論文ID: CR-23-0071
論文ID: CR-23-0071
Background: Whether drug therapy slows the growth of abdominal aortic aneurysms (AAAs) in the Japanese population remains unknown.
Methods and Results: In a multicenter prospective open-label study, patients with AAA at the presurgical stage (mean [±SD] AAA diameter 3.27±0.58 cm) were randomly assigned to treatment with candesartan (CAN; n=67) or amlodipine (AML; n=64) considering confounding factors (statin use, smoking, age, sex, renal function), with effects of blood pressure control minimized setting a target control level. The primary endpoint was percentage change in AAA diameter over 24 months. Secondary endpoints were changes in circulating biomarkers (high-sensitivity C-reactive protein [hs-CRP], malondialdehyde–low-density lipoprotein, tissue-specific inhibitor of metalloproteinase-1, matrix metalloproteinase [MMP] 2, MMP9, transforming growth factor-β1, plasma renin activity [PRA], angiotensin II, aldosterone). At 24 months, percentage changes in AAA diameter were comparable between the CAN and AML groups (8.4% [95% CI 6.23–10.59%] and 6.5% [95% CI 3.65–9.43%], respectively; P=0.23]. In subanalyses, AML attenuated AAA growth in patients with comorbid chronic kidney disease (CKD; P=0.04) or systolic blood pressure (SBP) <130 mmHg (P=0.003). AML exhibited a definite trend for slowing AAA growth exclusively in never-smokers (P=0.06). Among circulating surrogate candidates for AAA growth, PRA (P=0.02) and hs-CRP (P=0.001) were lower in the AML group.
Conclusions: AML may prevent AAA growth in patients with CKD or lower SBP, associated with a decline in PRA and circulating hs-CRP.
Patients with smaller-size abdominal aortic aneurysms (AAAs), which are generally defined by a maximum short-axis AAA diameter <30 mm,1 receive no survival benefit from surgical intervention; thus, these presurgical stage AAAs (psAAA) are treated with medical therapy, primarily antihypertensive medications. Although up to 70% of small aneurysms continue to grow, which increases the risk of rupture,2 it is unknown what type of drugs can effectively delay the growth of AAAs.
Given that angiotensin II is used to create mouse models of AAA,3 it is possible that angiotensin II receptor blockers (ARBs) may prevent the expansion of AAAs. In the case of a thoracic aortic aneurysm, losartan has been shown to reduce the rate of aortic root dilatation in young patients with Marfan’s syndrome.4 In contrast, a recent clinical randomized study reported that telmisartan did not affect the growth of small AAAs over a period of 2 years among participants recruited from Australia, the Netherlands, and the US.5 However, that study was designed as a placebo-controlled study, which resulted in differences in blood pressure levels between the control and treatment arms, which may be a confounding bias towards AAA growth. Furthermore, considering another study reported racial disparity in terms of clinical outcomes for AAA,6 the impact of ARBs on AAA growth may be different in the Japanese population compared with Western populations.
The aim of the present study was to test the impact of popular antihypertensive drugs (i.e., ARBs and calcium channel blockers [CCBs]) on Japanese psAAA patients using a study design that minimized the influence of blood pressure control on AAA expansion. We also explored possible mechanisms contributing to AAA growth by measuring circulating biomarker candidates (renin-angiotensin system [RAS], vascular remodeling, inflammation, oxidative stress).
The present study tested the impact of an ARB (candesartan [CAN] and CCB (amlodipine [AML]) on the expansion of psAAA (Figure 1). Details of the trial protocol are available in the Supplementary File. The sample size was determined based on the prevalence of AAA and previously published clinical studies that have examined changes in AAA diameter using other agents,4,7,8 assuming a risk rate of 5%, a power of 80%, and a dropout rate of 10%. Based on these considerations, at least 100 patients would have to be enrolled in each group to detect a significant difference between the 2 groups.
