Abstract
Background: Alcohol septal ablation (ASA) and septal myectomy (SM) are 2 options for septal reduction therapy (SRT) to treat medication-resistant symptomatic obstructive hypertrophic cardiomyopathy (HCM). Because differences in mortality rates after these different SRT methods have not been extensively investigated in real-world settings, in this study compared the 1-year mortality rates after ASA and SM using population-based database.
Methods and Results: Utilizing New York Statewide Planning and Research Cooperative System (SPARCS) data from 2005 to 2016, we performed a comparative effectiveness study of ASA vs. SM in patients with HCM. The outcome was all-cause death up to 360 days after SRT. We constructed a multivariable logistic regression model and performed sensitivity analysis with propensity score (PS)-matching and inverse probability of treatment weighting (IPTW) methods. We identified 755 patients with HCM who underwent SRT: 348 with ASA and 407 with SM. The multivariable analysis showed that all-cause deaths were significantly fewer in the ASA group at 360 days after SRT (adjusted odds ratio=0.34; 95% confidence interval [CI] 0.13-0.84; P=0.02). The PS-matching and IPTW methods also supported a lower mortality rate in the ASA group at 360 days post-SRT.
Conclusions: In this population-based study of patients with HCM who underwent SRT in a real-world setting, the 1-year all-cause mortality rate was significantly lower in patients who underwent ASA compared with SM.
Hypertrophic cardiomyopathy (HCM) is one of the most common inherited cardiac diseases and has a prevalence of 1 in 200–500 in the USA.1,2 Left ventricular outflow tract (LVOT) obstruction is present in 70–75% of patients with HCM and can cause a variety of cardiovascular symptoms such as syncope/presyncope, chest pain, and shortness of breath.3,4 Septal reduction therapy (SRT) reduces the LVOT obstruction by decreasing the thickness of the interventricular septum.2 Alcohol septal ablation (ASA) and septal myectomy (SM) are the 2 major options of SRT for patients with medication-resistant symptomatic obstructive HCM.
Prior studies from high-volume/tertiary care (HVTC) centers suggest that SM has a very low short-term mortality rate (≤1%) and a long-term mortality rate that is not significantly different from or lower than the post-ASA mortality rate.5–9 For instance, a study from the Mayo Clinic, one of the top HVTC centers in the USA, reported a 30-day mortality rate after SM of 0.3%.8 Accordingly, the 2020 AHA/ACC HCM Guideline recommends SM as the first-line choice and ASA as the second-line option to be conducted only if SM is contraindicated or the surgical risk is high.10 By contrast, population-based studies including non-HVTC centers have consistently reported much higher mortality rates with SM.11,12 For example, a population-based study using the US Nationwide Inpatient Sample reported an in-hospital mortality rate of 5.2%.11 Another population-based study of an elderly HCM population using the Medicare database reported an in-hospital mortality rate of 4.5% and a 30-day mortality rate of 5.1%.13 These findings suggest more deaths after SM performed at non-HVTC centers than at HVTC centers.
On the other hand, mortality rates after ASA appear similar between HVTC and non-HVTC centers. In both the study from the Mayo Clinic and the one using the US Nationwide Inpatient Sample, the short-term mortality rate was 0.7% after ASA.8,11 The Medicare study showed a post-ASA in-hospital mortality rate of 1.5%.13 Thus, the risk-benefit balance between ASA vs. SM may be different when non-HVTC centers are included in the analysis. Indeed, 3 prior studies including all hospitals in the USA reported a significantly higher mortality rate for SM compared with ASA up to 3 months after SRT.11–13 However, those 3 studies focused on short-term mortality rates11,12 or only included patients aged >65 years.13 Therefore, we designed the present population-based comparative effectiveness study to investigate the all-cause 1-year mortality rates for ASA vs. SM in patients with HCM inclusive of all ages.
Methods
We used population-based datasets from the New York Statewide Planning and Research Cooperative System (SPARCS) databases for 2005–2016.14 SPARCS is a comprehensive all-payer data reporting system that includes patient-level detail on clinical characteristics, diagnoses and treatments, services, and charges for each hospital inpatient stay and outpatient (ambulatory surgery, emergency department [ED], and outpatient services) visit in New York State.14 Details of the databases have been published previously.15–19 The Institutional Review Boards of Columbia University Irving Medical Center approved this study and the investigation conformed with the principles outlined in the Declaration of Helsinki .20 All subjects gave written informed consent to participate in the study.
