biomarkers The results for biomarkers are shown in Table 3. The changes in LDL-C from baseline were −12.2 mg/dL (95% CI, −18.2 to −6.2) at Week 4, −10.6 mg/dL (95% CI, −15.8 to −5.5) at Week 8, −10.4 mg/dL (95% CI, −17.9 to −2.9) at Week 12, and −12.1 mg/dL (95% CI, −19.2 to −4.9) at the last evaluation. The changes in T-Chol from baseline were −11.1 mg/dL (95% CI, −18.0 to −4.1) at Week 4, −9.5 mg/dL (95% CI, −15.7 to −3.3) at Week 8, −11.9 mg/dL (95% CI, −18.9 to −5.0) at Week 12, and −12.5 mg/dL (95% CI, −19.6 to −5.4) at the last evaluation. The LDL-C and TChol levels significantly decreased from baseline at Weeks 4, 8, and 12 and the last evaluation. The changes in TG, PRA and eGFR were not significantly different after elobixibat administration; however, BNP was significantly increased from baseline at the last evaluation (22.65 pg/mL [95% CI, 0.19 to 45.11]).
Table 3.
Time Course of Biomarkers
| |
Baseline |
Week 4 |
Week 8 |
Week 12 |
Last evaluation |
| T-Chol, mg/dL |
| Mean±SD, n |
152.6±37.5
(n=18) |
141.5±39.4
(n=18) |
135.1±40.4
(n=14) |
132.7±39.8
(n=14) |
140.1±39.9
(n=18) |
| Change (95% CI) |
– |
−11.1
(−18.0 to −4.1) |
−9.5
(−15.7 to −3.3) |
−11.9
(−18.9 to −5.0) |
−12.5
(−19.6 to −5.4) |
| HDL-C, mg/dL |
| Mean±SD, n |
54.4±17.5
(n=18) |
57.4±18.3
(n=18) |
54.4±18.0
(n=14) |
53.2±17.8
(n=14) |
54.7±18.3
(n=18) |
| Change (95% CI) |
– |
3.0
(1.0 to 5.0) |
0.4
(−3.4 to 4.3) |
−0.8
(−4.1 to 2.5) |
0.3
(−2.5 to 3.2) |
| LDL-C, mg/dL |
| Mean±SD, n |
81.8±29.6
(n=18) |
69.6±30.5
(n=18) |
63.9±28.8
(n=14) |
64.1±31.7
(n=14) |
69.8±32.1
(n=18) |
| Change (95% CI) |
– |
−12.2
(−18.2 to −6.2) |
−10.6
(−15.8 to −5.5) |
−10.4
(−17.9 to −2.9) |
−12.1
(−19.2 to −4.9) |
| TG, mg/dL |
| Mean±SD, n |
84.1±32.0
(n=18) |
81.1±32.1
(n=18) |
92.4±41.7
(n=14) |
83.1±31.4
(n=14) |
83.6±32.4
(n=18) |
| Change (95% CI) |
– |
−3.0
(−14.1 to 8.1) |
11.8
(−9.8 to 33.4) |
2.6
(−10.0 to 15.1) |
−0.5
(−10.6 to 9.6) |
| BNP, pg/mL |
| Mean±SD, n |
90.29±206.14
(n=18) |
103.76±187.94
(n=18) |
119.53±254.85
(n=14) |
116.12±236.72
(n=14) |
112.94±212.61
(n=18) |
| Change (95% CI) |
– |
13.47
(−15.18 to 42.13) |
16.72
(−1.42 to 34.86) |
13.31
(−3.21 to 29.84) |
22.65
(0.19 to 45.11) |
| ALD, pg/mL |
| Mean±SD, n |
68.99±134.57
(n=16) |
65.91±89.76
(n=15) |
39.12±73.26
(n=12) |
54.89±109.94
(n=11) |
61.63±106.70
(n=17) |
| Change (95% CI) |
– |
−5.91
(−48.48 to 36.66) |
−27.70
(−82.48 to 27.08) |
−15.95
(−52.94 to 21.04) |
−4.53
(−30.22 to 21.17) |
| PRA, ng/mL/h |
| Mean±SD, n |
11.39±25.66
(n=16) |
10.78±23.55
(n=16) |
8.68±19.20
(n=12) |
9.36±21.06
(n=12) |
9.34±18.47
(n=17) |
| Change (95% CI) |
– |
−0.63
(−3.66 to 2.39) |
−3.49
(−10.99 to 4.01) |
−2.78
(−9.07 to 3.51) |
−1.49
(−6.25 to 3.27) |
| eGFR, mL/min/1.73 m2 |
| Mean±SD, n |
55.957±21.443
(n=18) |
55.403±20.248
(n=18) |
56.020±22.705
(n=14) |
55.558±20.832
(n=14) |
56.914±20.513
(n=18) |
| Change (95% CI) |
– |
−0.553
(−3.111 to 2.005) |
2.393
(−0.126 to 4.912) |
1.931
(−0.206 to 4.068) |
0.957
(−1.753 to 3.667) |
The results in FAS (1) are presented. ALD, plasma aldosterone level; BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; PRA, plasma renin activity; T-Chol, total cholesterol; TG, triglyceride. Other abbreviations as in Table 2.