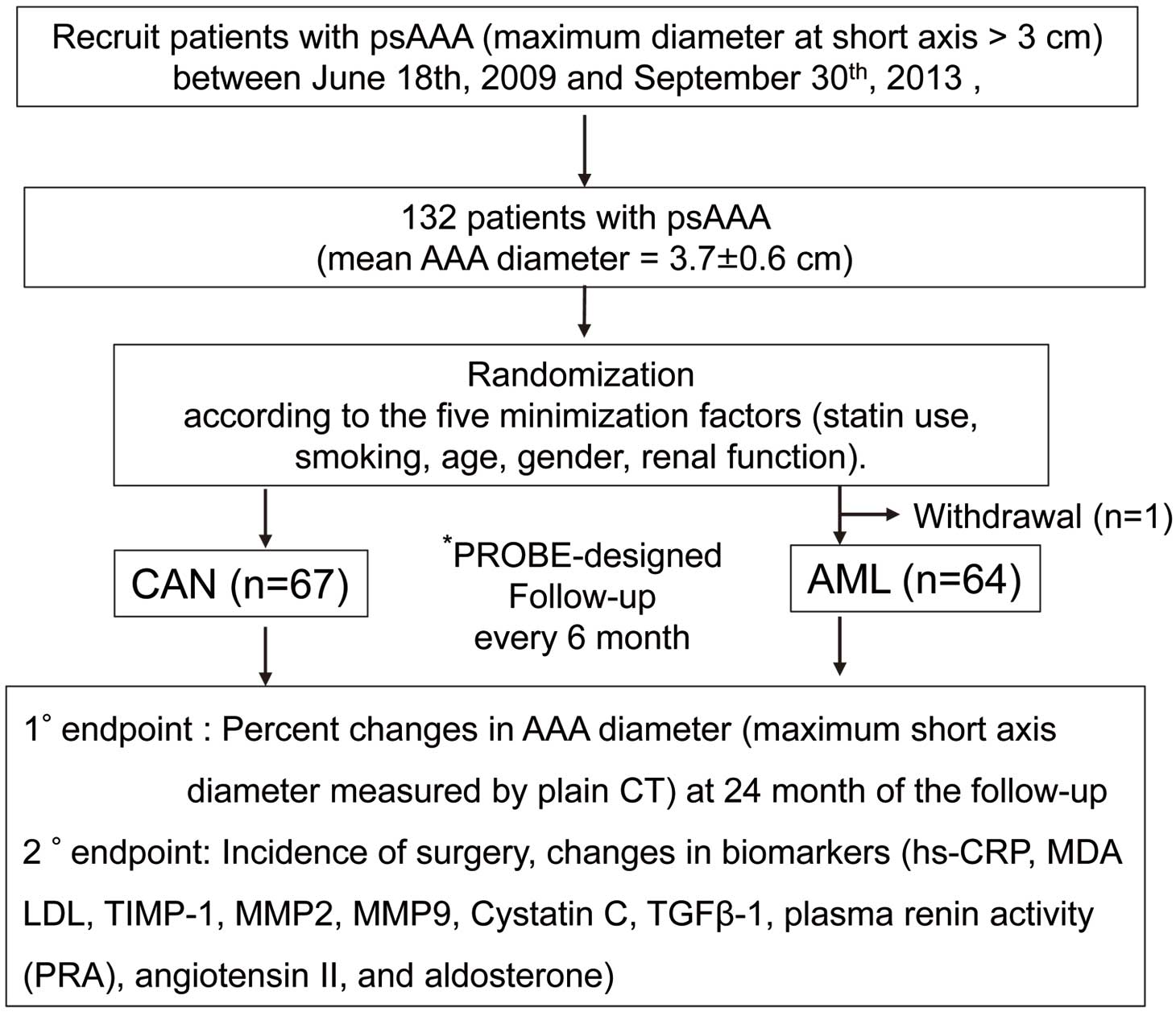
Study overview. *PROBE: prospective randomized open blinded end-point design. 1°, primary; 2°, secondary; AAA, abdominal aortic aneurysm; AML, amlodipine; CAN, candesartan; CT, computed tomography; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; MDA, malondialdehyde; MMP, matrix metalloproteinase; PRA, plasma renin activity; psAAA, abdominal aortic aneurysm at the presurgical stage; TGF-β1, transforming growth factor-β1; TIMP1, tissue-specific inhibitor of metalloproteinase 1.
Briefly, the inclusion criteria for the study are as follows: patients with aortic aneurysms in the preoperative stage (short-axis diameter from 3.0 to <4.5 cm); age ≥40 years; male and postmenopausal female; and systolic blood pressure (SBP) levels (office blood pressure values measured on the upper arm using a cuff, except for the case of white coat hypertension) ≥120 mmHg (untreated with antihypertensive drugs) or not applicable (patients on antihypertensive treatment). Participants prescribed antihypertensive medications such as ARBs, angiotensin-converting enzyme inhibitors, mineralocorticoid receptor antagonists, and CCBs were allowed to enroll in the study with consent to change their oral medications according to the study drug assignment. The exclusion criteria were as follows: pregnancy; premenopausal women; patients with vasospastic coronary artery disease taking CCBs; any past medical history of malignancy within the previous 5 years; patients with end-stage renal diseases, including those on hemodialysis; and drug allergies to ARB and CCB. In the case of patients with white coat hypertension or masked hypertension, the attending physicians referred to the patients’ home blood pressure records to confirm their eligibility for the study. Blood pressure values in the selection criteria referred to office blood pressure values measured on the upper arm using a cuff. The procedure for blood pressure measurements was not restricted to either Korotkoff sounds or oscillatory devices, but changing the procedure or device used during the study period was prohibited.
The present study was performed using a randomized open-label multicenter and prospective (prospective randomized open blinded end-point [PROBE]) design.9,10 There was no preregistration for this trial, and patients were enrolled consecutively. Patients were randomized using a computer algorithm (MINIM; an MS-DOS program for running minimization in clinical trials11,12) according to the 5 confounding factors (statin use, smoking, age, sex, renal function; chronic kidney disease [CKD] defined as estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2) that presumably affect AAA expansion (Supplementary Table). Patients were recruited between June 18, 2009 and September 30, 2013 and randomized to either the CAN or AML group using MINIM.
Once enrolled, the control range of SBP was prespecified as 120–135 mmHg to minimize the effects of blood pressure. Therefore, patients who were not being with antihypertensive agents and whose SBP was <120 mmHg at entry were excluded. During the study, the initial dose of each study drug was determined by the attending physicians, taking into consideration baseline blood pressure in individual patients. If blood pressure control may become insufficient (i.e., SBP >135 mmHg), the addition of antihypertensive agents other than the study drugs was permitted and was left to the discretion of the attending physician under consideration of safety and study criteria.
