2025 年 7 巻 3 号 p. 212-215
2025 年 7 巻 3 号 p. 212-215
Background: Despite the development of effective pulmonary vasodilators, the prognosis for patients with pulmonary hypertension (PH) remains poor, particularly in medication-refractory patients. Catheter-based pulmonary artery denervation (PADN) is an emerging therapeutic strategy targeting the sympathetic nervous system in various types of PH. However, data on its safety and efficacy in refractory patients with PH who truly require non-pharmacotherapy are lacking. Here, we describe a phase II, investigator-initiated, open-label, single-arm trial (Japan Registry of Clinical Trials jRCTs052200017) to evaluate the efficacy and safety of PADN over a 2-year observation period.
Methods and Results: Twenty participants will be enrolled and will undergo PADN. The primary endpoint is the time from PADN to the first occurrence of the composite events of death, lung transplantation, and worsening of PH. The safety endpoints are the occurrence of adverse events related to PADN and bradycardia requiring treatment. The exploratory endpoints include right ventricular function evaluated using cardiac magnetic resonance imaging and Short Form-36 score.
Conclusions: The findings of this study will lead to the adoption of PADN for patients with limited treatment options.
Pulmonary hypertension (PH), a progressive disease of the pulmonary vasculature, results in increased pulmonary vascular resistance, elevated pulmonary arterial pressure (PAP), and right ventricular (RV) failure.1,2 Despite the development of effective pulmonary vasodilators, the prognosis for patients with PH remains poor, especially for patients with severe PH. According to the COMPERA registry, 29% (459/1,588) of patients died within 5 years after the diagnosis.3 Additionally, among the high-risk group classified by COMPERA 2.0, a 4-stratum risk-assessment model based on refined cut-off levels for functional class, 6-min walk distance (6MWD), and B-type natriuretic peptide (BNP)/N-terminal proBNP, the survival rates at 5 years were only 28.2% using the Kaplan-Meier model.4 Therefore, alternative treatments for reducing PAP in medication-refractory patients are urgently required.
Sympathetic overactivation has been identified as a potential therapeutic target in PH. Catheter-based pulmonary artery denervation (PADN) is an emerging therapeutic strategy that targets the sympathetic nervous system in various types of PH, including idiopathic pulmonary arterial hypertension,5–7 chronic thromboembolic PH,8 and combined PH associated with left heart failure.9 However, safety and efficacy data for medication-refractory patients with PH who require non-pharmacotherapy are lacking. Additionally, no data are available regarding the long-term efficacy of PADN. Therefore, in this study, we aim to conduct an open-label, single-arm trial in patients with medication-refractory PH over a 2-year observation period.
The aim of the PADN for medication refractory PH (PARPH) study is to explore the safety and efficacy of PADN in patients with medication refractory PH. This is a prospective, open-label, single-arm clinical trial being conducted at the National Cerebral and Cardiovascular Center (NCVC) from June 1, 2020, to March 31, 2026. The patient flowchart of the study is shown in Figure. The enrollment period was from June 1, 2020, to March 31, 2023. Hemodynamic follow ups will be performed at 3, 12, and 24 months after PADN (Table 1).
