2023 Volume 63 Issue 3 Pages 159-162
2023 Volume 63 Issue 3 Pages 159-162
Sjögren’s syndrome (SJS) is a common autoimmune disease. Generally, posterior reversible encephalopathy syndrome (PRES) is often concomitant with autoimmune disease; however, PRES rarely complicates SJS. Thus, the detailed clinical course of cases with SJS and PRES remains unknown. We present the case of a 71-year-old female patient with primary SJS, whose magnetic resonance (MR) images showed bilateral vasogenic edema in the basal ganglia, brainstem, and cerebellum. Cerebrospinal fluid (CSF) examination revealed increased IgG index and higher interleukin-6 and anti-SSA-autoantibody levels. Management of her blood pressure combined with corticosteroid therapy improved her neurological symptoms, including abnormal CSF and MR imaging findings.
Sjögren’s syndrome (SJS) is a common autoimmune disease. Although SJS affects the salivary and lacrimal glands, it often causes various neurological complications in the central nervous system (CNS), such as cerebral vasculitis, and encephalopathy1)2). However, because there are no definite diagnostic criteria for CNS involvement in SJS, many clinical features remain unclear3). In comparison, posterior reversible encephalopathy syndrome (PRES) is a clinicoradiologic syndrome that presents reversible bilateral vasogenic edema typically in the parietooccipital and posterior lobes4). It is not unusual that these abnormal findings can be appeared in the cerebellum, whereas they are less common in the basal ganglia and brainstem5). PRES is often associated with many causes, such as immunosuppression, hypertension, eclampsia, and autoimmune disease4). Systemic lupus erythematosus is one of the established causes of PRES and is associated with 0.7–1.4% of PRES cases6)–8). However, only two cases of PRES with SJS have been reported9)10). Therefore, the detailed clinical course and features of these syndromes remain to be elucidated. In this report, we present the case of a patient with atypical PRES in the basal ganglia, thalamus, brainstem, and cerebellum with CNS involvement in SJS.
A 71-year-old female patient was admitted to our hospital for unsteadiness and weakness in her left lower extremity for three days. The patient’s medical history included hypertension, which was well controlled by nifedipine, betaxolol, and azilsartan; SJS, which was diagnosed ten years ago based on the results of 99mTc-pertechnetate salivary gland scintigraphy; and the presence of anti-Ro/SSA antibodies. The patient did not receive immunosuppressants for the treatment of SJS. She had a family history of hypertension affecting her mother and two siblings. On arrival at the hospital, the patient was alert and conscious. She had severe hypertension (197/108 mmHg), sinus tachycardia with a rate of 97 beats per minute without fever. Neurological findings showed muscle weakness in both the iliopsoas and biceps femoris muscles (manual muscle test scores right/left of 4/4). Moreover, the left Hoffmann and Tromner reflexes were positive, and truncal ataxia was observed. Laboratory tests revealed elevated serum levels of creatinine (1.13 mg/dl), C-reactive protein (0.451 mg/dl), rheumatoid factor (29.0 IU/ml), and IgG (6675 mg/dl). No abnormal blood cell findings or liver dysfunction was observed. Autoantibody levels, including antinuclear antibodies (10,240-fold), anti-Ro/SSA autoantibodies (128-fold), and anti-Ro/SSB autoantibodies (64-fold) were also elevated. Other autoantibodies, including anti-double-stranded DNA antibody, anti-cyclic-citrullinated peptide antibody, and anti-aquaporin 4 antibody were not present. A blood gas analysis revealed a normal anion gap and the urine drug screening was negative. The cerebrospinal fluid (CSF) test findings showed elevated levels of total protein (137.6 mg/dl), IgG index (1.06), anti-SSA antibody (1,040 U/ml), anti-SSB antibody (259 U/ml), interleukin-6 (IL-6) (28.4 pg/ml), and positive oligoclonal IgG bands in contrast to normal cell count and myelin basic protein levels. Whole-body CT did not reveal any malignancies. Brain MRI demonstrated small lateral ventricles and narrowing of the sulci. Moreover, there were diffuse symmetrical hyperintensities of fluid-attenuated inversion recovery (FLAIR) and an apparent diffusion coefficient map in the bilateral cerebral white matter, basal ganglia, thalamus, brainstem, cerebellum, and right subcortical temporal lobe (Fig. 1A–F). These findings were presumed to be due to diffuse swelling of the brain parenchyma. The patient was started on antihypertensive treatment, which resulted in partial improvement in brain MRI (Fig. 1G–I). Analysis of the patient’s CSF indicated a high IgG index (1.07) on day 16, when the patient was treated using intravenous methylprednisolone pulse 1 g/day for 3 days, followed by oral prednisone 1 mg/kg. On day 32, brain MRI revealed marked improvement of diffuse swelling of the brain parenchyma and the hyperintense signals in the bilateral basal ganglia, brainstem, thalamus, and cerebellum (Fig. 1J–L). Abnormal intensities in the bilateral cerebral white matter remained, suggesting chronic cerebral ischemia. In addition, CSF analysis on day 46 revealed an overall improvement in total protein (32.9 mg/dl), the IgG index (0.82), anti-SSA antibody (77.4 U/ml), anti-SSB antibody (27.1 U/ml), and anti-IL-6 levels (1.51 pg/ml). Although mild truncal ataxia persisted, lower-extremity muscle strength improved, and the Hoffmann and Tromner reflexes on the left side were negative.