The primary outcome was the percentage change in the maximum orthogonal AAA diameter (measured by plain multislice computed tomography [CT]) at the 24-month follow-up. Secondary outcomes were the incidence of surgical repair, cardiovascular events, all-cause death, and changes in biomarkers (high-sensitivity C-reactive protein [hs-CRP], malondialdehyde (MDA)–low-density lipoprotein (LDL), tissue-specific inhibitor of metalloproteinase 1 [TIMP1], matrix metalloproteinase [MMP] 2, MMP9, cystatin C, transforming growth factor [TGF]-β1, plasma renin activity [PRA], angiotensin II, and aldosterone) and physiological indices. The enrolled patients were followed up every 6 months for changes in AAA diameter, blood pressure, and circulating biomarkers.
AAA MeasurementBaseline and follow-up measures of AAA diameter (every 6 months) by plain and multislice CT were performed by 3 independent researchers (2 clinical nurses and 1 cardiologist who is a coauthor [T. Mitsui]) who were blinded to group allocation, as per procedures that have been reported previously.13 All measurements were performed in the central laboratory at Nagoya University Hospital (for details, see University Hospital Medical Information Network [UMIN] Clinical Trials Registry: UMIN000002216). Static digital images in the transverse plane of the AAA were obtained at the point of maximum diameter. Each image was anonymized before measurement and transferred to the core laboratory for analysis in a blinded manner. The baseline characteristics of each arm are presented in Table 1. Furthermore, we assessed the risk of AAA growth, defined as percentage change in diameter >20% compared with baseline diameter, according to the 5 confounding factors.
Baseline Characteristics
| All patients | CAN (n=67) | AML (n=64) | P value | |
|---|---|---|---|---|
| Age at baseline (years) | 73±9 | 72±10 | 73±8 | 0.519 |
| Male sex (%) | 85.6 | 85.1 | 86.2 | 0.111 |
| Body weight (kg) | 61.9±11.3 | 61.3±11.9 | 62.5±10.7 | 0.589 |
| eGFR (mL/min/1.73 m2) | 62.9±15.9 | 65.4±16.4 | 60.2±15.1 | 0.691 |
| LDL (mg/dL) | 110.4±30.1 | 112.2±34.1 | 108.3±25.1 | 0.881 |
| SBP (mmHg) | 130.2±16.8 | 129.5±17.7 | 130.9±1.0 | 0.631 |
| DBP (mmHg) | 74.2±12.7 | 73.2±12.1 | 75.2±13.2 | 0.938 |
| AAA diameter (cm) | 0.262 | |||
| Mean±SD | 3.72±0.58 | 3.66±0.58 | 3.78±0.57 | |
| Median [IQR] | 3.69 [2.78–6.38] | 3.56 [2.78–5.48] | 3.82 [2.79–6.38] | |
| No. patients | 131 | 67 | 64 | |
| Without β-blockers | 0.890 | |||
| Mean±SD | 3.75±0.60 | 3.74±0.67 | 3.76±0.70 | |
| Median [IQR] | 3.70 [2.78–6.38] | 3.68 [2.78–5.48] | 3.80 [2.79–6.38] | |
| No. patients | 93 | 49 | 44 | |
| HbA1c (%) | 5.6±0.6 | 5.6±0.5 | 5.6±0.7 | 0.68 |
| CAD | 1 (0.76) | 1 (1.49) | 0 (0.0) | 0.23 |
| Smoking | 94 (72.0) | 48 (71.6) | 46 (72.3) | 0.549 |
Unless indicated otherwise, data are given as n (%) or mean±SD. P values for comparisons between the AML and CAN groups were obtained from t‐tests. AAA, abdominal aortic aneurysm; AML, amlodipine; CAD, coronary artery disease; CAN, candesartan; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; IQR, interquartile range; LDL, low-density lipoprotein; SBP, systolic blood pressure.
Blood Biochemical Analysis
At the baseline and 6-monthly follow-up visits, blood samples were collected for analysis of circulating biomarkers (i.e., hs-CRP, MDA-LDL, TIMP1, MMP2, MMP9, TGF-β1, PRA, angiotensin II, and aldosterone). All analyses were conducted at an external laboratory (SRL, Inc., Tokyo, Japan) to exclude technical bias.
Statistical AnalysisAll analyses were conducted according to intention-to-treat principles. The effects of CAN or AML on AAA growth were assessed using linear mixed models. To compensate for the impact of missing data in the longitudinal dataset at a small scale (n=131), we used the mixed-effects model for repeated measures (MMRM)14 for data analysis to minimize inflation of type I error rates for tests of regression coefficient parameters. In addition, exploratory analyses were performed, such as correlation analysis for each factor, causal analysis of event occurrence, and multiple regression analysis. The Cox proportional hazards model estimated hazard ratios (HRs) comparing CAN with AML and 95% confidence intervals (CIs). Multivariate Cox regression or analysis of covariance (ANCOVA) was used to assess the secondary outcomes. Studies of percentage changes in AAA diameter and surgical repair risk were exclusively conducted by the biostatistician (A.H.) in a blinded manner. Subgroup analyses were performed by T.M. and Y.K.B. using JMP Pro version 16.1.0 (SAS Institute Inc., Cary, NC, USA). All statistical tests were 2-sided, and a P<0.05 was considered significant. Continuous data are presented as the mean±SD or as the median with interquartile range (IQR).