We took the following steps to identify all patients with HCM who underwent either ASA or SM. First, we used the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes 425.1x, and 10th Revision, Clinical Modification (ICD-10-CM) diagnosis codes I42.1 and I42 to identify patients with HCM. Second, among these patients, we identified those who underwent either ASA or SM, using the ICD-9-CM procedure code 37.34 and the ICD-10-CM codes 025M3ZZ and 025L3ZZ for ASA and the ICD-9-CM code 37.33 and ICD-10-CM codes 02BL0ZZ and 025L0ZZ for SM.11,21 We included patients who underwent either ASA or SM from January 1, 2007 to December 31, 2014 to allow for a 2-year follow-up before and after SRT. Lastly, among these patients with HCM who underwent either ASA or SM, we further identified all-cause deaths at 7, 15, 30, 180, and 360 days from SRT. We excluded patients with HCM who underwent multiple SRTs during the study period.19,22–27
We retrieved data from SPARCS for patient demographics (age, sex, and race/ethnicity), source of payment (Medicare, Medicaid, private insurance, self-pay, or other), ICD-9-CM and ICD-10-CM diagnoses, procedures, year of SRT, comorbidities defined by Elixhauser comorbidity measures, and the number of ED visits or unplanned hospitalizations for any reason within the 2 years before SRT.19,22–26 We used the baseline characteristics information recorded on admission for SRT. The primary outcome measure was all-cause death at 7, 15, 30, 180, and 360 days from SRT.
Statistical Analysis
For comparisons of the baseline characteristics between patients with ASA and those with SM, the Mann-Whitney-Wilcoxon or χ2
test was used as appropriate. The number of patients and risk of the outcome event were determined at 7, 15, 30, 180, and 360 days from SRT. Unadjusted and adjusted odds ratios (ORs) were computed by fitting logistic regression models, with SM group as the reference, for each time period. Multivariable models were adjusted for patient demographics (age, sex, and race/ethnicity), source of payment, comorbidities defined by Elixhauser comorbidity measures on admission for SRT, and the number of ED visits or unplanned hospitalizations for any reason within the 2 years before SRT.
Several sensitivity analyses were performed to determine the robustness of our inferences. First, PS-matched analysis was performed to address possible confounding by indication.28,29 PS was computed with the use of a logistic regression model to estimate the propensity that a patient would undergo ASA. The variables included in the logistic regression model for the PS matching were the same variables included in the multivariable models. Patients who underwent ASA were matched to patients who received SM according to PS at a 1 : 1 ratio. The matching was performed without replacement, using calipers (width=0.1) of the standard deviation of the logit of the PS. Second, the inverse probability of treatment weighting (IPTW) method was used to determine the treatment effects of ASA and SM without removal of outliers using the caliper widths.
Results
A total of 755 patients with HCM who underwent SRT (348 (46%) with ASA and 407 (54%) with SM) were included in the current study. The baseline characteristics before PS matching are presented in Table 1. At baseline, patients who underwent ASA had a significantly lower prevalence of valvular disease and fluid and electrolyte disorders, and higher prevalence of arrhythmia compared with those who underwent SM. The PS-matching method yielded 143 PS-matched pairs. After PS matching, the baseline characteristics of the 2 groups were well balanced as indicated by a standardized mean difference <0.2 in all covariates (Table 2).
Table 1.