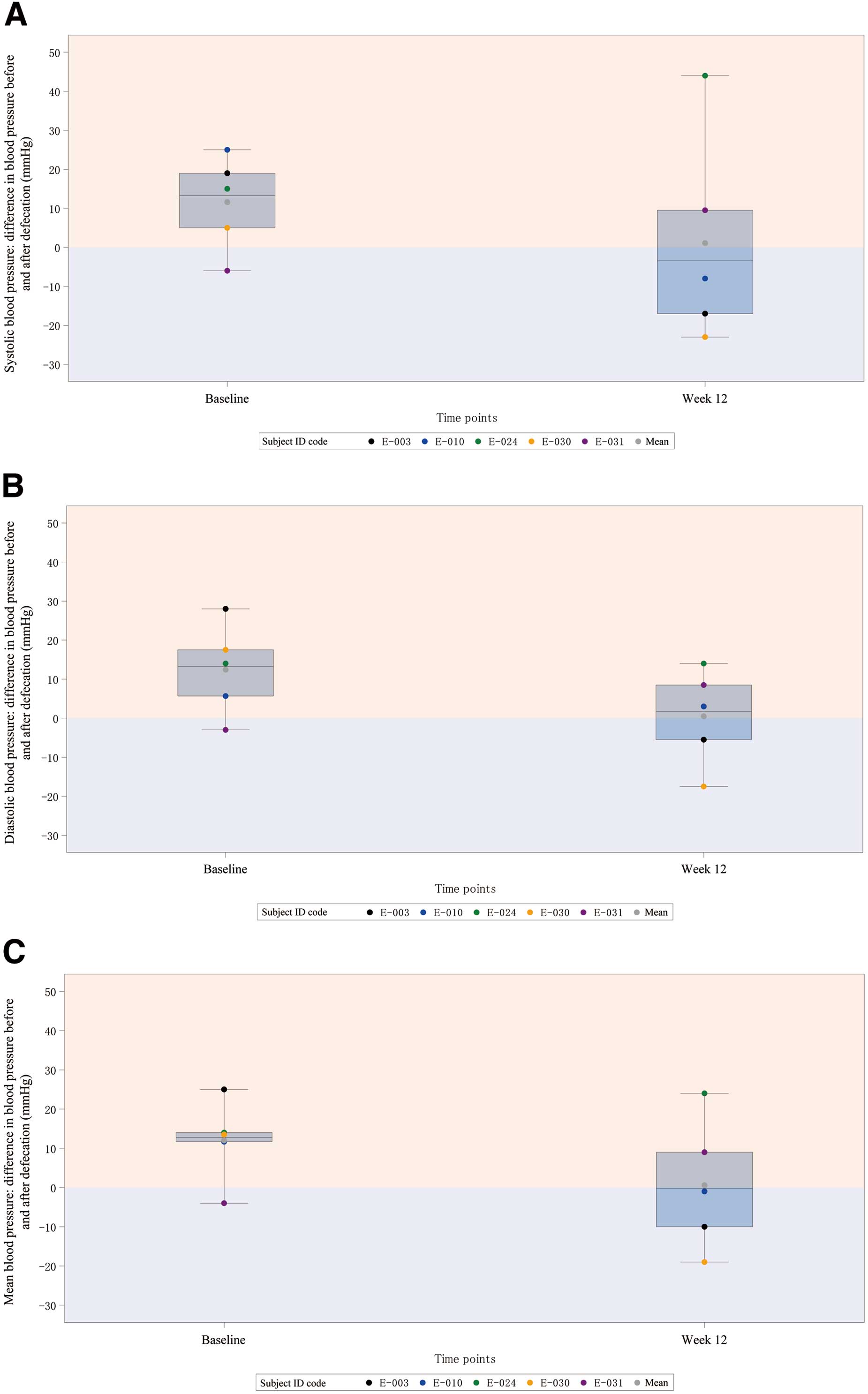
ABPM The changes in BP by ABPM before and after defecation from baseline to Week 12 were as follows: systolic blood pressure, −10.50 mmHg; diastolic blood pressure, −11.93 mmHg; and mean blood pressure, −11.43 mmHg. Although these were not significant, the difference in BP before and after defecation tended to decrease by approximately 10 mmHg for all variables (systolic blood pressure, diastolic blood pressure, and mean blood pressure) at Week 12 from baseline (Figure 3).