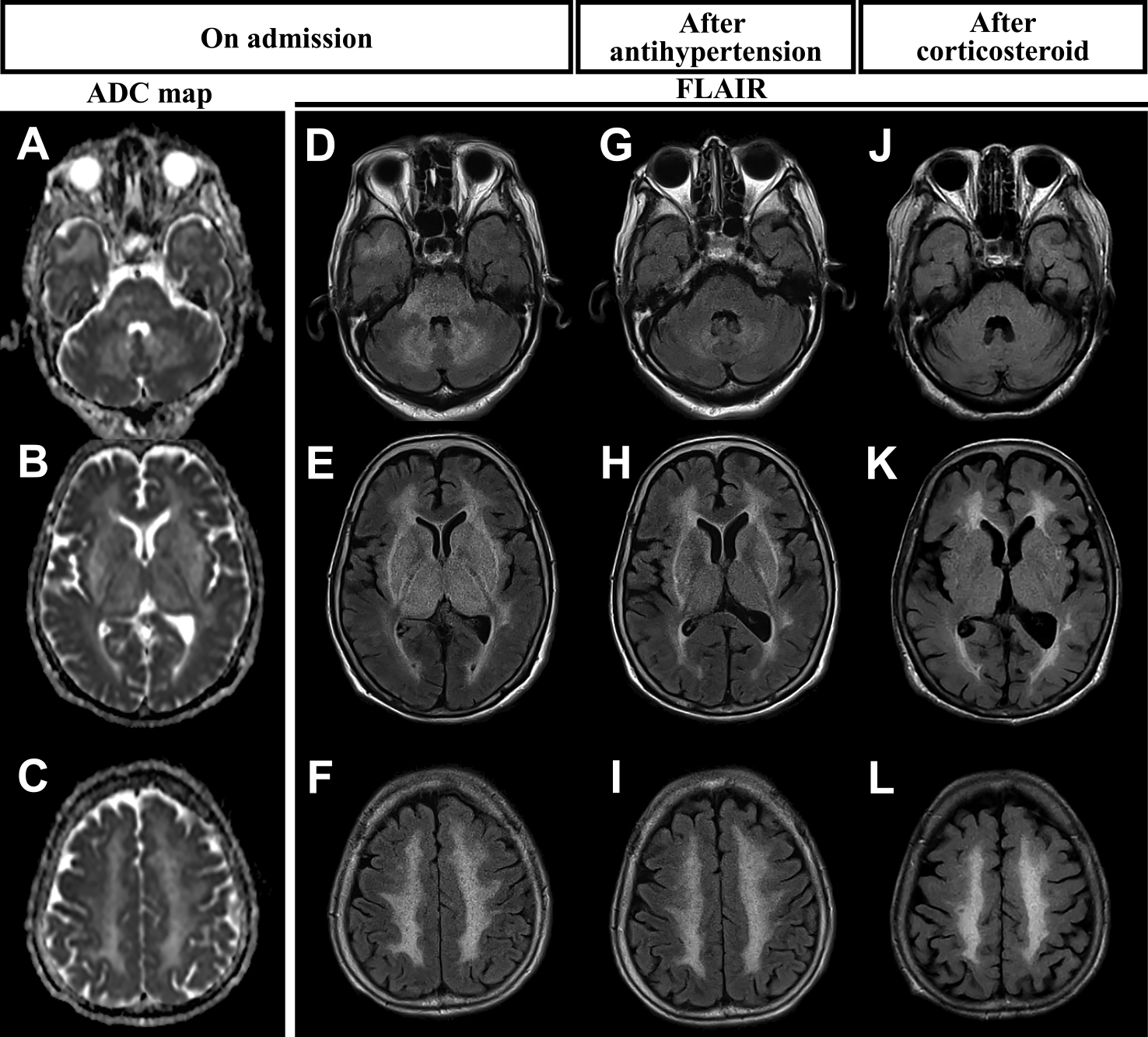
ADC map (A–C) and FLAIR images (D–F) of the patient on admission (day 2), FLAIR images after antihypertensive therapy (day 14) (G–I), and after corticosteroid therapy (day 68) (J–L) are shown.
We report the case of a patient presenting with diffuse bilateral symmetrical PRES, for which the suspected causes were hypertension and CNS involvement in SJS. For the patient’s treatment, the management of blood pressure in addition to corticosteroid therapy improved the neurological symptoms and abnormal MR images.
