論文ID: CR-21-0027
論文ID: CR-21-0027
Background: Atrial fibrillation (AF) and mitral regurgitation (MR) are frequently combined in patients with heart failure (HF). However, the effect of AF on the prognosis of patients with HF and MR remains unknown.
Methods and Results: We studied 867 patients (mean age 73 years; 42.7% female) with acute decompensated HF (ADHF) in the NARA-HF registry. Patients were divided into 4 groups based on the presence or absence of AF and MR at discharge. Patients with severe MR were excluded. The primary endpoint was the composite of cardiovascular (CV) death and HF-related readmission. During the median follow-up of 621 days, 398 patients (45.9%) reached the primary endpoint. In patients with MR, AF was associated with a higher incidence of the primary endpoint regardless of left ventricular function; however, in patients without MR, AF was not associated with CV events. Cox multivariate analyses showed that the incidence of CV events was significantly higher in patients with AF and MR than in patients with MR but without AF (hazard ratio 1.381, P=0.036). Similar findings were obtained in subgroup analysis of patients with AF and only mild MR.
Conclusions: The present study demonstrated that AF is associated with poor prognosis in patients with ADHF with mild to moderate MR, but not in those without MR.
Atrial fibrillation (AF) and mitral regurgitation (MR) are frequently observed comorbidities in patients with heart failure (HF), and both affect the prognosis of these patients.1–11 Previous studies have found that approximately 30% of patients with HF have MR of mild, moderate, or severe degree, and approximately 35% have AF; a substantial proportion of these patients are assumed to have both AF and MR.
Functional MR (FMR) has been combined with left ventricular (LV) dilatation and dysfunction classically and, in the case of HF patients with a reduced ejection fraction (EF), FMR is associated with worse outcomes.5,8,9,11 Atrial FMR, which occurs in patients with AF but normal LV size and function, was recently reported to be associated with adverse outcomes even if its severity is mild.9 Two-thirds of HF patients with preserved EF experience AF. The coexistence of AF links to worse outcomes because it causes greater left atrium (LA) remodeling, natriuretic peptide elevation, and exertional intolerance.12–16
Prior retrospective and prospective observational studies have reported that AF is associated with poor prognosis in HF patients with either educed EF or preserved EF. Thus, a complex interplay exists among AF, MR, and HF that forms a vicious cycle leading to poor prognosis.13–15
In the present study, we investigated the impact of AF on prognosis in acute decompensated HF (ADHF) in patients with or without MR.
Consecutive patients with ADHF admitted to our hospital between January 2007 and December 2016 were enrolled in the Nara Registry and Analyses for HF3 (NARA-HF3) and were included in the present study. Patients who died during the index hospitalization and patients with severe MR who underwent or were planned for elective mitral valvular surgery were excluded. In addition, patients who did not undergo echocardiographic examination at the time of discharge were also excluded because the severity of MR in these patients is unknown.
Patients were grouped according to the presence or absence of MR and AF. MR was defined as the presence of mild or moderate MR at the time of discharge. Patients were divided into 4 groups: Group 1, no MR and no AF; Group 2, no MR with AF; Group 3, MR but no AF; and Group 4, both MR and AF.
The NARA-HF study17–20 recruited consecutive patients who were emergency admissions to the Department of Cardiovascular Medicine, Nara Medical University for ADHF between January 2007 and December 2016. The diagnosis of HF was based on the Framingham study criteria.21 Patients with acute myocardial infarction (MI), acute myocarditis, and acute HF with acute pulmonary embolism were excluded from this registry. AF was defined as a history of either chronic or paroxysmal AF. In addition, patients with an episode of paroxysmal AF (PAF) documented by electrocardiography (ECG) during the index hospitalization were included.