Baseline Characteristics of Patients With HCM Undergoing ASA or SM
| Characteristics* |
ASA
(n=348) |
SM
(n=407) |
P value |
| Age (years) |
61±14 |
62±15 |
0.72 |
| Male |
175 (50) |
167 (41) |
0.01 |
| Race/ethnicity |
|
|
0.57 |
| Non-Hispanic white |
222 (63.8) |
248 (60.9) |
|
| Non-Hispanic black |
19 (5.5) |
35 (8.6) |
|
| Hispanic |
19 (5.5) |
20 (4.9) |
|
| Asian |
6 (1.7) |
7 (1.7) |
|
| Other |
82 (23.6) |
97 (23.8) |
|
| Primary insurance |
|
|
0.07 |
| Medicare |
120 (34.5) |
103 (25.3) |
|
| Medicaid |
31 (8.9) |
35 (8.6) |
|
| Private |
51 (14.7) |
62 (15.2) |
|
| Self-funded |
1 (0.3) |
2 (0.5) |
|
| Other |
145 (41.7) |
205 (50.4) |
|
| Selected comorbidities |
|
|
|
| Chronic heart failure |
155 (44.5) |
225 (55.3) |
0.004 |
| Arrhythmia |
278 (79.9) |
269 (66.1) |
<0.001 |
| Valvular disease |
86 (24.7) |
332 (81.6) |
<0.001 |
| Pulmonary circulation disorder |
36 (10.3) |
70 (17.2) |
0.009 |
| Peripheral vascular disorder |
34 (9.8) |
42 (10.3) |
0.90 |
| Hypertension |
227 (65.2) |
249 (61.2) |
0.25 |
| Chronic pulmonary disease |
45 (12.9) |
61 (15.0) |
0.48 |
| Diabetes mellitus |
55 (15.8) |
70 (17.2) |
0.68 |
| Fluid and electrolyte disorders |
26 (17.5) |
139 (34.2) |
<0.001 |
| Obesity |
45 (12.9) |
61 (15.0) |
0.48 |
*Data are expressed as number (percentage) or mean±standard deviation. ASA, alcohol septal ablation; HCM, hypertrophic cardiomyopathy; SM, septal myectomy.
Table 2.
Baseline Characteristics of Patients With HCM Who Underwent ASA and Propensity Score-Matched Patients Who Underwent SM
| Characteristics* |
ASA
(n=143) |
SM
(n=143) |
P value |
Standardized
mean difference |
| Age (years) |
63±14 |
63±14 |
0.65 |
0.05 |
| Male |
62 (43.4) |
66 (46.2) |
0.72 |
0.06 |
| Race/ethnicity |
|
|
0.82 |
0.15 |
| Non-Hispanic white |
95 (66.4) |
89 (62.2) |
|
|
| Non-Hispanic black |
7 (4.9) |
11 (7.7) |
|
|
| Hispanic |
6 (4.2) |
6 (4.2) |
|
|
| Asian |
2 (1.4) |
1 (0.7) |
|
|
| Other |
33 (23.1) |
36 (25.2) |
|
|
| Primary insurance |
|
|
0.68 |
0.18 |
| Medicare |
46 (32.2) |
44 (30.8) |
|
|
| Medicaid |
13 (9.1) |
10 (7.0) |
|
|
| Private |
19 (13.3) |
25 (17.5) |
|
|
| Self-funded |
0 (0) |
1 (0.7) |
|
|
| Other |
65 (45.5) |
63 (44.1) |
|
|
| Selected comorbidities |
| Chronic heart failure |
71 (49.7) |
70 (49.0) |
>0.99 |
0.01 |
| Arrhythmia |
105 (73.4) |
105 (73.4) |
>0.99 |
<0.001 |
| Valvular disease |
82 (57.3) |
86 (60.1) |
0.72 |
0.06 |
| Pulmonary circulation disorder |
22 (15.4) |
25 (17.5) |
0.75 |
0.06 |
| Peripheral vascular disorder |
20 (14.0) |
14 (9.8) |
0.36 |
0.13 |
| Hypertension |
92 (64.3) |
92 (64.3) |
>0.99 |
<0.001 |
| Chronic pulmonary disease |
46 (32.2) |
48 (33.6) |
0.90 |
0.03 |
| Diabetes mellitus |
29 (20.3) |
27 (18.9) |
0.88 |
0.04 |
| Fluid and electrolyte disorders |
21 (14.7) |
24 (16.8) |
0.75 |
0.06 |
| Obesity |
17 (11.9) |
19 (13.3) |
0.86 |
0.04 |
*Data are expressed as number (percentage) or mean±standard deviation. Abbreviations as in Table 1.