In all, 132 Japanese patients with AAA were enrolled in the present study (Figure 1). One patient withdrew consent immediately after enrollment; therefore, 131 patients were examined after follow-up at 6, 12, 18, and 24 months. Of these 131 patients, 67 were treated with CAN and 64 were treated with AML (Figure 1). The baseline characteristics of the patients are summarized in Table 1. At study entry, the mean age of patients in the CAN and AML groups 72±10 years (range 44–88 years) and 73±8 years (range 50–86 years), respectively. The mean AAA diameter was 3.66±0.58 cm (median 3.56 cm; IQR 2.78–5.48 cm) in the CAN group and 3.78±0.57 cm (median 3.82 cm; IQR 2.79–6.38 cm) in the AML group (P=0.262). Notably, more than 70% of the study population was male with past or current smoking habits (Table 1). There was no difference in blood pressure control levels between the CAN and AML groups during study period, including at baseline (Supplementary Figure 1).
Primary OutcomesThe primary risk with AAA is rupture, which causes critical consequences with a high mortality rate.1,15 Accordingly, the primary outcome in the present study was the percentage change in AAA diameter as a surrogate of AAA growth. Although there was a trend for a higher percentage increase in AAA diameter in the CAN than AML group (8.4% [95% CI 6.23%, 10.59%] vs. 6.5% [95% CI 3.65%, 9.43%], respectively; Figure 2A), the difference (1.87%; 95% CI −1.22%, 4.95%) was not statistically significant (P=0.233). Furthermore, there was no significant difference in SBP between the CAN and AML groups (P=0.800; Supplementary Figure 1). After patient enrollment in the study, the attending physicians adjusted individual patient’s antihypertensive medication during the study to maintain target SBP control (i.e., 120–135 mmHg) considering safety and study criteria (e.g., choosing a drug class other than an ARB that could control participants taking the CCB). The mean dose of CAN was 7.5±2.7 mg/day and the mean dose of AML was 5.1±2.0 mg/day.

Primary outcome. (A) Percentage changes in the maximum orthogonal abdominal aortic aneurysm (AAA) diameter (measured by plain multislice computed tomography) at the 24-month follow-up. The difference between the candesartan (CAN; red circles) and amlodipine (AML; blue triangles) groups (at the time point of 24 months) was 1.87% (95% confidence interval [CI] −1.22%, 4.95%; P=0.233). At 24 months, the percentage change in AAA diameter was +8.4% (95% CI 6.23%, 10.59%) in the CAN group and +6.5% (95% CI 3.65%, 9.43%) in the AML group. (B) Subanalysis of the effect of β-blockers on the percentage change in AAA diameter (Table 1). There was no significant difference between the CAN and AML groups in patients who were not taking β-blockers (Table 1), despite a trend indicating that AML suppressed AAA growth more strongly than CAN (P=0.18). Data are the mean±SD.
A previous study demonstrated the ability of β-blockers to retard the progression of a thoracic aortic aneurysm according to Class IB recommendations in clinical guidelines,16 and several pilot studies have demonstrated the controversial role of β-blockers in AAA progression;17 thus, in the present study we performed a subanalysis to evaluate the impact of β-blockers on percentage changes in AAA diameter (Table 1; Figure 2B). There was no significant difference between the CAN and AML groups in patients who were not taking β-blockers (Table 1), despite a trend for AML to suppress AAA growth more strongly than CAN (P=0.18; Figure 2B).
We next performed a risk assessment of factors that could contribute to percentage changes in AAA diameter using the Cox hazard model (Table 2). Younger age (<75 years; P=0.07 in multivariate analysis) and larger AAA diameter at baseline (>3.7 cm; odds ratio [OR] 3.09, P=0.07 in multivariate analysis) were found to be possible risk factors for AAA expansion. Consistent with previous reports,1,18 current smoking and CKD exhibited trends as primary risk factors for AAA expansion in this Japanese AAA cohort (Table 2).
Risk Assessment for AAA Expansion
| Parameter | Category | No. patients | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |||
| Medication | ARB | 67 | 1 | 1 | ||
| CCB | 64 | 0.65 (0.16–2.61) | 0.5407 | 0.40 (0.10–1.58) | 0.190 | |
| SBP (mmHg) | <129 | 64 | 1 | 1 | ||
| ≥129 | 67 | 0.59 (0.15–2.37) | 0.4546 | 0.70 (0.18–2.76) | 0.611 | |
| Age (years) | <75 | 65 | 1 | 1 | ||
| ≥75 | 66 | 0.18 (0.03–1.08) | 0.061 | 0.18 (0.03–1.17) | 0.072 | |
| Sex | Male | 112 | 1 | 1 | ||
| Female | 19 | 0.32 (0.02–6.11) | 0.4453 | 1.15 (0.05–24.05) | 0.928 | |
| Smoking | Never | 37 | 1 | 1 | ||
| Current | 45 | 3.30 (0.50–21.74) | 0.2137 | 2.25 (0.39–13.18) | 0.367 | |
| Past | 49 | 1.28 (0.16–10.41) | 0.8169 | 0.89 (0.12–6.58) | 0.905 | |
| Statin | No | 64 | 1 | 1 | ||
| Yes | 67 | 1.55 (0.38–6.25) | 0.5407 | 1.22 (0.32–4.73) | 0.769 | |
| eGFR (mL/min/1.73 m2) | <60 | 64 | 1 | 1 | ||
| ≥60 | 67 | 2.64 (0.58–11.99) | 0.2078 | 2.47 (0.61–9.90) | 0.203 | |
| AAAd at baseline (cm) | <3.7 | 1 | 1 | |||
| ≥3.7 | 131 | 1.99 (0.74–5.38) | 0.1752 | 3.09 (0.91–10.45) | 0.070 | |
All covariates were entered into the adjusted model. Associations are reported for the outcome, namely the percentage change in the maximum orthogonal abdominal aortic aneurysm diameter (AAAd; measured by plain multislice computed tomography) at the 24-month follow-up vs. baseline diameter. The mean value SBP at baseline was set as the cut-off value (129 mmHg; minimum 94 mmHg, maximum 143 mmHg). ARB, angiotensin receptor blocker; CCB, calcium channel blocker; CI, confidence interval; OR, odds ratio. Other abbreviations as in Table 1.