Transthoracic EchocardiographyEchocardiographic examinations were performed using the Sonos 7500 system (Philips, Best, Netherlands) or the Acuson Sequoia system (Siemens, Erlangen, Germany). The results were interpreted by experienced attending doctors in the echocardiography laboratory. The severity of MR was evaluated by qualitative color Doppler imaging as reported previously.22 Briefly, MR severity was graded as “mild” if the color flow jet area was <4 cm2 or <20% of the LA area, as “moderate” if the color flow jet area was ≤40% of the LA area, and as “severe” if the color flow jet area was >10 cm2 or >40% of the LA area.22 LVEF was calculated by the modified Simpson’s method. LV end-diastolic diameter, LV end-systolic diameter, and LA diameter were measured via 2D or M-mode echocardiography. Preserved EF was defined as an LVEF ≥50% at discharge, whereas reduced EF was defined as LVEF <50% at discharge.
EndpointsThe primary endpoint was a cardiovascular (CV) event, defined as a composite endpoint of CV death and HF-related readmission. CV death was defined as death due to HF, MI, vascular disease, stroke, or sudden CV death. When this information was unavailable in the medical records, clinicians blinded to a patient’s clinical status telephoned patients or their families to collect this information. Secondary endpoints included the individual component outcomes of CV death and HF-related readmission. Of 867 patients, 519 patients (59.9%) were followed-up from the medical records at Nara Medical University by cardiologists, 91 patients (10.5%) were followed-up by cardiologists at affiliated hospitals, and 185 patients (21.3%) were followed-up by generalists. A prognosis survey of 72 patients (8.3%) was conducted by contacting patients or their families by telephone.
Statistical AnalysisNormally distributed data are presented as the mean±SD, whereas non-normally distributed data are presented as the median and interquartile range. Differences between groups were analyzed using the Chi-squared test for categorical variables. Student’s t-test (normally distributed data) or the Wilcoxon rank-sum test (non-normally distributed data) were used for comparisons of continuous variables between 2 groups. Cumulative event-free rates during follow-up were assessed using the Kaplan-Meier method. Univariate and multivariate analyses of event-free survival were performed using the Cox proportional hazard models. An unadjusted model and a model adjusted for covariates that were significantly associated with the primary endpoint in the univariate analysis were used to determine the variables independently associated with the primary endpoint. The covariates adjusted for in the multivariate model were age, sex, body mass index (BMI), diabetes (DM), prior MI, smoking, B-type natriuretic peptide (BNP) concentration, estimated glomerular filtration rate (eGFR), LA diameter, MR, and AF. Two-sided P<0.05 was considered statistically significant. All analyses were performed using JMP software for Mac version 14 (SAS Institute, Cary, NC, USA).
Ethical ConsiderationsThis study was approved by the Ethics Committee of Nara Medical University (Approval no. 1176-5), and written informed consent was obtained from all patients in accordance with the Declaration of Helsinki’s Ethical Principles for Medical Research Involving Human Subjects.
Of the 1,074 patients who were enrolled in the NARA-HF3 study, 207 patients were excluded from the present study: 49 patients died in hospital during the index hospitalization, 50 patients underwent or were planned for elective mitral valvular surgery, and 108 patients did not undergo echocardiographic examination at discharge. The remaining 867 patients with ADHF were included in the study. MR was absent in 347 patients (40%) and present in 520 (60%); AF was present in 313 patients (36%) and absent in 554 patients (64%). Accordingly, patients were divided into 4 groups: no MR and no AF (Group 1; n=249), no MR with AF (Group 2; n=98), MR but no AF (Group 3; n=305), and both MR and AF (Group 4; n=305; Figure 1). Of the AF patients, 102 patients were categorized as PAF.

Study flow chart. AF, atrial fibrillation; MR, mitral regurgitation.
The baseline characteristics of the study population are presented in Table 1. Regardless of the presence or absence of MR, patients with AF were older and had larger LA diameters. They had a higher prevalence of prior stroke or transient ischemic attack and a lower prevalence of DM and ischemic heart disease as HF etiology. There were no significant differences among the 4 groups in eGFR and hemoglobin, BNP, and sodium concentrations at discharge.