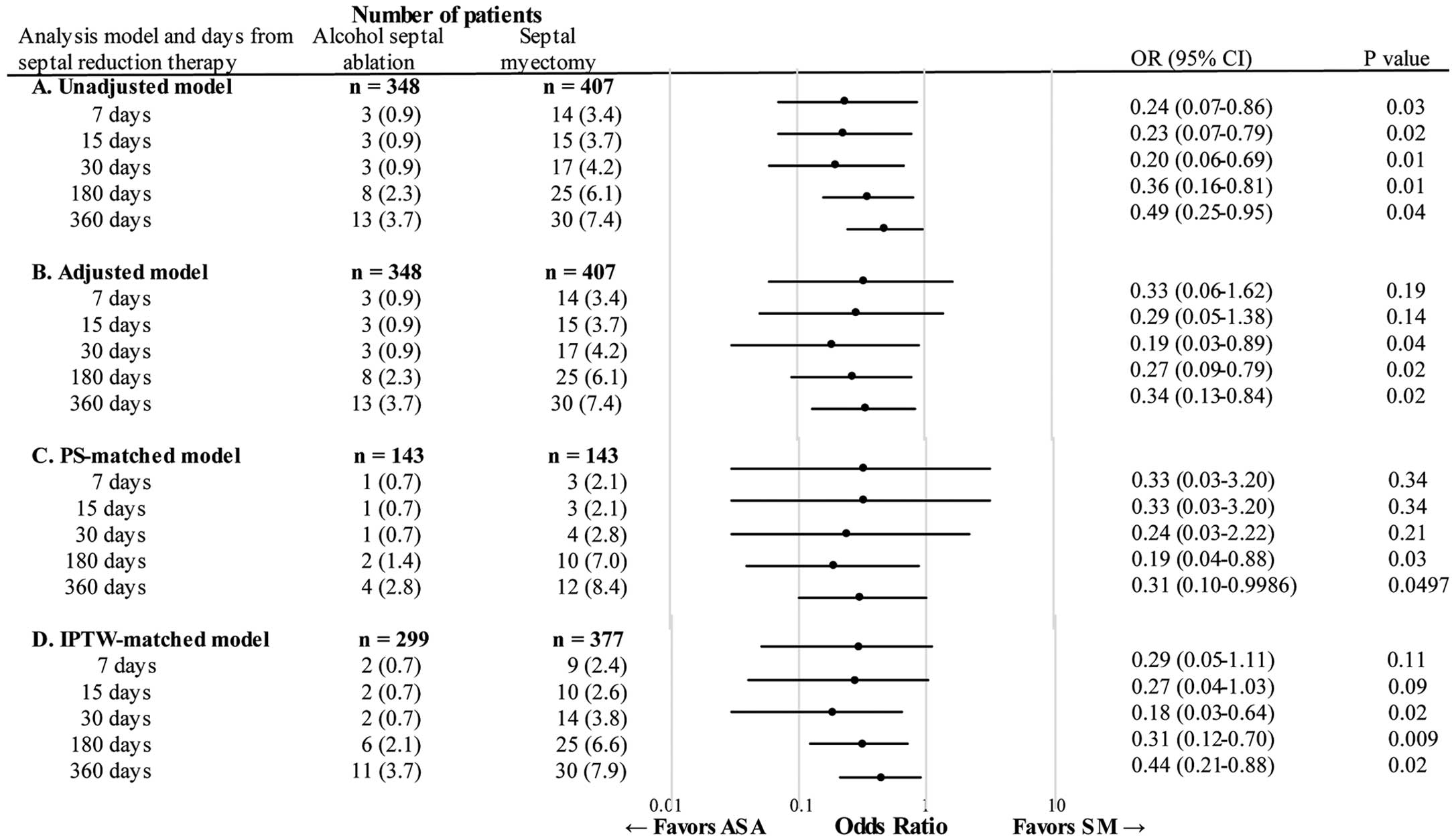
All-cause deaths post-SRT are displayed in Figure. In the unadjusted analysis, deaths in the ASA group were fewer than in the SM group at all post-SRT time periods (e.g., crude mortality rate at 360 days, 3.7% vs. 7.4%; unadjusted OR=0.49; 95% confidence interval [CI] 0.25–0.95; P=0.04; Figure A). In the multivariable analysis, the mortality rate was significantly lower in the ASA group at 30 days (adjusted OR=0.19; 95% CI 0.03–0.89; P=0.04), 180 days (adjusted OR=0.27; 95% CI 0.09–0.79; P=0.02), and 360 days (adjusted OR=0.34; 95% CI 0.13–0.84; P=0.02) after SRT (Figure B). Similarly, with PS matching the mortality rate was lower in the ASA group at both 180 days (adjusted OR=0.19; 95% CI 0.04–0.88; P=0.03) and 360 days (adjusted OR=0.31; 95% CI 0.10–0.9986; P=0.0497) post-SRT (Figure C). The IPTW model showed findings consistent with the multivariable analysis and the PS-matched model (Figure D).