We next compared the impact of drug treatment on AAA expansion according to confounding factors (Figure 3). AML effectively attenuated AAA growth in the patient population with CKD (defined as eGFR <60 mL/min/1.73 m2; −2.36; P=0.037) or lower SBP (<130 mmHg [mean SBP at baseline]; −4.26; P=0.0029]. Interestingly, the smoking habit, including past smoking, may attenuate the preventive effect of AML on AAA growth (−2.20%; P=0.063; Figure 3).
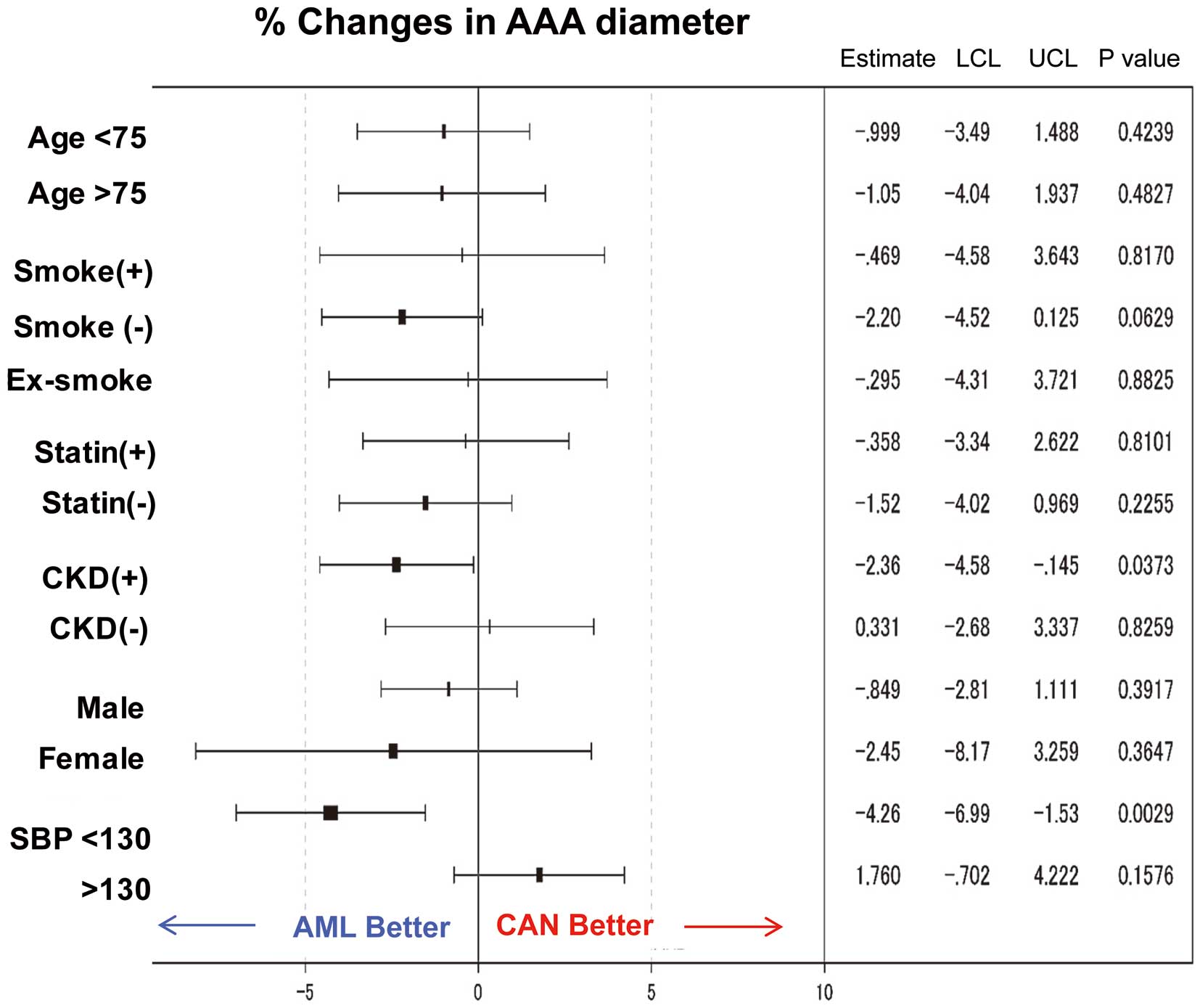
Subgroup analysis of percentage changes in abdominal aortic aneurysm (AAA) diameter. Forest plots of hazard ratios for confounding factors (age, smoking history, statin treatment, sex, and hypertension) associated with AAA growth. The impact of confounding factors was tested in terms of percentage changes in AAA diameter following treatment with candesartan (CAN) or amlodipine (AML). CKD, chronic kidney disease; LCL, lower confidence limit; SBP, systolic blood pressure; UCL, upper confidence limit.