| No MR | MR | |||||
|---|---|---|---|---|---|---|
| No AF (n=249) | AF (n=98) | P value | No-AF (n=305) | AF (n=215) | P value | |
| Demographics | ||||||
| Age (years) | 69.6±13.1 | 72.7±11.5 | 0.045 | 72.6±13.0 | 76.0±9.6 | 0.001 |
| Male sex | 152 (61.0) | 48 (49.0) | 0.041 | 168 (55.1) | 129 (60.0) | 0.264 |
| BMI (kg/m2) | 24.4±4.6 | 24.2±4.1 | 0.635 | 22.8±4.1 | 23.2±3.7 | 0.267 |
| NYHA FC III–IV | 220 (88.7) | 85 (86.7) | 0.612 | 279 (91.8) | 189 (89.2) | 0.315 |
| HF etiology | ||||||
| IHD | 101 (40.6) | 18 (18.4) | <0.001 | 142 (46.6) | 62 (28.8) | <0.001 |
| DCM | 27 (10.8) | 16 (16.3) | 0.173 | 61 (20.0) | 34 (15.8) | 0.221 |
| Comorbidities | ||||||
| COPD | 14 (5.7) | 4 (4.2) | 0.581 | 21 (7.0) | 15 (7.0) | 0.997 |
| Hypertension | 205 (82.3) | 71 (72.5) | 0.044 | 237 (77.7) | 153 (72.2) | 0.152 |
| Diabetes | 125 (50.2) | 35 (35.7) | 0.014 | 137 (44.9) | 77 (36.3) | 0.050 |
| Prior MI | 57 (22.9) | 13 (13.3) | 0.038 | 100 (32.9) | 55 (25.6) | 0.072 |
| Prior stroke | 17 (6.8) | 20 (20.4) | 0.001 | 26 (8.5) | 33 (15.4) | 0.017 |
| Smoker | 148 (59.9) | 48 (49.0) | 0.065 | 168 (55.3) | 123 (59.4) | 0.351 |
| Laboratory data | ||||||
| Hemoglobin (g/dL) | 11.4±2.1 | 11.8±2.4 | 0.075 | 11.5±5.3 | 12.4±8.3 | 0.140 |
| eGFR (mL/min/1.73 m2) | 43.0±26.7 | 43.8±23.6 | 0.779 | 44.4±26.2 | 42.6±32.1 | 0.379 |
| BNP (pg/mL) | 209 [97.3–470.3] | 181.6 [101.9–359.1] | 0.303 | 327.1 [178.4–608.3] | 281.3 [151.4–528.2] | 0.163 |
| HbA1c (%) | 6.1±1.3 | 5.8±1.1 | 0.199 | 5.8±1.1 | 5.8±1.0 | 0.774 |
| Sodium (mEq/L) | 138.1±3.6 | 138.0±4.2 | 0.798 | 137.7±4.4 | 137.5±3.9 | 0.544 |
| Echocardiogram | ||||||
| LVEF (%) | 49.1±17.3 | 47.8±15.3 | 0.490 | 40.9±15.9 | 45.6±16.7 | 0.001 |
| LAD (mm) | 41.6±6.2 | 47.6±9.8 | <0.001 | 42.9±6.9 | 48.5±8.5 | <0.001 |
| Medications at discharge | ||||||
| ACEI or ARB | 215 (86.4) | 78 (79.6) | 0.126 | 276 (90.8) | 181 (84.2) | 0.023 |
| β-blocker | 142 (57.3) | 63 (65.0) | 0.189 | 199 (65.3) | 143 (66.5) | 0.764 |
| Aldosterone antagonist | 85 (35.0) | 38 (39.6) | 0.429 | 95 (31.7) | 86 (41.0) | 0.031 |
| Diuretics | 187 (75.1) | 89 (81.6) | 0.186 | 231 (75.7) | 86 (87.9) | <0.001 |
| Antiplatelet drug | 115 (46.2) | 33 (33.7) | 0.033 | 170 (55.7) | 81 (37.7) | <0.001 |
| Digoxin | 2 (0.8) | 17 (17.5) | <0.001 | 8 (2.7) | 40 (18.6) | <0.001 |
| Amiodarone | 8 (3.2) | 3 (3.1) | 0.942 | 14 (4.6) | 24 (11.2) | 0.005 |
| Statin | 84 (33.9) | 20 (20.6) | 0.014 | 128 (42.0) | 54 (25.1) | <0.001 |
| Anticoagulant | 27 (10.9) | 7 (77.3) | <0.001 | 60 (19.7) | 171 (79.5) | <0.001 |
Unless indicated otherwise, data are given as the mean±SD, median [interquartile range], or n (%). ACEI, angiotensin-converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, B-type natriuretic peptide; COPD, chronic obstructive pulmonary disease; DCM, dilated cardiomyopathy; eGFR, estimated glomerular filtration rate (Modified Diet in Renal Disease formula); HF, heart failure; IHD, ischemic heart disease; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MR, mitral regurgitation; NYHA FC, New York Heart Association functional class.