Discussion
Summary of Findings
In this comparative effectiveness study using population-based data of 755 patients with HCM, the all-cause mortality rate was lower among patients who underwent ASA compared with SM at both 180 days and 360 days after SRT. This difference was consistently observed in the multivariable, PS-matched, and IPTW analyses. To our knowledge, this is the first population-based comparative effectiveness study inclusive of all ages that has examined the all-cause 1-year mortality rates after ASA and SM in real-world settings.
Results in Context
To date, studies comparing mortality rates after ASA and SM have been mainly performed at HVTC centers and have reported very low post-SM short-term mortality rates (≤1%) that are either lower than or not significantly different from post-ASA mortality rates.5–9 On the other hand, there is a large discrepancy in terms of mortality rates after SM between HVTC and non-HVTC centers; prior studies that included non-HVTC centers reported much higher mortality rates for SM.11,12 For example, population-based studies using the US Nationwide Inpatient Sample and Medicare databases reported in-hospital mortality rates as high as 4.5–5.2%.11,13 Our study showed a 7-day mortality rate after SM of 3.4%, which is in agreement with prior population-based studies,11,12 and collectively the study findings indicate that the mortality rates after SM at non-HVTC centers may be much higher than at HVTC centers.
Unlike the large difference in mortality rates after SM, those for ASA seem similar between HVTC and non-HVTC centers. For instance, the study using the US National Inpatient Sample documented 0.7% mortality rate after ASA,11 and another study using the National Readmission Database reported a post-ASA in-hospital mortality rate of 0.9%.12 The in-hospital mortality rate was 1.5% in the study using the Medicare database.13 These data are similar to that in a previous study from the Mayo Clinic (0.7%),8 the Multicenter North American Registry (1.0%),9 and the present study (7-day mortality rate after ASA 0.9%). Thus, the risk-benefit balance of SM vs. ASA may be more favorable to ASA at non-HVTC centers when compared with HVTC centers. Indeed, both of the studies using the US Nationwide Inpatient Sample and National Readmission Database showed >4-fold higher mortality rates after SM compared with ASA.11,12 However, those studies assessed only short-term mortality rates up to 30 days. The Medicare study also showed significantly higher mortality rates up to 30 days (hazard ratio 1.96; 95% CI 1.32–2.92; P<0.001) and a non-significant trend towards higher mortality rates with SM up to 2 years after SRT (P=0.2); however, that Medicare study only included patients >65 years of age.13 In this context, the present population-based study examining 1-year mortality rates inclusive of all ages adds to the body of knowledge by demonstrating that ASA is associated with fewer deaths than SM when non-HVTC centers are included.
Comparison With Previous Meta-Analyses
There have been a few meta-analyses investigating differences in all-cause mortality rates for ASA vs. SM. For example, in 2016 a meta-analysis that included 10 studies with a total of 805 patients who underwent ASA and 1,019 with SM, there was no difference between ASA and SM in terms of 30-day all-cause mortality rates.30 In a meta-analysis conducted in 2019, data of 22 ASA cohorts (n=4,213) and 23 SM cohorts (n=4,240) were analyzed and the 30-day mortality rate was lower for ASA than for SM (2.0% vs. 1.2%, P=0.009).31 These findings are inconclusive likely because the risk difference depends on the proportion of studies from HVTC centers vs. non-HVTC centers included in the analysis. Thus, even with meta-analyses, it remained controversial as to whether ASA is associated with lower 30-day all-cause mortality rates than SM in the real world, and if so, whether the difference remains after 30 days. The current study provides additional insights to the lower mortality rates with ASA compared with SM up to 1 year when all institutions are included regardless of volume.
Advantages of the Study Design and Methods
A large, high-quality randomized controlled trial (RCT) is the ideal for comparing the efficacy and safety of ASA and SM, but can be difficult to conduct because of resource and time constraints. Furthermore, it has been reported that subjects participating in RCTs may be highly selected or behave differently than the general population in real-world settings.29 For example, a previous study showed that enrollment of 1,200 suitable candidates (600 in each treatment arm) would require screening as many as 34,000 consecutive patients with obstructive HCM,32 a number that far exceeds the total of all cohorts currently being followed at major centers in North America and Europe. By contrast, the SPARCS databases capture all ED visits and hospitalizations in New York State, which strengthens the external validity of the present study because it used a large general population-based dataset from real-world settings.
