論文ID: CR-19-0126
論文ID: CR-19-0126
Background: Myocardial viability assessment in revascularization of ischemic heart failure remains controversial. This study evaluated the prognostic utility of cardiac magnetic resonance (CMR) late gadolinium enhancement (LGE) in ischemic heart failure.
Methods and Results: This study retrospectively analyzed subjects with ischemic heart failure and left ventricular ejection fraction (LVEF) ≤35%, who underwent CMR at a single center in 2004–2014 before undergoing coronary artery bypass grafting (CABG) or optimal medical therapy (OMT). Analyses were stratified by treatment. Myocardial segments were deemed non-viable if LGE exceeded 50% wall thickness. Overall and anterior viability were assessed. Outcomes were all-cause mortality, cardiovascular (CV) mortality and major adverse CV events. Among 165 subjects (mean (±SD) age 57.5±8.5 years, 152 males), 79 underwent CABG and 86 received OMT. A greater number of non-viable segments was significantly associated with higher all-cause and CV mortality in the CABG group (adjusted hazard ratios 1.17 [95% confidence interval {CI} 1.01–1.37; P=0.04] and 1.25 [95% CI 1.01–1.56; P=0.045], respectively), but not in the OMT (P>0.05) group. Anterior wall viability did not affect outcomes.
Conclusions: The extent of myocardial viability assessed by LGE appeared to identify patients with a differential survival benefit from CABG in this retrospective, small cohort study. These findings raise interesting hypotheses that need to be validated in larger prospective studies.
Despite major treatment advances, ischemic heart failure (HF) remains a significant cause of morbidity and mortality worldwide. Coronary artery revascularization may improve left ventricular contractility and function, as well as clinical outcomes in patients with viable myocardium,1 but the usefulness of myocardial viability assessment in decision making regarding revascularization in ischemic HF remains controversial. In the Surgical Treatment for IsChemic Heart failure (STICH) trial, there were fewer incident deaths from cardiovascular (CV) and all causes among subjects with impaired left ventricular ejection fraction (LVEF; ≤35%) who underwent coronary artery bypass grafting (CABG),2 an effect that persisted until the end of extended follow-up.3 Contrary to prior non-randomized studies,4,5 there was no interaction between the presence of myocardial viability assessed using dobutamine stress echocardiography (DSE) and/or single photon emission computed tomography (SPECT) with the treatment effects of CABG or optimal medical therapy (OMT) on mortality and CV morbidity.6 These findings were corroborated in the smaller Heart Failure Revascularisation Trial (HEART), in which the presence of viable myocardium did not confer survival benefit in percutaneously or surgically revascularized subjects with ischemic HF compared with conservative management.7
Late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR) accurately depicts the transmural extent of myocardial infarct8,9 and correlates well with myocardial perfusion-metabolism mismatch,10 the conventional gold standard for myocardial viability. A <50% extent of transmural LGE is predictive of left ventricular segmental contractile recovery after revascularization.9,11 Of note, the use of LGE for myocardial viability assessment was not widespread at the inception of the STICH and HEART studies, and there is sparse literature on its effect on clinical outcomes after coronary revascularization or with contemporary OMT, unlike with DSE and SPECT. The aim of this study was to investigate the association between myocardial viability assessed by LGE and clinical outcomes after either CABG or OMT in patients with ischemic HF.
This was a single-center retrospective analysis of clinical outcomes in consecutive ischemic HF patients who had undergone myocardial viability assessment using CMR as part of workup for consideration of surgical revascularization. The recruitment period was from 1 September 2004 to 28 February 2014. All subjects had severely impaired LVEF (≤35%) on CMR. In each patient, the decision for subsequent CABG or OMT was reached based on clinical team review of clinical status, multimodality imaging results (including LGE), and the patient’s wishes. This study has been registered with the Research Registry (www.researchregistry.com, UIN: 4711) and was approved by the SingHealth Centralised Institutional Review Board (2014/604/C). The study was conducted in accordance with the Declaration of Helsinki. Clinical data, baseline demographics, CV risk factors, significant comorbidities, cardiac catheterization reports, CMR findings, and procedural details of CABG were garnered from a review of patients’ medical charts.