Safety The incidence of adverse events was 17% (3/18 patients), and all adverse events were assessed as being related to the study drug. No serious adverse events were recorded. Adverse events comprised diarrhea in 11% of patients (2/18 patients) and constipation in 6% (1/18 patients); all were of mild-to-moderate severity. The diarrhea resolved in 1 patient and abated in the other; the constipation in 1 patient abated. Adverse events leading to discontinuation of the study treatment were single instances of diarrhea and constipation. There was no clinically relevant change in laboratory values.
Discussion
This study evaluated the efficacy and safety of elobixibat administered for 12 weeks for chronic constipation in Japanese patients with HF. The results revealed no statistically significant change in SBM, but significantly reduced straining during defecation and significantly decreased serum LDL-C and T-Chol levels.
One patient in NYHA class I who did not meet the inclusion criterion was mistakenly enrolled in the study. The rationale for defining patients with NYHA Class II–III as an inclusion criterion was “to select patients with stable symptoms of HF.” The patient was not excluded from analysis because this patient was considered unlikely to influence evaluation of the change in SBM, defined as the primary endpoint of the study.
In a previous Japanese long-term study, drugs for constipation were prohibited except for rescue medications 2 weeks before administration of elobixibat until the end of the study. The change in SBM increased significantly from baseline to Week 12, at 3.53/week.15 However, drugs that patients had been taking for constipation at enrolment were continued in the present study: ≥1 of several drugs for constipation (magnesium oxide, stimulant laxatives, and lubiprostone) were used by 61% of the patients, and 89% of the patients concomitantly received these drugs for constipation during the study period. Due to the effects of these concomitant medications, the number of patients meeting the definition of FAS (2) was as few as 3. Although there was no statistically significant difference in SBM from baseline to Week 12 in FAS (2), the change from baseline to Week 12 was equivalent to that in the previous Japanese long-term study;15 the current small sample size might have resulted in the absence of a significant difference.
Patients with chronic constipation experience not just decreased bowel movement frequency but also various symptoms, including straining and the sensation of incomplete bowel evacuation. We included patients with chronic constipation meeting the Rome IV criteria16 and did not specify a limitation for SBM at enrolment. Consequently, the mean SBM at baseline was 7.0/week and normal in FAS (1), and the SBM frequency was 7.6–8.2/week after elobixibat treatment, suggesting good control. Elobixibat increased the CSBM by 1.3 to 1.9/week, which implied that the sensation of incomplete bowel evacuation was improved. Furthermore, elobixibat significantly improved the degree of straining during defecation. Based on these findings, we deduce that elobixibat improved various symptoms of constipation.
Straining during defecation can elevate BP and increase the risk of cardiovascular events1 by increasing the afterload, leading to pulmonary edema. Elobixibat significantly reduced the degree of straining during defecation from Week 4 to Week 12. Furthermore, although not significant, the difference in BP before and after defecation tended to decrease by approximately 10 mmHg for all variables (systolic blood pressure, diastolic blood pressure, and mean blood pressure) at Week 12 from baseline. This was assumed to be as a result of decreased straining during defecations following administration of elobixibat, which prevented changes in BP between before and after defecation.
Elobixibat inhibits bile acid reabsorption and enhances bile acid synthesis by the liver, resulting in reduced serum LDL-C levels.12,15,17 The results of epidemiological studies have shown that the relative risk of coronary artery disease (CAD) rises with increases in LDL-C and TChol.18–20 In addition, the Lipid Research Clinics Coronary Primary Prevention Trial (LRC-CPPT) has reported that colestyramine treatment reduced LDL-C by 12.6% and the incidence of CAD by 19%.21 In our study, the finding that elobixibat decreased LDL-C by 14.8% suggests that this drug may also contribute to improvement of lipid abnormalities and might reduce the development of CAD compared with other laxatives. However, because there was no restriction on the use of concomitant medications such as statins in this study, an effect of these concomitant medications cannot be excluded.