Secondary Outcomes
A secondary outcome of the present study was the incidence of surgical repair at 24 months after entry. Five patients (1 in the ARB arm, 4 in the AML arm) underwent surgical repair. We compared risk factors for surgery in terms of medication (CAN or AML), SBP (median 129 mmHg), age (median 75 years), sex, smoking, statin use, CKD, and AAA diameter at baseline (Table 3). Unexpectedly, AML treatment had a trend for higher surgical risk than CAN (HR 2.615; 95% CI 0.721–9.492; P=0.144). Current smoking was the most substantial risk for the incidence of surgery (HR 5.751; 95% CI 0.314–28.01; P=0.03). Although not statistically significant, statin use (HR 0.355; 95% CI 0.046–2.731; P=0.3199) and lowering blood pressure (HR 0.562; 95% CI 0.145–2.183; P=0.405) may reduce surgical risk.
Risk Assessment for Surgical Repair
| Parameter | Category | HR (95% CI) | Pr(>ChiSq) |
|---|---|---|---|
| Medication | ARB | 1 | |
| CCB | 2.615 (0.721–9.492) | 0.144 | |
| SBP (mmHg) | <129 | 1 | |
| ≥129 | 0.562 (0.145–2.183) | 0.405 | |
| Age (years) | <75 | 1 | |
| ≥75 | 1.198 (0.181–7.928) | 0.851 | |
| Sex | Male | 1 | |
| Female | 1.432 (0.088–23.427) | 0.801 | |
| Smoking | No | 1 | |
| Yes | 5.751 (1.181–28.005) | 0.030 | |
| Ex-smoker | 1.627 (0.314–8.425) | 0.562 | |
| Statin use | No | 1 | |
| Yes | 0.355 (0.046–2.731) | 0.320 | |
| eGFR (mL/min/1.73 m2) | <60 | 1 | |
| ≥60 | 0.729 (0.131–4.044) | 0.718 | |
| AAAd at baseline (cm) | <3.7 | 1 | |
| ≥3.7 | 1.467 (0.202–10.627) | 0.705 |
Associations are reported for the outcome, namely the incidence of surgical repair during the 24-month follow-up period vs. no evidence of surgical intervention. AAAd, abdominal aortic aneurysm diameter at baseline; HR, hazard ratio; Pr(>ChiSq), probability of obtaining a χ2 value greater than the one shown under the null hypothesis. Other abbreviations as in Tables 1,2.
To investigate pathophysiology in our study population, we performed subgroup analysis of surrogate biomarkers that are associated with atherosclerosis and vascular remodeling, specifically the RAS pathway (PRA, angiotensin II, aldosterone; Figure 4A–C), oxidative stress (MDA-LDL; Figure 4D), inflammation (hs-CRP; Figure 4E), cell growth/proliferation (TGF-β; Figure 4F), and matrix remodeling (cystatin C, TIMP1, MMP2, MMP9; Supplementary Figure 2).
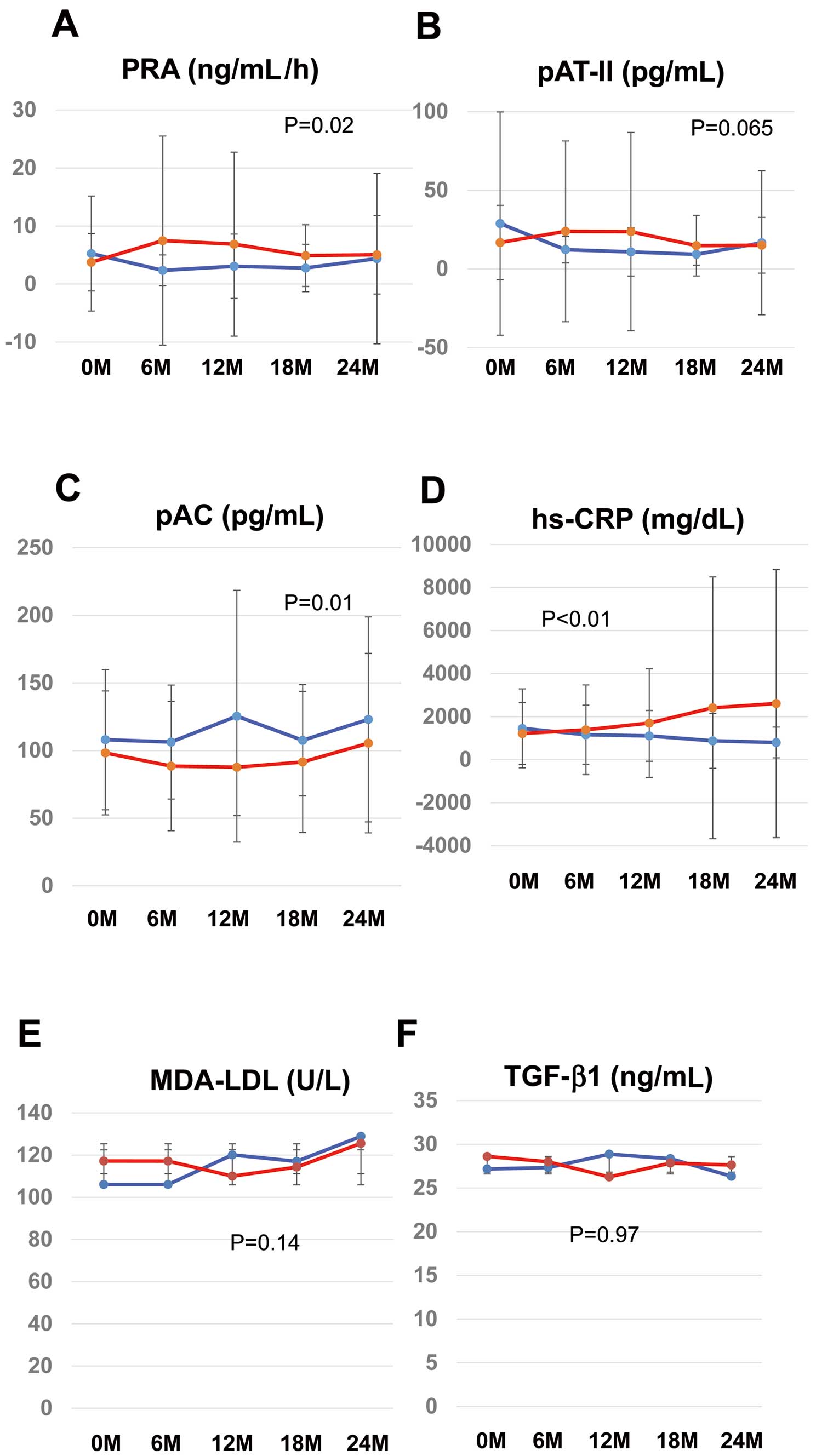
Secondary outcomes in the candesartan (CAN; red) and amlodipine (AML; blue) groups: (A) plasma renin activity (PRA), (B) plasma angiotensin II (pAT-II), (C) plasma aldosterone concentration (pAC), (D) inflammation (high-sensitivity C-reactive protein [hs-CRP]), (E) oxidative stress (malondialdehyde (MDA)–low-density lipoprotein [LDL]), and (F) remodeling/cell growth (transforming growth factor [TGF]-β1). Analysis of covariance (ANCOVA) was used for the assessment of secondary outcomes. All statistical tests were 2-sided, and P<0.05 was considered significant. Data are the mean±SD.
During the 2-year follow-up period, PRA was lower in the AML than CAN group (Figure 4A; P=0.02), but plasma angiotensin II concentrations tended to be higher in the AML group (Figure 4B; P=0.065). In contrast, the plasma aldosterone activity was significantly higher in the AML than CAN group (Figure 4C; P=0.01).
Oxidative stress and inflammation are essential factors for the progression of atherosclerosis, including AAA.19 AML treatment decreased circulating concentrations of hs-CRP (Figure 4A), but MDA-LDL and TGF-β levels remained unchanged (Figure 4B,C). Regarding the series of markers related to matrix remodeling, neither CAN nor AML had any significant effect over the 2-year follow-up (Supplementary Figure 2).