In addition to rigorous adjustment for potential confounders, the PS-matched analysis and IPTW method improve the internal validity because they reduce between-group differences at baseline and allow for a more accurate determination of the effectiveness of different interventions.28,29,33 Indeed, the PS-matched groups in our dataset had similar characteristics at baseline, ensuring less biased examination of the treatment effect.
Study Limitations
Our study has several potential limitations. First, misclassification may have occurred when using administrative data. However, the SPARCS databases have been widely used in prior studies and the quality has been extensively tested.15–19 To ensure data accuracy and avoid misclassification, the SPARCS databases were developed by the New York State Department of Health in conjunction with the Vital Statistics Birth Registry, the Vital Statistics Death Registry, and the Bureau of Biometrics, in cooperation with trend analyses conducted by biometrics, and they are subject to periodic quality checks.14 In addition, the inclusion criteria of the present study required both HCM diagnosis codes and SRT procedure codes to increase specificity. Second, because this was an observational cohort study, it does not prove causality and may not provide evidence of the same strength as an RCT. Third, the SPARCS databases did not capture SRTs that were performed outside New York State. Finally, the SPARCS databases did not record some important clinical variables such as symptoms, past medical history, medication, cause of death, blood tests such as B-type natriuretic peptide levels, renal function, and anemia, cardiac imaging data (e.g., LV maximal wall thickness, type of HCM, pressure gradient, and LVEF) and improvement in heart failure and LV remodeling. Thus, there may be some imbalances in these parameters between the 2 groups even with PS matching and IPTW.
Conclusions
In this comparative effectiveness study of 755 patients with HCM using large population-based datasets in real-world settings, all-cause mortality rates were significantly lower at both 180 days and 360 days after SRT in patients who underwent ASA compared with SM. This observation, when compared with studies from HVTC centers reporting higher or non-differential mortality rates for ASA vs. SM suggests that the risk-benefit balance of ASA vs. SM may differ between HVTC centers and others.
Sources of Funding
Y.J.S. is supported by NIH R01 HL157216 and R01 HL168382, the American Heart Association National Clinical and Population Research Awards, the American Heart Association Career Development Award, Korea Institute of Oriental Medicine (K18190 and W22005), Feldstein Medical Foundation, Columbia University Irving Medical Center Irving Institute for Clinical & Translational Research Precision Medicine Pilot Award, and Columbia University Irving Medical Center Lewis Katz Cardiovascular Research Prize. M.P.R. is supported by NIH UL1 TR001873 and K24 HL107643. M.S.M. is supported by NIH K24 AG036778. The funding organizations did not have any role in the study design, collection, analysis, or interpretation of data, writing of the manuscript, or in the decision to submit the article for publication. The researchers were independent from the funding organizations.
Declaration of Interest
None declared.
IRB Information
Name of the ethics committee: Columbia University Irving Medical Center Institutional Review Board (Reference no. AAAR5756)
Data Availability
The data that support the findings of this study are available from the New York Statewide Planning and Research Cooperative System (https://www.health.ny.gov), but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of the New York Statewide Planning and Research Cooperative System.
References
- 1.
Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet 2013; 381: 242–255, doi:10.1016/s0140-6736(12)60397-3.
- 2.
Maron BJ. Clinical course and management of hypertrophic cardiomyopathy. N Engl J Med 2018; 379: 655–668, doi:10.1056/NEJMra1710575.
- 3.
Geske JB, Ong KC, Siontis KC, Hebl VB, Ackerman MJ, Hodge DO, et al. Women with hypertrophic cardiomyopathy have worse survival. Eur Heart J 2017; 38: 3434–3440, doi:10.1093/eurheartj/ehx527.
- 4.
Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT, et al. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation 2006; 114: 2232–2239, doi:10.1161/CIRCULATIONAHA.106.644682.
- 5.