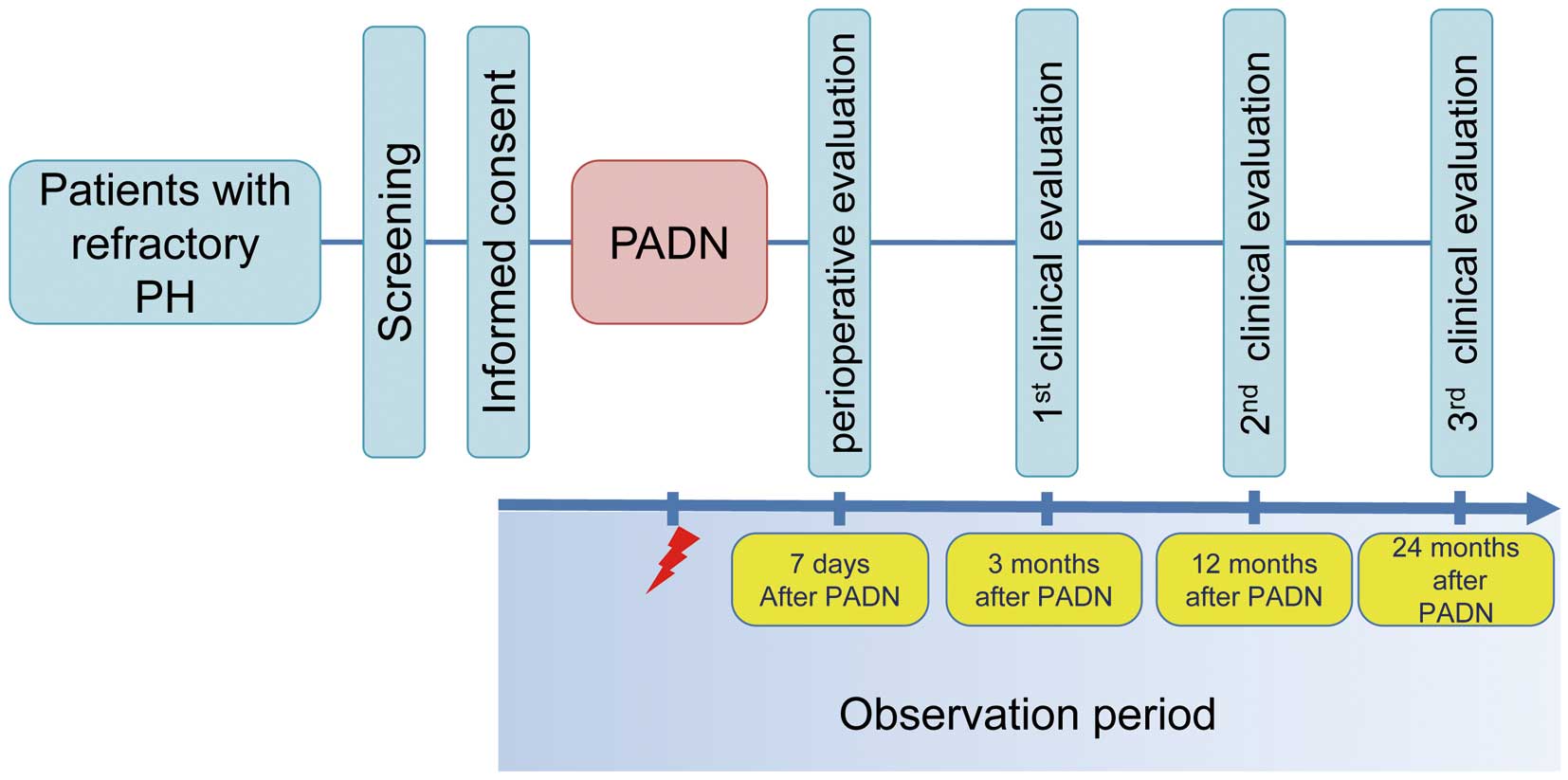
Patient flowchart of the study period. PADN, pulmonary artery denervation; PH, pulmonary hypertension.
Study Schedule and Evaluation of Endpoints
| Pre-PADN | 7 days after PADN |
3 months after PADN |
12 months after PADN |
24 months after PADN |
|
|---|---|---|---|---|---|
| Participants characteristics | ● | ||||
| Disease status (WHO-FC, PH drugs, vital signs) |
● | ● | ● | ● | ● |
| Laboratory test | ● | ● | ● | ||
| Chest X-ray | ● | ● | ● | ||
| 6-min walk distance | ● | ● | ● | ||
| Cardiac MRI | ● | ● | ● | ||
| Right heart catheterization | ● | ● | ● | ||
| SF-36 | ● | ● | ● | ||
| Events |  |
||||
| Complications |  |
||||
MRI, magnetic resonance imaging; PADN, pulmonary artery denervation; PH, pulmonary hypertension; SF-36, Short Form-36; WHO-FC, World Health Organization functional class.
Eligibility Criteria
Among the patients with PH,10,11 refractory patients who fulfill the following criteria are eligible: (1) mean PAP ≥25 mmHg; (2) World Health Organization functional class ≥III under the medications fully administered, including endothelin receptor antagonist, prostacyclin analog and phosphodiesterase type 5 inhibitor, according to Japanese guidelines; and (3) age ≥16 and <80 years.