During a median follow-up of 621 days, 398 patients (45.9%) reached the primary endpoint: 95 patients in Group 1, 36 in Group 2, 137 in Group 3, and 130 in Group 4. In all, 310 patients had HF-related readmission (72, 27, 109, and 102 patients in Groups 1, 2, 3, and 4, respectively) and CV deaths were recorded for 190 patients (45, 19, 67, and 59 patients in Groups 1, 2, 3, and 4, respectively; Table 2).
| Group 1 (n=249) |
Group 2 (n=98) |
Group 3 (n=305) |
Group 4 (n=215) |
Total (n=876) |
|
|---|---|---|---|---|---|
| CV death and HF-related readmission |
95 (38) | 36 (37) | 137 (45) | 130 (60) | 398 (45) |
| HF-related readmission | 72 (29) | 27 (28) | 109 (36) | 102 (47) | 310 (35) |
| CV death | 45 (18) | 19 (19) | 67 (22) | 59 (27) | 190 (22) |
Data are given as n (%). Patients were divided into 4 groups: Group 1, no MR and no AF; Group 2, no MR with AF; Group 3, MR but no AF; and Group 4, both MR and AF. CV, cardiovascular. Other abbreviations as in Table 1.
The primary endpoint was observed more frequently in Group 4 (MR/AF) than Group 3 (MR/no AF; 137 patients [46%] vs. 130 patients [61%], respectively; P<0.001). Kaplan-Meier curves of comparisons of the primary composite endpoint and each endpoint component among the 4 groups are shown in Figure 2. The cumulative rate of event-free survival was lowest in Group 4 compared with the other 3 groups (log-rank, P<0.001).

Kaplan-Meier plots showing the time to (A) the composite endpoint of cardiovascular death or heart failure-related readmission, (B) heart failure-related readmission, and (C) cardiovascular death in patients without either mitral regurgitation (MR) or atrial fibrillation (AF).
Table 3 shows the unadjusted and adjusted hazard ratios (HRs) for the primary endpoint and each component in the 4 groups. Compared with Group 3 (MR/no AF) as a reference, Group 4 (MR/AF) had a significantly higher risk for the primary endpoint. After multivariable analysis adjusted for age, sex, BMI, DM, prior MI, smoking, BNP concentration, eGFR and LA diameter, all of which were significantly associated with the primary endpoint in the univariate analysis, the presence of both MR and AF was associated with an increased risk for the primary endpoint (HR 1.608; 95% confidence interval [CI] 1.155–2.249). Interestingly, AF was associated with an increased risk of the primary endpoint in patients with MR (HR 1.381; 95% CI 1.022–1.866), but not in those without MR (HR 1.064; 95% CI 0.679–1.632).