CMR ImagingAll patients underwent CMR on a 1.5-T CMR scanner (Avanto; Siemens). Cine CMR and, after gadolinium contrast administration, LGE sequences were performed at standard long-axis 2-, 3-, and 4-chamber and a parallel stack of contiguous short-axis slices. LVEF and left ventricular volumes were calculated using standard methods.12 The CMR wall motion score index was calculated from the 17-segment model, with each segment assigned a score based on systolic motion, as follows: 1: normal; 2, hypokinesia; 3, akinesia; 4, dyskinesia; 5, aneurysmal/scar.13 A CMR LGE score index was calculated from the 17-segment model, with each segment assigned a score based on the extent of hyperenhancement as follows: 0, 0% hyperenhancement; 1, 1–25% hyperenhancement; 2, 26–50% hyperenhancement; 3, 51–75% hyperenhancement; 4, 76–100% hyperenhancement. Anterior segments were defined as basal anteroseptal, basal anterior, mid-anteroseptal, mid-inferoseptal, mid-anterior, apical septal, apical anterior, apical lateral, apical inferior, and apex. Non-viable segments were defined as segments having >50% hyperenhancement. Primary analysis was performed with viability as a continuous variable. Secondary analysis was performed using viability as a dichotomous variable with a cut-off of ≥4 non-viable segments defined as non-viable. Sensitivity analyses were also performed at a range of other cut-off values (5, 6, and 7 non-viable segments).
OutcomesSubjects were followed up until a common end-of-study date, namely 1 January 2015. The primary outcome measure was all-cause mortality. Secondary outcome measures were CV mortality and major adverse CV events (MACE) comprising CV death, non-fatal myocardial infarct, non-fatal stroke, and coronary revascularization with either CABG or percutaneous coronary intervention (PCI). Outcomes were obtained from national registries and a review of patients’ health records.
Statistical AnalysisSubjects were stratified into CABG and OMT groups for analysis. As these groups were non-randomized with inherent biases related to the complex clinical decision-making process, all analyses were performed separately in each group. Baseline demographics and risk factors were reported and compared between subjects with dichotomous left ventricular overall viable vs. non-viable status using descriptive statistics, Fisher’s exact test or a t-test as appropriate. Survival time was measured from recruitment to the date of outcomes or date of censor (1 January 2015). Hazard ratios (HRs) for outcomes and their corresponding 95% confidence intervals (CIs) for overall and anterior territory viability status were derived using a Cox proportional hazards model.14 We tested the constant propositional hazards on the basis of Schoenfeld residuals and no violation to the proportional hazards assumption was observed. Two-tailed P<0.05 was considered statistically significant. Stata version 13 (StataCorp, College Station, TX, USA) was used for all analyses.
Of a total of 165 enrolled subjects (mean (±SD) age 57.5±8.5 years, 152 males), 79 underwent CABG and 86 received OMT. The mean (±SD) duration of follow-up was 3.9±2.7 years. Table 1 summarizes the baseline demographics and clinical characteristics of the study population.