We found that CAN and AML had comparable efficacies on the growth rate of psAAA (Figure 2). However, subgroup analysis demonstrated that AML had a better impact on percentage changes in AAA diameter in the patient population with CKD or better control of blood pressure (i.e., SBP <130 mmHg; Figure 3), which may be explained, in part, by its antioxidative effects20 or improvement in endothelial function via the protein kinase C/endothelial nitric oxide synthase pathway.21
Furthermore, we found that younger patients (age <75 years; P=0.06) or patients with a larger AAA diameter at baseline (>3.7 cm; P=0.07) may be at higher risk of AAA growth (Table 2). Smoking is one of the primary risks for AAA rupture;22 we consistently found that smoking was an immediate risk for surgical repair (HR 5.75; P=0.03; Table 3).
One of the strengths of this study is the exploratory analysis regarding the surrogate biomarkers of AAA growth. To date, several surrogate markers for AAA growth and rupture risks have been explored.22 Circulating biomarkers22 are convenient and non-invasive for the assessment of AAA risk if the diagnostic sensitivity and specificity of each of the biomarkers are high enough. Unfortunately, fewer studies have investigated circulating markers associated with AAA growth, and there is no consensus regarding circulating markers introduced into clinical practice. In the present study, we selected the biomarkers associated with AAA pathophysiology, such as vascular remodeling (MMPs, TIMP, cystatin C), RAS system (PRA, plasma aldosterone, plasma angiotensin II), inflammation (hs-CRP), cell growth/proliferation (TGF-β), or oxidative stress (MDA-LDL), to understand the different effects of CAN and AML on psAAA growth. Interestingly, AAA growth has a trend for growing slower in the AML group than those CAN (Figure 2A), with PRA (Figure 4A; P=0.02) and hs-CRP (Figure 4B; P<0.001) levels being significantly lower in the AML than CAN group. In contrast, the risk of surgical repair was higher in the AML group (Table 2), in which plasma aldosterone concentrations were unexpectedly elevated (Figure 4C; P=0.011). A previous study revealed that antihypertensive medication affects circulating RAS activity in hypertensive patients with primary aldosteronism.23 ARB augments PRA24,25 and reduces plasma aldosterone, presumably stimulating renin secretion by interrupting angiotensin II feedback inhibition.23 In contrast, CCB did not affect PRA. Subanalysis of the Multiethnic Study of Atherosclerosis (MESA) study revealed that PRA is one of the primary risks for atherosclerotic vascular disease (ASCVD),26 with plasma aldosterone independent of ASCVD incidence. In contrast, aldosterone agonism is suggested as another risk factor for aortic dissection and rupture.27 In the present study, we found that PRA was lower in the AML than CAN group. In contrast, plasma aldosterone concentrations were higher in the AML than CAN group (Figure 4C). Furthermore, hs-CRP concentrations were significantly lower in the AML group, suggesting that the reduced PRA presumably contributed to the attenuation of AAA growth via its impact on chronic inflammation (Figure 1). Notably, the RAS system controls a biological feedback loop between vascular inflammation; for instance, angiotensin II upregulates interleukin-6, CRP, or angiotensinogen through the Janus tyrosine kinases (JAK)/signal transducers and activators of transcription (STAT)/p300 pathway.28 Interestingly, inflammatory signaling plays a vital role in the regulation of (pro)renin expression and activity.29 Activated inflammatory signaling, including proinflammatory cytokines, may serve as a repressor of (pro)renin expression in (pro)renin-producing juxtaglomerular apparatus cells or renal connecting tubules and collecting ducts of the kidney.30 In the present study, we found that comorbid CKD was a factor that significantly attenuated AAA growth in the AML-treated patients (P=0.04; Figure 3), indicating that the amlodipine-mediated anti-inflammatory effect may result in the suppression of renin production (i.e., a decline in PRA) that may contribute to aneurysm remodeling.