We also investigated the effects of elobixibat on cardiorenal-related biomarkers. BNP levels were significantly increased at the last evaluation, although there was no significant change in BNP at weeks 4, 8, or 12. Furthermore, the ALD or PRA level was not significantly changed, despite numerically decreasing. Based on these findings, the significant increase in BNP levels at the last evaluation was not found to be a clinically meaningful change; thus, we assume that elobixibat does not adversely affect ALD, PRA, or BNP. It is also unlikely to adversely affect renal function because there was no significant change in eGFR.
Based on clinical studies of elobixibat hydrate tablets, the adverse events observed in this study and their incidence were predictable; elobixibat could be safely administered to treat chronic constipation in HF patients and we anticipate that our study findings will expand the treatment options for HF patients with chronic constipation.
Study Limitations
First, because the sample size was small, statistical power was not sufficient. Second, the study had an open-label, single-arm design and did not compare efficacy or safety with placebo or other laxatives. Therefore, a placebo effect cannot be discounted, and efficacy or safety cannot be compared with other laxatives. However, we believe the placebo effect was not significant because the subjects were patients with chronic constipation who had continued constipation symptoms for >6 months, and 61% were under treatment with other laxatives. Finally, this study was conducted at a single center, and recruitment bias cannot be excluded.
Acknowledgments
We thank the subinvestigators and staff at the Department of Cardiovascular Medicine, Kumamoto University.
Funding
This study was funded by EA Pharma Co., Ltd. and Mochida Pharmaceutical Co., Ltd.
Disclosures
K.T. reports remuneration for lectures from Abbott Medical Co., Ltd., Amgen K.K., AstraZeneca K.K., Bayer Yakuhin Ltd., Daiichi Sankyo Co., Ltd., Medtronic Japan Co., Ltd., Kowa Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Novartis Pharma K.K., Otsuka Pharmaceutical Co., Ltd., Pfizer Japan Inc., and Janssen Pharmaceutical K.K.; trust research/joint research funds from CSL Behring K.K., JIMRO Co., Ltd., Alexion Pharmaceuticals Inc., AnGes Inc., PPD-Shin Nippon Biomedical Laboratories K.K., Sugi Bee Garden (International) Co., Ltd., Daiichi Sankyo Co., Ltd., Bayer Yakuhin Ltd., Pfizer Japan Inc., Bristol-Myers K.K., Mochida Pharmaceutical Co., Ltd., and EA Pharma Co., Ltd.; scholarship funds from AMI Co., Ltd., Abbott Medical Co., Ltd., Boehringer Ingelheim Japan, Daiichi Sankyo Co., Ltd., ITI Co., Ltd., Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., and Takeda Pharmaceutical Co., Ltd.; and affiliations with endowed departments from Abbott Japan Co., Ltd., Boston Scientific Japan K.K., Fides-one Inc., GM Medical Co., Ltd., ITI Co., Ltd., Kaneka Medix Co., Ltd., Nipro Corporation, Terumo Co., Ltd., Abbott Medical Co., Ltd., Fukuda Denshi Co., Ltd., Japan Lifeline Co., Ltd., and Medtronic Japan Co., Ltd. K.T. is a member of Circulation Reports’ Editorial Team. Y.M. reports affiliation with a donated course funded by Nishinihon Hospital. The other authors have no conflicts of interest to disclose. All authors have approved the final version of the manuscript.
IRB Information
The study was approved by the Kumamoto University Certified Clinical Research Review Board (certification No.: CRB7200002).
Data Availability
For any types of analysis, the deidentified participant data will be shared on reasonable request to the corresponding author. The research protocol and statistical analysis plan will not be shared. The requested data will become available immediately following publication, no end date. The data will be shared as SAS format files via Email, or URL link for downloading.
Supplementary Files
Please find supplementary file(s);
https://doi.org/10.1253/circrep.CR-23-0099
References
- 1.
Ishiyama Y, Hoshide S, Mizuno H, Kario K. Constipation-induced pressor effects as triggers for cardiovascular events. J Clin Hypertens (Greenwich) 2019; 21: 421–425.
- 2.
Bekelman DB, Havranek EP, Becker DM, Kutner JS, Peterson PN, Wittstein IS, et al. Symptoms, depression, and quality of life in patients with heart failure. J Card Fail 2007; 13: 643–648.