| Whole cohort (n=165) |
CABG (n=79) | OMT (n=86) | |||||
|---|---|---|---|---|---|---|---|
| Viable (n=64) |
Non-viable (n=15) |
P-value | Viable (n=49) |
Non-viable (n=37) |
P-value | ||
| Age (years) | 58±8.8 | 58±6.9 | 54±9.9 | 0.13 | 60±10.0 | 56±9.0 | 0.065 |
| Male sex | 152 (92) | 59 (92) | 14 (93) | 1.00 | 44 (90) | 35 (95) | 0.69 |
| Ethnicity | <0.01 | 0.02 | |||||
| Chinese | 106 (64) | 49 (77) | 5 (33) | 31 (63) | 21 (57) | ||
| Malay | 34 (21) | 7 (11) | 4 (27) | 16 (33) | 7 (19) | ||
| Indian | 21 (13) | 7 (11) | 6 (40) | 1 (2) | 7 (19) | ||
| Other | 4 (2) | 1 (1) | 0 (0) | 1 (2) | 2 (5) | ||
| BMI (kg/m2) | 24.8±4.2 | 24.6±4.2 | 24.5±4.4 | 0.72 | 24.9±4.1 | 25.0±4.3 | 0.98 |
| Comorbidities | |||||||
| Hypertension | 87 (53) | 36 (56) | 8 (53) | 1.00 | 24 (49) | 19 (51) | 1.00 |
| Hyperlipidemia | 102 (62) | 47 (73) | 10 (67) | 0.75 | 25 (51) | 20 (54) | 0.83 |
| Diabetes | 83 (50) | 31 (48) | 10 (67) | 0.26 | 25 (51) | 17 (46) | 0.67 |
| COPD | 5 (3) | 2 (3) | 1 (7) | 0.47 | 1 (2) | 1 (3) | 1.00 |
| Smoker | 86 (52) | 36 (56) | 13 (87) | 0.04 | 19 (39) | 18 (49) | 0.39 |
| Prior MI | 95 (58) | 34 (53) | 12 (80) | 0.08 | 25 (51) | 24 (65) | 0.27 |
| Prior PCI | 135 (82) | 56 (88) | 14 (93) | 1.00 | 26 (73) | 29 (79) | 0.62 |
| Prior CABG | 0 (0) | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – |
| Prior symptomatic HF | 71 (43) | 28 (44) | 6 (40) | 1.00 | 20 (41) | 17 (46) | 0.67 |
| Prior valvular heart disease* | |||||||
| Mitral regurgitation | 84 (51) | 36 (56) | 7 (47) | 0.18 | 25 (51) | 16 (43) | 0.92 |
| Mitral stenosis | 0 (0) | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – |
| Aortic regurgitation | 11 (7) | 5 (8) | 1 (7) | 1.00 | 4 (8) | 1 (3) | 0.78 |
| Aortic stenosis | 1 (1) | 1 (2) | 0 (0) | 1.00 | 0 (0) | 0 (0) | – |
| Tricuspid regurgitation | 49 (0) | 18 (28) | 5 (33) | 0.21 | 18 (37) | 8 (22) | 0.10 |
| Atrial fibrillation | 13 (8) | 5 (8) | 1 (7) | 1.00 | 4 (8) | 3 (8) | 1.00 |
| Prior stroke | 26 (16) | 9 (14) | 1 (7) | 0.68 | 12 (24) | 4 (11) | 0.16 |
| Renal impairment | 15 (9) | 5 (8) | 0 (0) | 0.58 | 6 (12) | 4 (11) | 1.00 |
| Creatinine (μmol/L) | 106±51 | 106±73 | 99±27 | 0.72 | 108±30 | 104±32 | 0.62 |
| Peripheral vascular disease | 4 (2) | 0 (0) | 1 (7) | 0.19 | 2 (4) | 1 (3) | 1.00 |
| Presentation | 0.02 | <0.01 | |||||
| STEMI | 24 (15) | 5 (8) | 3 (20) | 4 (8) | 12 (32) | ||
| NSTEMI | 57 (35) | 33 (52) | 1 (7) | 21 (43) | 2 (5) | ||
| No. vessels with 75% stenosis | |||||||
| Left main | 50 (30) | 24 (38) | 4 (27) | 0.56 | 13 (27) | 9 (24) | 1.00 |
| DVD, DVD, and left main | 30 (18) | 10 (16) | 3 (20) | 0.70 | 8 (17) | 9 (24) | 0.42 |
| TVD, TVD, and left main | 135 (82) | 54 (84) | 12 (80) | 41 (84) | 28 (76) | ||
| CABG details | |||||||
| No. grafts | 3±0.8 | 3±0.8 | 0.98 | ||||
| LIMA graft | 60 (94) | 11 (73) | 0.038 | ||||
| LIMA and RIMA graft | 1 (2) | 0 | 1.00 | ||||
| Mitral valve replacement | 6 (9) | 3 (20) | 0.36 | ||||
| Aortic valve replacement | 0 (0) | 0 (0) | – | ||||
| Medications | |||||||
| Antiplatelet agents | 119 (72) | 44 (69) | 12 (80) | 0.53 | 41 (34) | 22 (59) | 0.015 |
| ACEIs/ARBs | 99 (60) | 35 (55) | 11 (73) | 0.25 | 35 (71) | 18 (49) | 0.044 |
| β-blockers | 102 (62) | 39 (61) | 9 (60) | 1.00 | 34 (69) | 20 (54) | 0.18 |