There was a critical limitation in this study that needs to be considered regarding the blood sampling conditions for the measurement of RAS activity. Plasma aldosterone measurements must be made in patients after a washout period for all hypertensive and prior resting to avoid false-positive and false-negative results.23 In the present study, sampling of plasma aldosterone is suggested to take a rest (i.e., lay down) for at least 30 min prior to taking the blood, which may have affected plasma aldosterone concentrations. Further investigations are warranted. Another study limitation is insufficient regulation of medication due to safety considerations. Ideally, if patients were taking any similar class of antihypertensive medication (e.g., ARBs, angiotensin-converting enzyme inhibitors, and CCBs), the drugs were supposed to be washed out by cessation for several months; however, we anticipated the risk of AAA expansion due to drug washout, so we allowed patients to be enrolled in the study with consent to change their oral medications according to study assignment. The last limitations are the small number of enrollments and the missing data in the longitudinal analysis, which causes underpowered results in terms of statistical significance. There is quite limited evidence regarding the prevalence of AAA; it is low, ranging from 0.5% to 3.2% of the population, or approximately 3 cases per 100,000 people per year, as noted in the Japanese guideline.8 Because of the low prevalence of AAA, the recent multinational study recruiting participants from Australia, the Netherlands, and the US over a period of 5 years, faced a similar issue of small numbers, with a total number 210 participants (telmisartan, n=107; placebo, n=103).5 In this study, we set the target number of AAA patients to recruit at 200, with 100 patients in each group. However, we were not able to recruit this number of patients.
In conclusion, the present study provides evidence of a beneficial role of AML in the medical management of psAAA in the Japanese population to attenuate chronic inflammation, presumably via suppression of PRA (Figure 5). AML may be another choice for prophylaxis management of psAAA. Both hs-CRP and PRA are plausible candidates for monitoring AAA growth in the presurgical stage.

Summary of this study. In this multicenter prospective open-labeled study, the effects of candesartan (CAN; n=67) or amlodipine (AML; n=64) on presurgical abdominal aortic aneurysm (AAA) growth and associated biomarkers in the Japanese population were evaluated. AML may prevent AAA growth in patients with chronic kidney disease (CKD) or lower systolic blood pressure (SBP; <130 mmHg), associated with a decline in plasma renin activity (PRA) and circulating high-sensitivity C-reactive protein (hs-CRP). Parts of the figure were drawn using pictures from Servier Medical Art. ARB, angiotensin receptor blocker; BP, blood pressure; CCB, calcium channel blocker; CT, computed tomography; LDL, low-density lipoprotein; MDA, malondialdehyde; MMPs, matrix metalloproteinases; PRA, plasma renin activity; psAAA, abdominal aortic aneurysm at the presurgical stage; RAS, renin-angiotensin system; TGF-β, transforming growth factor-β; Tx, treatment.
The authors thank the principal investigators, trial coordinators, and participants who contributed to the trial.
This study was supported by the Clinical Research Grant of the Department of Cardiology, Nagoya University Graduate School of Medicine, which includes research grants provided by Pfizer and Takeda. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Y.K.B. reports receiving honoraria from Pfizer and Daiichi-Sankyo outside the submitted work. T. Murohara reports receiving lecture honoraria from Pfizer, Novartis, Daiichi-Sankyo, Takeda, and Mitsubishi-Tanabe outside the submitted work. No other disclosures are reported. T. Murohara, Y.K.B. are members of Circulation Reports’ Editorial Team.
T. Mitsui, Y.K.B. had full access to all the data in the study. As a result, they take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: T. Murohara, K.N., K.K., Y.K.B; Acquisition, analysis, or interpretation of the data: all authors; Drafting the manuscript: T. Mitsui, Y.K.B., T. Murohara; Critical revision of the manuscript for important intellectual content: Y.K.B., K.F., R.M., T. Murohara; Statistical analysis: A.H., T. Mitsui, Y.K.B.
This study was approved by the Institutional Review Board of Nagoya University Graduate School of Medicine (Reference no. 208014).
The deidentified participant data will not be shared.
Please find supplementary file(s);
https://doi.org/10.1253/circrep.CR-23-0071