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Demographics | ||||
| Age (per 1 year) | 1.024 (1.015–1.033) | <0.001 | 1.017 (1.005–1.028) | 0.004 |
| Male sex | 1.221 (1.001–1.494) | 0.050 | 0.008 (0.822–1.546) | 0.463 |
| BMI (per 1 kg/m2) | 0.974 (0.951–0.998) | 0.031 | 0.987 (0.956–1.018) | 0.407 |
| HF etiology | ||||
| IHD | 1.209 (0.987–1.476) | 0.066 | ||
| DCM | 0.817 (0.609–1.075) | 0.152 | ||
| Comorbidities | ||||
| COPD | 1.383 (0.905–2.021) | 0.129 | ||
| Hypertension | 1.195 (0.942–1.533) | 0.144 | ||
| Diabetes | 1.235 (1.013–1.504) | 0.037 | 1.312 (1.034–1.663) | 0.026 |
| Prior MI | 1.539 (1.241–1.898) | <0.001 | 1.340 (1.037–1.723) | 0.025 |
| Stroke | 1.251 (0.916–1.670) | 0.155 | ||
| Smoking | 1.279 (1.047–1.567) | 0.016 | 1.348 (0.980–1.864) | 0.067 |
| Laboratory data | ||||
| Hemoglobin (per 1 g/dL) | 0.974 (0.931–1.007) | 0.155 | ||
| eGFR (per 1 mL/min/1.73 m2) | 0.994 (0.991–0.998) | 0.004 | 0.996 (0.991–1.001) | 0.136 |
| BNP (per 100 pg/mL) | 1.027 (1.011–1.041) | 0.002 | 1.026 (1.002–1.048) | 0.036 |
| HbA1c (per 1%) | 1.052 (0.952–1.153) | 0.312 | ||
| Sodium (per 1 mEq/L) | 0.990 (0.965–1.017) | 0.449 | ||
| Echocardiography | ||||
| LVEF (per 1%) | 1.000 (0.995–1.006) | 0.875 | ||
| LA diameter (per 1 mm) | 1.019 (1.006–1.033) | 0.005 | 1.015 (0.999–1.031) | 0.063 |
| MR/AFA | ||||
| No MR, no AF | 0.753 (0.578–0.977) | 0.033 | 0.859 (0.627–1.171) | 0.337 |
| No MR with AF | 0.724 (0.494–1.033) | 0.076 | 0.914 (0.591–1.377) | 0.674 |
| MR but no AF | Reference | Reference | ||
| MR and AF | 1.589 (1.248–2.024) | <0.001 | 1.381 (1.022–1.866) | 0.036 |
Data are reported as hazard ratios (HRs) with 95% confidence intervals (CIs). Multivariate analyses were adjusted for age, BMI, diabetes, prior MI, smoking, BNP concentration, eGFR, left atrium (LA) diameter, MR, AF. AIn the case of analyses for MR/AF, HRs were calculated among 4 groups with the MR but no AF group as the reference. For other factors in the column for univariate analysis, unadjusted HRs are provided. Abbreviations as in Table 1.
As a sensitivity analysis, the same analyses were performed in 315 patients with only mild MR after excluding patients with moderate MR. The cumulative rate of event-free survival was lowest in Group 4 (MR/AF) compared with the other 3 groups (log-rank, P<0.001; Figure 3).

Kaplan-Meier plots showing the time to (A) the composite endpoint of cardiovascular death or heart failure-related readmission, (B) heart failure-related readmission, and (C) cardiovascular death in patients without either mitral regurgitation (MR) or atrial fibrillation (AF), with either mild MR or AF, and with both mild MR and AF.
Figure 4 shows the impact of AF on the prognosis of patients in different subgroups. Among patients in the MR group, AF was associated with a significantly higher risk for the primary endpoint in patients aged >65 and <75 years. There was no significant difference regarding sex, the presence of ischemic heart disease, LVEF (≥50% or <50%), the presence of DM, β-blocker use, or the degree of MR (mild or moderate). Patients with prior stroke did not have a significantly higher risk for the primary endpoint. There were no interactions. The proportionality assumption was met for the AF variable in all models.