| Aldosterone antagonist | 53 (32) | 18 (28) | 5 (33) | 0.76 | 20 (41) | 10 (27) | 0.25 |
| Warfarin | 17 (10) | 6 (9) | 3 (20) | 0.36 | 5 (10) | 3 (8) | 1.00 |
| Statin | 111 (67) | 42 (66) | 10 (67) | 1.00 | 37 (76) | 22 (59) | 0.16 |
| OHA | 65 (39) | 22 (34) | 6 (40) | 0.77 | 24 (49) | 13 (35) | 0.27 |
| Insulin | 8 (5) | 2 (3) | 1 (7) | 0.47 | 3 (6) | 2 (5) | 1.00 |
| CMR | |||||||
| LVEF (%) | 25.9±12.0 | 28.4±13.7 | 25.2±12.8 | 0.34 | 25.0±11.7 | 23.0±8.2 | 0.56 |
| WMSI | 2.5±0.6 | 2.2±0.6 | 2.8±0.6 | 0.0052 | 2.4±0.5 | 2.9±0.7 | 0.00001 |
| LVEDV | 259±77.9 | 246±66.3 | 261±119 | 0.97 | 267±87.1 | 270±59.5 | 0.53 |
| LVESV | 198±77.9 | 182±68.5 | 205±119.1 | 0.92 | 205±84.6 | 211±58.2 | 0.44 |
| No. non-viable segments | 3±3.1 | 1±0.9 | 5±1.7 | <0.0001 | 1±1.0 | 7±2.3 | <0.0001 |
Unless indicated otherwise, data are given as the mean±SD or as n (%). *Moderate or worse. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; CABG, coronary artery bypass grafting; CMR, cardiac magnetic resonance imaging; COPD, chronic obstructive pulmonary disease; DVD, double vessel disease; HF, heart failure; LIMA, left internal mammary artery; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; MI, myocardial infarction; NSTEMI, non-ST segment elevation myocardial infarction; OHA, oral hypoglycemic agent; OMT, optimal medical therapy; PCI, percutaneous coronary intervention; RIMA, right internal mammary artery; STEMI, ST-elevation myocardial infarction; TVD, triple vessel disease; WMSI, wall motion score index.
The number of outcome events per group is given in Table 2. On univariate analysis, a unit increase in the number of non-viable left ventricular wall segments was significantly associated with higher incident all-cause mortality (HR 1.19; 95% CI 1.01–1.41; P=0.04) and CV mortality (HR 1.29; 95% CI 1.03–1.60; P=0.03) in the CABG group, but not in the OMT group. This remained significant after adjustment for confounders of age, body mass index, atrial fibrillation, and diabetes for overall mortality (adjusted [a] HR 1.17; 95% CI 1.01–1.37; P=0.04) and after adjustment for confounders of age, body mass index, and diabetes for CV mortality (aHR 1.25; 95% CI 1.01–1.56; P=0.045). A unit increase in left ventricular non-viable wall segments was associated with a non-significant trend for increased MACE events in the CABG group (HR 1.13; 95% CI 0.99–1.30; P=0.07), but not in the OMT group (HR 1.01; 95% CI 0.92–1.10; P=0.85). Analyses using dichotomous left ventricular overall viability status at a threshold of 4 non-viable segments demonstrated a significant effect on CV mortality in the CABG group (HR 3.58; 95% CI 1.08–11.9; P=0.04); this remained significant after adjustment for the above confounders (aHR 3.86; 95% CI 1.11–13.4; P=0.03). However, this dichotomous cut-off did not demonstrate a significant effect on all-cause mortality in either the CABG (HR 2.34; 95% CI 0.90–6.13; P=0.30) or OMT (HR 0.74; 95% CI 0.37–1.48; P=0.40) group. There was also no significant effect on MACE using this dichotomous cut-off in either group. Additional sensitivity analyses performed at other cut-off values (5, 6, and 7 non-viable segments) also did not yield any significant findings (Table 3; Figures 1,2).