Primary SJS often causes complications of CNS, including cerebral vasculitis and encephalitis1)–3). However, an accurate diagnosis of the CNS’s involvement in SJS is difficult owing to the lack of diagnostic criteria and consensus3). CNS involvement in SJS is strongly suggested when laboratory findings of intrathecal IgG synthesis, such as raised IgG index and one or more oligoclonal bands in the CSF, are demonstrated2)3)9). Furthermore, several reports indicate that anti-SSA-autoantibodies in CSF can lead to CNS involvement in SJS. In fact, patients with anti-SSA-autoantibodies in the CSF presented with larger brain lesions and more severe CNS complications than those without1). Megevan et al. reported that all three patients with CNS involvement in SJS showed both intrathecal IgG synthesis and anti-SSA-autoantibodies in the CSF11). In addition, as Tetsuka et al. suggested, anti-SSA-autoantibodies in the CSF could induce cerebellar degeneration12). In the present case, the patient was diagnosed with primary SJS approximately 10 years before our study, and intrathecal IgG synthesis was demonstrated by an elevated IgG index and more oligoclonal bands in the CSF. In addition, anti-SSA autoantibodies were present in the CSF. These findings strongly suggest that the patient developed CNS involvement in SJS. Therefore, we concluded that her CNS symptoms were due to involvement in SJS.
In this case, the patient presented with reversible bilateral symmetrical hyperintensities in the basal ganglia, thalamus, brainstem, and cerebellum and reversible hyperintensity in the right subcortical temporal lobe in FLAIR images compatible with PRES. Although PRES typically shows reversible bilateral vasogenic edema in the parietooccipital and posterior lobes4), previous studies demonstrated that vasogenic edema also appears in the cerebellum (12.5–30%), basal ganglia (4.2–22%), brainstem (4.2–17.7%), and thalamus (4–8.8%)5)13)–15). While the exact correlation between the location of the edema and the clinical course of PRES is still under investigation, there are several studies implying some relationships in limited conditions. For example, Fugate et al. reported that cerebellar involvement in PRES was significantly more frequent in patients with autoimmune diseases16) and Moon et al. showed that hypertension influences the involvement of the brainstem in PRES17). In our case, the patient was diagnosed with PRES with complicated autoimmune disease and hypertension, which is consistent with previous reports.
Although there is no specific treatment for PRES, it is known that the management of blood pressure and seizure are important. When there are other causes, such as immunosuppression and autoimmune disease, treatment for each cause is also essential. Fujieda et al. recommended, for PRES with SLE, that if 7-day treatments for hypertension and convulsion does not improve neurological manifestations, immunosuppressive treatment should be performed18). In our case, we first attempted to manage the patient’s blood pressure, resulting in a partial improvement in the MRI. Subsequently, corticosteroid therapy showed almost complete improvement in the MRI. These findings suggest that the combination of blood pressure management and immunosuppression therapy may not only be effective for PRES with SLE but also for SJS.
The precise pathophysiologic mechanisms behind PRES have yet to be fully elucidated and remain controversial19). Two major theories have been proposed.
One theory proposes that rapid rises in blood pressure eventually overcome the autoregulatory capabilities of the cerebral vasculature, causing vascular leakage and resultant vasogenic edema4)6)20). Impaired cerebral autoregulation is seen in the cases of acute rises in blood pressure, such as in cases of eclampsia and autonomic dysfunction4)21); hence, this is the possible pathogenesis of PRES developing in patients with autonomic dysfunction in GBS21)22). Similarly, many studies have reported evidence of autonomic dysfunction in primary SJS23), which may contribute to a wide range of symptoms, including the dysregulation of blood pressure. Accordingly, the acute hypertension in our case may also be explainable by primary SJS-related autonomic dysfunction, which in turn may have caused PRES.
The other theory is that endothelial dysfunction is the primary culprit, which may be caused by cytokines released from activated lymphocytes and monocytes in immune disorders. These cytokines can activate endothelial cells to secrete vasoactive factors, increase vascular permeability, and lead to interstitial brain edema4). A patient with SLE with PRES showed significant elevation of serum IL-6 compared to both a patient with SLE without PRES and healthy controls24). Therefore, it is reasonable to hypothesize that elevation of both serum and CSF IL-6 levels contributes to pathogenesis of PRES in addition to hypertension.
Corticosteroid therapy is a treatment option for CNS involvement in SJS2), and its effects may be judged by the IL-6 level in the CSF. In fact, two cases of meningitis in SJS revealed that corticosteroid therapy specifically improved IL-6 levels in the CSF25)26). Supported by previous reports, we can reasonably conclude that for our patient, the IL-6 level in the CSF was improved by corticosteroid therapy.
In addition, anti-SSA-autoantibody levels in the CSF improved. Although little has been done to examine whether anti-SSA-autoantibodies play a key role as a marker for treatment, our case implies that anti-SSA-autoantibodies, as well as IL-6, in CSF might be prospective biomarkers for CNS involvement in SJS.
Our case presented with central-variant PRES associated with CNS involvement in SJS. Management of blood pressure and corticosteroid therapy improved the IgG index, IL-6 levels, and anti-SSA-autoantibody levels in the CSF and neurological symptoms. This report provides a useful clue for the clinical diagnosis and appropriate treatment for PRES with SJS.
We would like to thank Editage (www.editage.com) for English language editing.
※The authors declare there is no conflict of interest relevant to this article.