- 3.
Blinderman CD, Homel P, Billings JA, Portenoy RK, Tennstedt SL. Symptom distress and quality of life in patients with advanced congestive heart failure. J Pain Symptom Manage 2008; 35: 594–603.
- 4.
Nordgren L, Sörensen S. Symptoms experienced in the last six months of life in patients with end-stage heart failure. Eur J Cardiovasc Nurs 2003; 2: 213–217.
- 5.
Imai M, Hirai M, Kuwahara Y, Iwase S, Nishimura N, Shimizu Y, et al. Influence of intrathoracic pressure at defecation on cardiovascular system. Jpn J Nurs Art Sci 2011; 10: 111–120.
- 6.
Sikirov BA. Cardio-vascular events at defecation: Are they unavoidable? Med Hypotheses 1990; 32: 231–233.
- 7.
Sumida K, Molnar MZ, Potukuchi PK, Thomas F, Lu JL, Yamagata K, et al. Constipation and risk of death and cardiovascular events. Atherosclerosis 2019; 281: 114–120.
- 8.
Salmoirago-Blotcher E, Crawford S, Jackson E, Ockene J, Ockene I. Constipation and risk of cardiovascular disease among postmenopausal women. Am J Med 2011; 124: 714–723.
- 9.
Honkura K, Tomata Y, Sugiyama K, Kaiho Y, Watanabe T, Zhang S, et al. Defecation frequency and cardiovascular disease mortality in Japan: The Ohsaki cohort study. Atherosclerosis 2016; 246: 251–256.
- 10.
Lembo A, Camilleri M. Chronic constipation. N Engl J Med 2003; 349: 1360–1368.
- 11.
Müller-Lissner SA, Kamm MA, Scarpignato C, Wald A. Myths and misconceptions about chronic constipation. Am J Gastroenterol 2005; 100: 232–242.
- 12.
Acosta A, Camilleri M. Elobixibat and its potential role in chronic idiopathic constipation. Therap Adv Gastroenterol 2014; 7: 167–175.
- 13.
Wingate DL, Krag E, Mekhjian HS, Phillips SF. Relationships between ion and water movement in the human jejunum, ileum and colon during perfusion with bile acids. Clin Sci Mol Med 1973; 45: 593–606.
- 14.
Bampton PA, Dinning PG, Kennedy ML, Lubowski DZ, Cook IJ. The proximal colonic motor response to rectal mechanical and chemical stimulation. Am J Physiol Gastrointest Liver Physiol 2002; 282: G443–G449.
- 15.
Nakajima A, Seki M, Taniguchi S, Ohta A, Gillberg PG, Mattsson JP, at al. Safety and efficacy of elobixibat for chronic constipation: Results from a randomised, double-blind, placebo-controlled, phase 3 trial and an open-label, single-arm, phase 3 trial. Lancet Gastroenterol Hepatol 2018; 3: 537–547.
- 16.
Drossman DA, Hasler WL. Rome IV-functional GI disorders: Disorders of gut–brain interaction. Gastroenterology 2016; 150: 1257–1261.
- 17.
Nakajima A, Seki M, Taniguchi S. Determining an optimal clinical dose of elobixibat, a novel inhibitor of the ileal bile acid transporter, in Japanese patients with chronic constipation: A phase II, multicenter, double-blind, placebo-controlled randomized clinical trial. J Gastroenterol 2018; 53: 525–534.
- 18.
Okamura T, Kokubo Y, Watanabe M, Higashiyama A, Miyamoto Y, Yoshimasa Y, et al. Low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol and the incidence of cardiovascular disease in an urban Japanese cohort study: The Suita study. Atherosclerosis 2009; 203: 587–592.
- 19.
Okamura T, Tanaka H, Miyamatsu N, Hayakawa T, Kadowaki T, Kita Y, et al. The relationship between serum total cholesterol and all-cause or cause-specific mortality in a 17.3-year study of a Japanese cohort. Atherosclerosis 2007; 190: 216–223.
- 20.
Sugiyama D, Okamura T, Watanabe M, Higashiyama A, Okuda N, Nakamura Y, et al. Risk of hypercholesterolemia for cardiovascular disease and the population attributable fraction in a 24-year Japanese cohort study. J Atheroscler Thromb 2015; 22: 95–107.
- 21.
The Lipid Research Clinics Coronary Primary Prevention Trial results. II: The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA 1984; 251: 365–374.