| CABG | OMT | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall viable (n=64) |
Overall non-viable (n=15) |
Anterior viable (n=68) |
Anterior non-viable (n=11) |
Overall viable (n=49) |
Overall non-viable (n=37) |
Anterior viable (n=56) |
Anterior non-viable (n=30) |
|
| All-cause mortality | 11 (17) | 7 (47) | 14 (21) | 4 (36) | 21 (43) | 13 (35) | 22 (39) | 12 (40) |
| CV mortality | 6 (9) | 5 (33) | 8 (12) | 3 (27) | 19 (39) | 10 (27) | 19 (34) | 10 (33) |
| MACE | 19 (30) | 9 (60) | 23 (34) | 5 (45) | 25 (51) | 17 (46) | 27 (48) | 15 (50) |
Data are given as n (%). “Non-viable” was defined as ≥4 non-viable segments. CV, cardiovascular; MACE, major adverse cardiac events. Other abbreviations as in Table 1.
| CABG | OMT | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All-cause mortality | MACE | CV mortality | All-cause mortality | MACE | CV mortality | |||||||
| HR (95% CI) |
P-value | HR (95% CI) |
P-value | HR (95% CI) |
P-value | HR (95% CI) |
P-value | HR (95% CI) |
P-value | HR (95% CI) |
P-value | |
| Overall viability | ||||||||||||
| Continuous* | 1.19 (1.01– 1.41) |
0.04 | 1.13 (0.99– 1.30) |
0.07 | 1.29 (1.03– 1.60) |
0.03 | 0.98 (0.89– 1.08) |
0.67 | 1.01 (0.92– 1.10) |
0.85 | 0.96 (0.86– 1.07) |
0.45 |
| Dichotomous | 2.34 (0.90– 6.13) |
0.08 | 2.02 (0.91– 4.49) |
0.09 | 3.58 (1.08– 11.9) |
0.04 | 0.74 (0.37– 1.48) |
0.40 | 0.88 (0.47– 1.63) |
0.68 | 0.65 (0.30– 1.40) |
0.27 |
| Anterior viability | ||||||||||||
| Continuous* | 1.13 (0.93– 1.38) |
0.21 | 1.09 (0.93– 1.28) |
0.28 | 1.19 (0.93– 1.52) |
0.18 | 0.99 (0.88– 1.12) |
0.88 | 1.01 (0.90– 1.12) |
0.91 | 0.99 (0.86– 1.13) |
0.84 |
| Dichotomous | 1.53 (0.50– 4.71) |
0.46 | 1.22 (0.46– 3.24) |
0.69 | 2.17 (0.57– 8.34) |
0.26 | 0.96 (0.48– 1.94) |
0.91 | 1.01 (0.53– 1.91) |
0.98 | 0.93 (0.43– 2.0) |
0.85 |
Primary analysis was performed with viability as a continuous variable. Secondary analysis was performed using viability as a dichotomous variable with a cut-off of ≥4 non-viable segments defined as non-viable. *Per unit increase in the number of non-viable segments. CI, confidence interval; HR, hazard ratio. Other abbreviations as in Tables 1,2.