Kimmelstiel C, Zisa DC, Kuttab JS, Wells S, Udelson JE, Wessler BS, et al. Guideline-based referral for septal reduction therapy in obstructive hypertrophic cardiomyopathy is associated with excellent clinical outcomes. Circ Cardiovasc Interv 2019; 12: e007673, doi:10.1161/CIRCINTERVENTIONS.118.007673.
- 6.
Nguyen A, Schaff HV, Hang D, Nishimura RA, Geske JB, Dearani JA, et al. Surgical myectomy versus alcohol septal ablation for obstructive hypertrophic cardiomyopathy: A propensity score-matched cohort. J Thorac Cardiovasc Surg 2019; 157: 306–315.e303, doi:10.1016/j.jtcvs.2018.08.062.
- 7.
Afanasyev AV, Bogachev-Prokophiev AV, Kashtanov MG, Astapov DA, Zalesov AS, Budagaev SA, et al. Myectomy versus alcohol septal ablation in patients with hypertrophic obstructive cardiomyopathy. Interact Cardiovasc Thorac Surg 2020; 31: 158–165, doi:10.1093/icvts/ivaa075.
- 8.
Cui H, Schaff HV, Wang S, Lahr BD, Rowin EJ, Rastegar H, et al. Survival following alcohol septal ablation or septal myectomy for patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2022; 79: 1647–1655, doi:10.1016/j.jacc.2022.02.032.
- 9.
Yandrapalli S, Harikrishnan P, Andries G, Aronow WS, Panza JA, Naidu SS. Differences in short-term outcomes and hospital-based resource utilization between septal reduction strategies for hypertrophic obstructive cardiomyopathy. J Invasive Cardiol 2022; 34: e8–e13.
- 10.
Ommen SR, Mital S, Burke MA, Day SM, Deswal A, Elliott P, et al. 2020 AHA/ACC Guideline for the diagnosis and treatment of patients with hypertrophic cardiomyopathy: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol 2020; 76: e159–e240, doi:10.1016/j.jacc.2020.08.045.
- 11.
Kim LK, Swaminathan RV, Looser P, Minutello RM, Wong SC, Bergman G, et al. Hospital volume outcomes after septal myectomy and alcohol septal ablation for treatment of obstructive hypertrophic cardiomyopathy: US Nationwide Inpatient Database, 2003–2011. JAMA Cardiol 2016; 1: 324–332, doi:10.1001/jamacardio.2016.0252.
- 12.
Lemor A, Villablanca PA, Hosseini Dehkordi SH, Mand R, Hernandez GA, Jain T, et al. Comparison of outcomes of alcohol septal ablation or septal myectomy for hypertrophic cardiomyopathy in patients </=65 years versus >65 years. Am J Cardiol 2020; 127: 128–134, doi:10.1016/j.amjcard.2020.04.018.
- 13.
Mentias A, Smedira NG, Krishnaswamy A, Reed GW, Ospina S, Thamilarasan M, et al. Survival after septal reduction in patients >65 years old with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2023; 81: 105–115, doi:10.1016/j.jacc.2022.10.027.
- 14.
Statewide Planning and Research Cooperative System (SPARCS). Data Governance Policy and Procedure Manual for Data Release. New York State Department of Health Office of Quality and Patient Safety. Version 3.0 (Updated May 2022). https://www.health.ny.gov/statistics/sparcs/training/docs/sparcs_dgc_manual.pdf (accessed November 7, 2023).
- 15.
Han BH, Tuazon E, M YW, Paone D. Multimorbidity and inpatient utilization among older adults with opioid use disorder in New York City. J Gen Intern Med 2022; 37: 1634–1640, doi:10.1007/s11606-021-07130-8.
- 16.
Burstein MD, Myneni AA, Towle-Miller LM, Simmonds I, Gray J, Schwaitzberg SD, et al. Outcomes following robot-assisted versus laparoscopic sleeve gastrectomy: The New York State experience. Surg Endosc 2022; 36: 6878–6885, doi:10.1007/s00464-022-09026-y.
- 17.
Kim KW, Brodeur PG, Mullen MA, Gil JA, Cruz AI Jr. Postoperative pain management following orthopedic spine procedures and consequent acute opioid poisoning: An analysis of New York State from 2009 to 2018. Spine (Phila Pa 1976) 2022; 47: 1270–1278, doi:10.1097/BRS.0000000000004395.
- 18.