The exclusion criteria are as follows: (1) systolic blood pressure <60 mmHg; (2) estimated glomerular filtration rate <30 mmHg; (3) allergy to disinfectants or local anesthetics; and (4) difficulty resting for more than 2 h.
InterventionsAll patients will receive PADN treatment using an irrigated radiofrequency ablation catheter (TactiCath; Abbott, Abbott Park, IL, USA) introduced from the right femoral vein. PADN will be performed with blood pressure monitoring of the femoral and pulmonary arteries. To ensure catheter positioning, we will use a 3-dimensional electro-anatomical mapping system (Ensite; Abbott, Abbott Park, IL, USA) to determine the geometry of the pulmonary artery (PA) constructed with the ring catheter. The lesion sets, which include the autonomic plexus around the PA, will be at the left and right at a level defined as 3–5 mm istal to the bifurcation and at the main PA trunk at a level 3–5 mm proximal to the bifurcation. The presence of the phrenic nerve at the ablation site will be confirmed in a timely manner using high-output pacing (20 V; 10 ms pulse width; 5 s). For ablation, radiofrequency energy will be delivered point-by-point at 10–30 W, with a temperature limit of 42℃ and an irrigation rate of 17 mL/min and a duration of 30–60 s per application. Acute success is defined as a decrease in systolic PAP of 10 mmHg.
Outcome MeasuresThe clinical, safety, and exploratory endpoints are summarized in Table 2. The primary endpoint is the time from PADN to the first occurrence of a composite event of death, lung transplantation, and worsening of PH. Worsening of PH is defined as the occurrence of at least one of the following: hospitalization due to right heart failure; a decrease in the 6MWD of at least 15% from baseline; a change from baseline to a higher World Health Organization functional class; or additional treatment for PH. The secondary endpoints are the time to each event of death, lung transplantation, and worsening of PH.
Clinical, Safety, and Exploratory Endpoints
| Clinical endpoints | Safety endpoints | Exploratory endpoints |
|---|---|---|
| • The primary endpoint is the time from PADN to the first composite event (death, lung transplantation, or worsening of PH) • The secondary endpoints are the time from PADN to worsening PH, lung transplantation, and death from any cause |
• Incidence of complications derived from PADN • Incidence of bradyarrhythmia requiring treatment |
• Changes in PAP, SaO2, SvO2, PaO2 due to PADN • Changes in BNP level due to PADN • Changes in biventricular function using CMR following PADN • Changes in QoL score according to SF-36 due to PADN |
BNP, B-type natriuretic peptide; CMR, cardiovascular magnetic resonance; PADN, pulmonary artery denervation; PaO2, partial pressure of oxygen in arterial blood; PAP, pulmonary arterial pressure; PH, pulmonary hypertension; QoL, quality of life; SAO2, arterial oxygen saturation; SF-36, Short Form-36; SvO2, mixed venous oxygen saturation.
The safety endpoints include the occurrence of adverse events related to PADN and bradycardia requiring treatment.
The exploratory endpoints are the changes from baseline to months 3, 12, and 24 after PADN in the following: (1) hemodynamic parameters, including PAP, arterial oxygen saturation, partial oxygen pressure in arterial blood, or mixed venous oxygen saturation; (2) serum BNP levels; (3) right or left ventricular volume evaluated using cardiovascular magnetic resonance (CMR); and (4) quality of life (QoL) using the Short Form-36 (SF-36) score.
Sample Size CalculationAccording to the prognosis record of 47 patients with refractory PH seen at the NCVC, a 12% 2-year event-free rate of death or hospitalization due to right heart failure was determined. This rate was used as the reference value to calculate the number of patients required for this trial. First, we considered that there would be a significant benefit if the 2-year event-free rate was improved to 30% by PADN. At least 17 patients will be required to set the upper limit of the 95% confidence interval (CI) of the hazard ratio with PADN for the composite event to the reference event without PADN being lower than 1. The target sample size was set at 20 patients, with a discontinuation or dropout rate of approximately 15%.
Data Collection and Statistical AnalysisThe investigators will maintain individual records for each patient as source data, including a copy of informed consent, medical records, laboratory data, imaging data, QoL scores, and other records or notes. Clinical data entry, management, and monitoring will be performed using the REDCap electronic data capture application (Vanderbilt University).