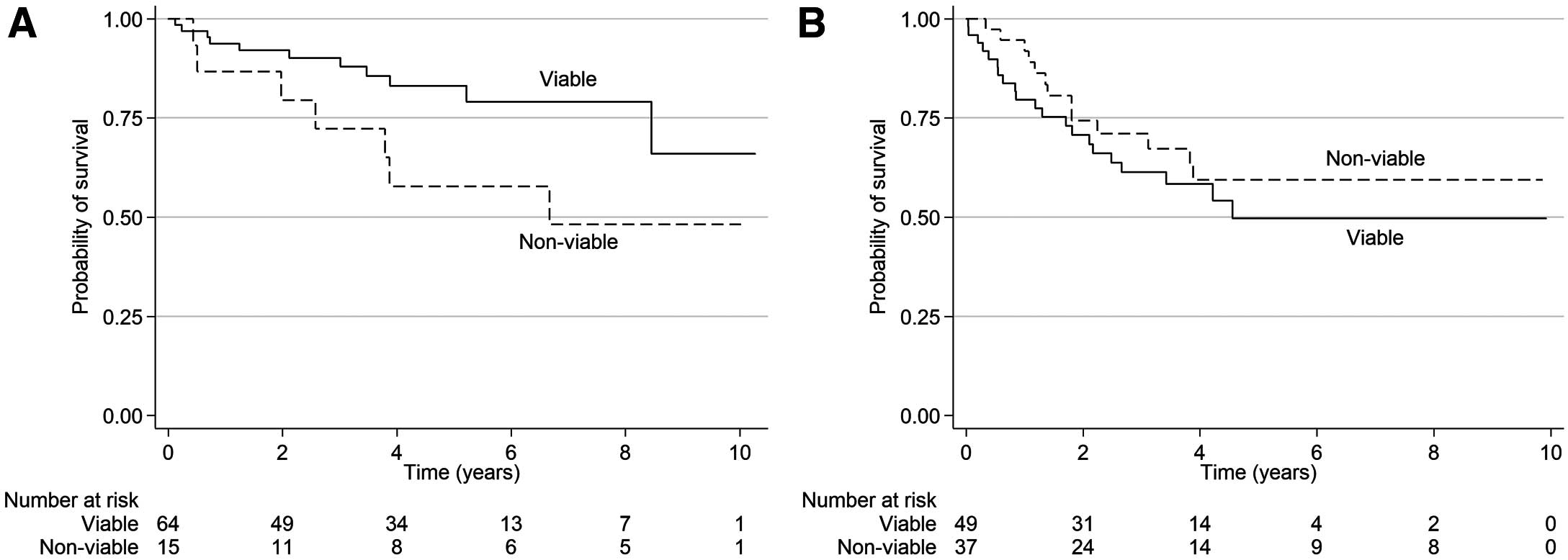
Kaplan-Meier curves for all-cause mortality in patients with viable vs. non-viable myocardium who underwent (A) coronary artery bypass grafting or (B) optimal medical therapy.

Kaplan-Meier curves for major adverse cardiac events (MACE)-free survival in patients with viable vs. non-viable myocardium who underwent (A) coronary artery bypass grafting or (B) optimal medical therapy.
The presence of anterior territory viability, either continuous or dichotomous, did not significantly affect all-cause mortality and MACE outcomes in either the CABG or OMT group (Table 3). Additional sensitivity analyses performed at other cut-off values did not yield any significant findings.
Comparison of CMR and SPECTIn all, 63 patients underwent both CMR and SPECT. Of the 19 patients with at least moderate ischemia on SPECT, CMR showed viability in 18. Of the 44 patients with nor or mild ischemia on SPECT, CMR showed viability in at least 28 patients. Interestingly, among the group of 14 patients with no or mild ischemia on SPECT but viability demonstrated on CMR who underwent CABG, there was 1 CV death (Table 4).