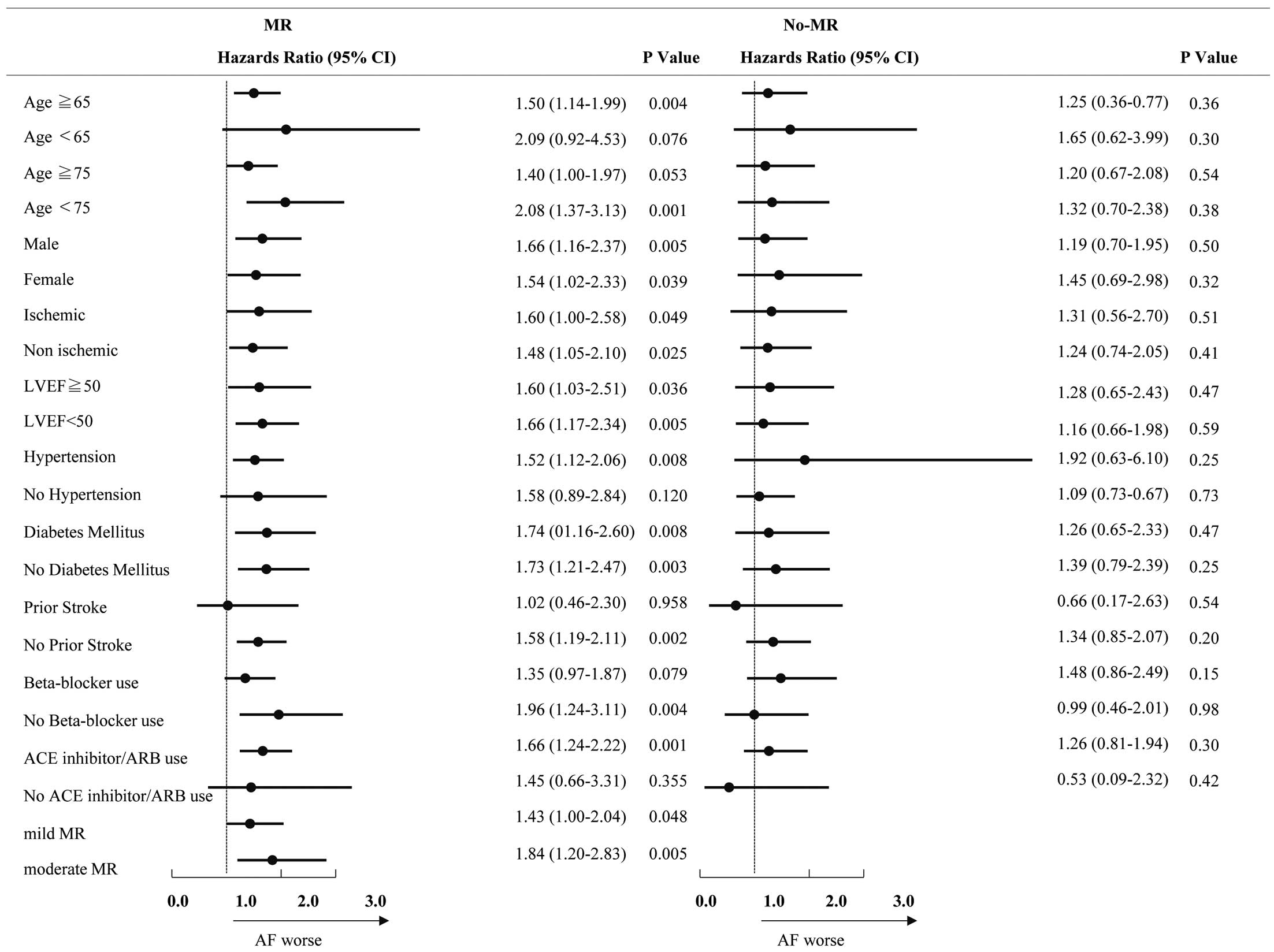
Hazard ratios and 95% confidence intervals (CIs) of patients with mitral regurgitation (MR) for prediction of cardiovascular death or heart failure-related readmission in patients with acute decompensated heart failure (ADHF). ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; LVEF, left ventricular ejection fraction.
The main finding of this study was that AF was associated with a higher incidence of CV events, defined as the composite endpoint of CV death and HF-related readmission, among patients with ADHF and MR regardless of LVEF, but not in patients without MR. Similar results were obtained for the incidence for either CV death or HF-related readmission.
The findings of this study are consistent with those of Ito et al, who reported that MR after HF treatment indicated a poor prognosis.15 Our findings suggest the need for intervention for AF and/or MR in patients with AF as well as mild or moderate MR. However, it remains unclear whether AF ablation is effective in improving long-term prognosis in patients with HF. Recently, AF ablation was reported to reduce CV events in patients with HF with reduced EF (HFrEF), but the effect of AF ablation has not been compared between patients with and without MR.23–26 The effects of AF ablation on the prognosis of patients with HF with preserved EF (HFpEF) has not been investigated.26 So, we performed subgroup analysis of patients according to HF type. In this study, 336 patients were classified as HFpEF (LVEF ≥50%) and 366 patients were classified as HFrEF (LVEF <40%). The cumulative rate of event-free survival was lowest in Group 4 (MR/AF) compared with the other 3 groups for both HFrEF and HFpEF patients (log-rank, P<0.001; Supplementary Figure 1).