He MZ, Do V, Liu S, Kinney PL, Fiore AM, Jin X, et al. Short-term PM2.5 and cardiovascular admissions in NY State: Assessing sensitivity to exposure model choice. Environ Health 2021; 20: 93, doi:10.1186/s12940-021-00782-3.
- 19.
Morita SX, Zhao Y, Hasegawa K, Fifer MA, Maurer MS, Reilly MP, et al. Effects of septal reduction therapy on acute cardiovascular events and all-cause mortality in patients with hypertrophic cardiomyopathy. Int Heart J 2021; 62: 1035–1041, doi:10.1536/ihj.21-095.
- 20.
Rickham PP. Human experimentation: Code of Ethics of the World Medical Association: Declaration of Helsinki. Br Med J 1964; 2: 177, doi:10.1136/bmj.2.5402.177.
- 21.
Center for Medicare, Medicaid Service. CMD ICD-10. www.cms.gov/medicare/coding/icd10 (accessed November 27, 2023).
- 22.
Shimada YJ, Tsugawa Y, Camargo CA Jr, Brown DFM, Hasegawa K. Effect of bariatric surgery on emergency department visits and hospitalizations for atrial fibrillation. Am J Cardiol 2017; 120: 947–952, doi:10.1016/j.amjcard.2017.06.026.
- 23.
Shimada YJ, Tsugawa Y, Iso H, Brown DF, Hasegawa K. Association between bariatric surgery and rate of hospitalisations for stable angina pectoris in obese adults. Heart 2017; 103: 1009–1014, doi:10.1136/heartjnl-2016-310757.
- 24.
Shimada YJ, Tsugawa Y, Brown DFM, Hasegawa K. Bariatric surgery and emergency department visits and hospitalizations for heart failure exacerbation: Population-based, self-controlled series. J Am Coll Cardiol 2016; 67: 895–903, doi:10.1016/j.jacc.2015.12.016.
- 25.
Hasegawa K, Tsugawa Y, Chang Y, Camargo CA Jr. Risk of an asthma exacerbation after bariatric surgery in adults. J Allergy Clin Immunol 2015; 136: 288–294.e288, doi:10.1016/j.jaci.2014.12.1931.
- 26.
Nguyen GC, Patel AM. Racial disparities in mortality in patients undergoing bariatric surgery in the U.S.A. Obes Surg 2013; 23: 1508–1514, doi:10.1007/s11695-013-0957-4.
- 27.
Shimada YJ, Gibo K, Tsugawa Y, Goto T, Yu EW, Iso H, et al. Bariatric surgery is associated with lower risk of acute care use for cardiovascular disease in obese adults. Cardiovasc Res 2019; 115: 800–806, doi:10.1093/cvr/cvy266.
- 28.
Benedetto U, Head SJ, Angelini GD, Blackstone EH. Statistical primer: Propensity score matching and its alternatives. Eur J Cardiothorac Surg 2018; 53: 1112–1117, doi:10.1093/ejcts/ezy167.
- 29.
Elze MC, Gregson J, Baber U, Williamson E, Sartori S, Mehran R, et al. Comparison of propensity score methods and covariate adjustment: Evaluation in 4 cardiovascular studies. J Am Coll Cardiol 2017; 69: 345–357, doi:10.1016/j.jacc.2016.10.060.
- 30.
Singh K, Qutub M, Carson K, Hibbert B, Glover C. A meta analysis of current status of alcohol septal ablation and surgical myectomy for obstructive hypertrophic cardiomyopathy. Catheter Cardiovasc Interv 2016; 88: 107–115, doi:10.1002/ccd.26293.
- 31.
Osman M, Kheiri B, Osman K, Barbarawi M, Alhamoud H, Alqahtani F, et al. Alcohol septal ablation vs. myectomy for symptomatic hypertrophic obstructive cardiomyopathy: Systematic review and meta-analysis. Clin Cardiol 2019; 42: 190–197, doi:10.1002/clc.23113.
- 32.
Olivotto I, Ommen SR, Maron MS, Cecchi F, Maron BJ. Surgical myectomy versus alcohol septal ablation for obstructive hypertrophic cardiomyopathy: Will there ever be a randomized trial? J Am Coll Cardiol 2007; 50: 831–834, doi:10.1016/j.jacc.2007.05.018.
- 33.
D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998; 17: 2265–2281, doi:10.1002/(sici)1097-
0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b.