| 63 patients underwent both CMR and SPECT | |||||||
|---|---|---|---|---|---|---|---|
| CABG (n=34) | OMT (n=29) | ||||||
| No or mild ischemia on SPECT (n=20) |
Moderate-severe ischemia on SPECT (n=14) |
No or mild ischemia on SPECT (n=24) |
Moderate-severe ischemia on SPECT (n=5) |
||||
| <4 Non-viable segments on CMR (n=14) |
≥4 Non-viable segments on CMR (n=6) |
<4 Non-viable segments on CMR (n=13) |
≥4 Non-viable segments on CMR (n=1) |
<4 Non-viable segments on CMR (n=14) |
≥4 Non-viable segments on CMR (n=10) |
<4 Non-viable segments on CMR (n=5) |
≥4 Non-viable segments on CMR (n=0) |
| 2 deaths (1 CV death) |
2 deaths (1 CV death) |
3 deaths (3 CV deaths) |
0 death (0 CV death) |
7 deaths (6 CV deaths) |
3 deaths (3 CV deaths) |
3 deaths (3 CV deaths) |
0 death (0 CV deaths) |
SPECT, single photon emission computed tomography. Other abbreviations as in Tables 1,2.
Few studies have investigated the association between myocardial viability assessment by LGE and clinical outcomes in patients with ischemic HF treated with either CABG or OMT.15 In the present study, an increasing number of non-viable wall segments on LGE was significantly associated with both all-cause and CV mortality in surgically revascularized patients, with a non-significant trend towards more MACE events. Anterior territory LGE extent did not have a significant effect on outcomes in either group.
In patients with severe ischemic left ventricular dysfunction (LVEF ≤35%), the post-CABG improvement in symptoms and survival16,17 is counterbalanced by recognized high perioperative mortality.18 STICH, the only contemporary randomized controlled trial of CABG plus OMT vs. OMT alone in severe ischemic cardiomyopathy (Hypothesis 1), showed neutral primary mortality outcomes on an intention-to-treat analysis, a result that had to be interpreted in light of the high degree of treatment cross-overs.2 Per-protocol analysis demonstrated mortality benefits in subjects who actually underwent CABG compared with OMT alone.2 This benefit favoring CABG persisted when mortality rates between the original randomization treatment arms were compared, after excluding survivors who withdrew, in the STICH Extension Study (STICHES) that followed subjects up to 10 years.3
In severe left ventricular dysfunction, selecting patients with sufficient viable myocardium that may recover contractile function after revascularization is a reasonable and appealing management approach. Various investigators reported that revascularization of viable myocardium significantly improved both global and segmental function,19,20 restored wall thickness, reversed adverse left ventricular remodeling,19 and prolonged survival.21 However, these are all non-randomized studies. The landmark prospective randomized controlled STICH trial showed a neutral interaction between myocardial viability and treatment (CABG plus OMT vs. OMT alone) for clinical outcomes (i.e., all-cause mortality, CV mortality), but showed a significant interaction between myocardial viability and a composite of CV mortality and admissions.6 In the present study, viability was assessed either by SPECT (viability defined as ≥11 viable segments based on relative tracer activity) or DSE (viability defined as ≥5 viable segments with improved contractility after dobutamine stress). The results remained consistent when the number of viable segments was treated as a continuous variable,2 as well as when anterior wall viability was considered separately. The results are consistent with those of prior studies. The Positron Emission Tomography and Recovery Following Revascularization (PARR-2) randomized controlled trial used positron emission tomography (PET) to assess viability, and did not show a reduction in CV outcomes in the group of patients who underwent PET.22 The randomized HEART was terminated early due to poor recruitment. In that underpowered study, no significant benefit was observed in subjects with ischemic cardiomyopathy (LVEF <35%) and a substantial volume of viable myocardium who underwent revascularization compared with those who were treated medically.7
In all the studies described above, LGE had not been used for the assessment of myocardial viability. LGE is an accurate tool for determining myocardial viability,23 whereby gadolinium-based contrast causes hyperenhancement of scarred infarcted tissue due to increased extracellular volume.8,9 Fibrosis <50% of wall thickness is deemed viable.24 Thus, viable and infarcted myocardium may be identified independent of wall motion and infarct age,8 and with close correlation to histologic findings.25 LGE has been well validated in both preclinical and clinical studies in animals and humans,26 and has been shown to be a strong prognostic factor for all-cause mortality and MACE.26,27