Statistical analyses will be conducted according to intention-to-treat principles. Survival curves for the primary and secondary endpoints will be estimated using the Kaplan-Meier method. For the primary endpoint, the hazard ratio to the reference, 12% of the 2-year event-free rate, with its 95% CI, will be calculated. The safety data will be analyzed descriptively. The detailed plan for the analysis will be prespecified in the statistical analysis plan, which will be finalized before the database lock. All statistical analyses will be conducted at the Center for Advancing Clinical and Translational Science National Cerebral and Cardiovascular Centers.
Ethics and Study OversightThis study is registered in the Japan Registry of Clinical Trials (jRCTs052200017). The study is being conducted in accordance with the Declaration of Helsinki, the Clinical Trials Act of Japan, and relevant laws and regulations. This study was approved by the certified review board of Osaka Metropolitan University, Japan (No. OCU0016). All participants will provide written informed consent and can withdraw their consent at any time.
Although the prognosis for patients with PH has improved with the development of pulmonary vasodilators, PH remains challenging to manage, particularly in patients who are refractory to optimal medication therapy and have extremely poor prognoses. The PARPH study, a novel prospective, open-label, single-arm clinical trial, is designed to address this unmet need by exploring the safety and efficacy of PADN in this vulnerable population. Another unique feature of the PARPH study is the 24-month follow-up period. PH is a chronic and progressive disease that requires long-term monitoring to accurately assess the effectiveness of the intervention. Most previous studies on PADN have focused mainly on outcomes up to 6 months, leaving a significant knowledge gap regarding sustained efficacy and safety. By extending the follow up to 24 months, our study aims to determine the long-term potential of PADN in patients with refractory PH.
In addition to clinical outcomes, this study evaluates CMR and QoL assessments to determine the broader impact of PADN. CMR can be used to evaluate RV function, which is a critical determinant of prognosis in PH.12 RV failure is a hallmark of disease progression in PH, and its improvement can improve prognosis.13,14 By including RV function as a key exploratory endpoint, the PARPH study seeks to elucidate the mechanistic benefits of PADN beyond hemodynamic measures. Furthermore, we included the SF-36 questionnaire to explore the patient-centered effects of PADN. SF-36 provides a standardized and comprehensive tool to assess the physical, emotional, and social dimensions of QoL.15,16 This approach aligns with the growing recognition that QoL is a critical metric in chronic disease management.
Study LimitationsThe present study has several limitations. First, this is an open-label single-arm trial. However, the single-arm design was based on the ethical and logistic challenges of recruiting a control group from a high-risk population. Second, the sample size is only 20 patients. However, this was calculated using retrospective survival data, ensuring that the study is adequately powered to detect clinically meaningful improvements. The exploratory nature of this study is a necessary first step toward validating the feasibility of PADN in refractory patients.
The PARPH study offers a unique perspective on the management of medication-refractory PH. By combining long-term follow up, RV function, and QoL assessments, this study will provide valuable insights into the impact of PADN as a potential therapeutic strategy. The findings from this study will build a foundation for future research and will lead to the adoption of PADN for patients with limited treatment options.
The authors thank Harue Takano and Yuko Iwai for their secretarial assistance. We thank Editage (www.editage.jp) for English language editing. The PARPH study is supported by the Intramural Research Fund for the National Cerebral and Cardiovascular Center and JSPS KAKENHI (Grant no. 21K08094 to T.O.).
T.O. is a member of Circulation Reports’ Editorial Team. T.O. received lecture fees from Janssen Pharmaceutical K.K., Bayer Yakuhin, Ltd, Nippon Shinyaku Co., Ltd, GlaxoSmithKline K.K., Pfizer Japan Inc., and Mochida Pharmaceutical Co., Ltd outside of the submitted work. The other authors declare no conflicts of interest.
Study design and conception: R.A., M.F.-D., Y.I., and T.O.; data collection: R.A., S.N., J.U., A.T., T.A., K.K., and T.O.; drafting the manuscript: R.A., and T.O.; statistical analyses: K.A., and H.Y. All authors have read and approved the final version of the manuscript. T.O. is the guarantor and is responsible for the overall content of the study.
The participant data will not be shared. The corresponding author will respond to inquiries regarding analyses.