Depending on AF burden, the impact of AF on ADHF may differ, but we have not considered AF burden in this study. Considering AF type, in a subgroup analysis of patients with PAF (n=102), the cumulative rate of event-free survival was lowest in Group 4 (MR/PAF) compared with the other 3 groups (log-rank, P<0.001; Supplementary Figure 2).
Regarding the treatment strategy for MR in patients with HF, the current guidelines recommend surgery for patients with severe MR, as well as those with moderate MR who undergo another concurrent open-heart surgery.27 However, there are no specific recommendations for the treatment of patients with MR associated with AF. Thus, the patients enrolled in the present study should be treated medically for MR, because patients with severe MR were excluded.
Given these guidelines and the fact that surgical intervention for MR is invasive, AF ablation would likely be a practical treatment strategy for HF and AF patients with mild to moderate MR. Considering that AF is not a risk factor for poor prognosis in patients without MR, further studies are needed to determine the effect of AF ablation on the long-term prognosis of patients with HF and MR.
We considered whether AF ablation would affect the results of the present analyses: most patients in the present study were treated with guideline-based medical therapy before and after discharge, and only 22 patients underwent catheter ablation after discharge. Only 12 of 22 patients who underwent AF ablation had mild to moderate MR. Thus, it is unlikely that AF ablation would affect the results of the present study.
Study LimitationsThis study has several limitations. First, the severity of MR was defined based on the qualitative color Doppler method recommended by the old guideline published in 200322 because the study period was prior to the publication of the updated guideline in 2017.28 The grade of MR, which was evaluated in the subacute phase of HF in the present study, may improve during the chronic phase after discharge with additional medical therapy. Second, AF was defined as either a history of AF or an episode of AF documented by ECG at any time during the admission period. Patients with new-onset AF after discharge were not included in this study. The impact of new-onset AF needs to be studied further. Third, exact mechanisms underlying MR, either ventricular, atrial or a combination of both, were not studied. Three-dimensional echocardiographic assessments of the mitral complex would be required to answer this question. Fourth, of 867 patients, 519 (59.9%) were followed-up from our medical records by cardiologists, 91 (10.5%) were followed-up by cardiologists at affiliated hospitals, and 185 (21.3%) were followed-up by generalists. A prognosis survey of 72 patients (8.3%) was conducted with patients and their families by telephone. Fifth, we used the individual clinical information at discharge in this study. As noted above, the MR grade may improve during the chronic phase after discharge with additional medical therapy. We do not know whether medical treatment was changed after discharge before the events.
The present study demonstrated that AF is associated with a poor prognosis in patients with ADHF with mild to moderate MR, regardless of LV function, suggesting that early management for AF, such as catheter ablation, would be recommended. However, a prospective randomized controlled study is needed to confirm the effects of AF ablation on prognosis in ADHF patients with MR.
The authors thank Y. Wada, Y. Kamada, and I. Yoshida for their support in the data-collection process.
This work was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science (MEXT KAKENHI; No. JP19155855), grants from Technology and the Ministry of Health, Labor, and Welfare of Japan (Health Labor Sciences Research No. 19189094 and 17933459; Comprehensive Research on Life-Style Related Disease including Cardiovascular Disease and Diabetes Mellitus), and grants from AMED (No. JP19ek0210080, JP19ek0210118, JP19ek0210121, JP19ek0210115 [Practical Research Project for Life-Style related Diseases including Cardiovascular Diseases and Diabetes Mellitus], No. JP19ek0109367 and JP19ek0109406 [Practical Research Project for Rare/Intractable Diseases], and No. JP19 km0405009 [Platform Program for Promotion of Genome Medicine]).
None.
The deidentified participant data will be shared upon reasonable request to contact the corresponding author. (The information regarding (2)–(6) should follow.)
Please find supplementary file(s);
http://dx.doi.org/10.1253/circrep.CR-21-0027