Several studies have suggested that CMR provides a better assessment of viability than SPECT and PET.23,26 SPECT overestimates scar tissue,28 causing significant variation from LGE in terms of the mean number of segments classified as scar.23 Conversely, PET underestimates scar tissue relative to CMR due to lower spatial resolution than CMR, with Klein et al reporting that 11% of segments defined as viable on PET having some degree of LGE on CMR, suggesting non-viability.10 In another study, Kuhl et al showed that LGE is more sensitive in predicting functional recovery than a combination of PET and SPECT (97% vs. 87%), and has a higher negative predictive value than the combination of PET and SPECT (93% vs. 77%), suggesting a possible advantage in identifying viability.29 In the present study we found that an increase in the number of non-viable segments on LGE was significantly associated with higher all-cause and CV mortality in patients who underwent CABG. The differences between the results of the present study and those of the STICH and PARR-2 trials may be accounted for, at least in part, by differences in the imaging modality of viability assessment, with LGE being more accurate. In the present study, of the 44 patients with no or mild ischemia on SPECT, 28 had viability demonstrated on CMR. Of these 28 patients, 14 underwent CABG, with only 1 CV mortality in this group. This explanation is strengthened by corroborative evidence. In a cohort of 144 patients with ischemic cardiomyopathy, Gerber et al found that, in the presence of dysfunctional viable myocardium as determined by LGE, revascularization had a significantly higher effect on survival than medical treatment (HR 4.56; 95% CI 1.93–10.90).15
The findings of the present study may potentially have some effect on patient selection for surgical revascularization among patients with ischemic HF, but this will have to be validated further. Although we did not find a specific dichotomous cut-off value for viability that affected all-cause mortality, a cut-off of 4 non-viable segments seemed to identify a group with less CV mortality with CABG. The use of viability as a continuous variable was also significant for both overall and CV mortality. Thus, CMR viability assessment may be used by the clinician in the overall patient assessment as an additional tool to aid in the complex decision-making process for revascularization in this fragile group. The decision for revascularization in this group of patients is always difficult and multifactorial. In cases where the decision may be less than straightforward, the presence of viability may provide the heart team with some evidence to lean towards revascularization. This has to be taken into account on a case-by-case basis, looking at the patient as a whole.
We also analyzed anterior territory viability because prior studies have shown that the prognostic benefit in revascularization arises primarily from revascularization of the left main or left anterior descending artery.30 In this study, anterior viability did not affect outcomes in either group, but the small number of patients may mean that the study is not powered to show any potential differences, and this needs to be validated in further studies. However, in the present study, overall viability appears to be a stronger predictor of outcomes than anterior viability in those who are revascularized by CABG. This may indicate the importance of the whole left ventricle rather than a particular distribution, especially in this group of patients with severe globally impaired left ventricular function.
Study LimitationsThere are several limitations that require consideration. First, this study is a retrospective review and has its inherent biases. CABG and OMT were non-randomized. Patient decision may have influenced treatment choice, and data on patients who were recommended to have CABG but decided on OMT were not available. Hence, we stratified the analysis of the cohort based on treatment received to study the effect of viability in each stratum. Second, this study was performed on a small cohort of patients, and the findings need to be confirmed in a randomized controlled trial with sufficient patient numbers and well-defined criteria for treatment assignment. Third, the use of CMR LGE in patients with advanced chronic kidney disease is limited in view of the potential of nephrogenic systemic fibrosis. Finally, data were not available for comparisons of patients who underwent both DSE and CMR; we did, however, perform a subset analysis on those who had concomitant SPECT and CMR.
In the present retrospective small cohort study, the extent of myocardial viability assessed by LGE appeared to identify patients with a differential survival benefit among those undergoing CABG, but not in medically treated patients. These findings raise interesting hypotheses that will need to be validated in larger prospective randomized controlled trials.
None.
This study did not receive any specific funding.
None.
This study was approved by the SingHealth Centralised Institutional Review Board (2014/604/C).