2014 Volume 39 Issue 2 Pages 101-112
2014 Volume 39 Issue 2 Pages 101-112
Stem cells routinely maintain the main epidermal components, i.e. the interfollicular epidermis, hair follicles, and sweat glands. Human sweat glands present throughout the body are glandular exocrine organs that mainly play a role in thermoregulation by sweating. Emerging evidence points to the presence of stem cells in sweat glands, but it remains unclear whether such stem cells exist in human sweat glands. Here, we attempted to gather evidence for stem cells in human sweat glands, which would be characterized by self-renewal ability and multipotency. First, we explored human sweat gland cells for expression of stem cell markers. CD29 and Notch, epidermal stem cell markers, were found to reside among α-smooth muscle actin-positive myoepithelial cells in human sweat glands. Next, sweat gland myoepithelial cells were isolated from human skin as a CD29hiCD49f hi subpopulation. The myoepithelial cell-enriched CD29hiCD49f hi subpopulation possessed the ability to differentiate into sweat gland luminal cells in sphere-forming assays. Furthermore, CD29hiCD49f hi subpopulation-derived sphere-forming cells exhibited long-term proliferative potential upon multiple passaging, indicating that the CD29hiCD49f hi myoepithelial subpopulation includes stem cells with self-renewal ability. These findings provide evidence that human sweat gland myoepithelial cells contain stem cells that possess both self-renewal ability and multipotency to differentiate into sweat glands.
The human epidermis mainly consists of three components: the interfollicular epidermis, hair follicles, and sweat glands. These components are maintained by stem cells characterized by self-renewal ability and multipotency. In the human interfollicular epidermis, clusters of stem cells have been identified in the basal layer of keratinocytes, based on their long-term self-renewal ability in culture. These cells express a range of markers, including high levels of integrin β1 (CD29), transmembrane proteoglycan MCSP, and EGFR antagonist LRIG1 (Jensen and Watt, 2006; Jones and Watt, 1993; Legg et al., 2003). In human hair follicles, the best-characterized stem cell population resides in a region known as the bulge. Human hair follicle stem cells, which express keratin 15 and CD200, regenerate the hair follicles, as evidenced by engraftment experiments (Lyle et al., 1998; Ohyama et al., 2006; Toyoshima et al., 2012). In contrast, much less is known about the stem cells in human sweat glands. Intriguingly, Lu et al. (2012) recently reported that mouse sweat gland myoepithelial cells can generate sweat glands in vivo. It is therefore conceivable that human sweat glands also contain a source of stem cells, analogous to those observed in mice.
Mammalian sweat glands are single tubes consisting of functionally distinct ductal and secretory portions (Fig. 1A). Sweat ducts are composed of a helical epidermal duct, straight dermal duct, and short coiled extension of the dermal duct that continues into the coiled secretory portion. The secretory portion, which is primed to respond to stimuli from sympathetic nerves, includes secretory luminal cells and encompassing myoepithelial cells (Saga, 2002). Mammalian sweat gland germs emerge from the basal layer of the epidermis and elongate downward into the dermis where they form glandular globules. The morphogenesis of sweat glands in human skin begins at 12 weeks of gestation, and is essentially completed by 22 weeks (Hashimoto et al., 1965). Because human sweat glands mainly function for the maintenance of body temperature by sweating, they are distributed throughout the body (Sato et al., 1989).

Expression patterns of sweat gland markers in human sweat glands. (A) (upper left) Diagram of the sweat gland structure in human skin; (upper center) diagram of a human skin cross-section; (lower center) diagram of the cell arrangement in a human sweat gland secretory portion consisting of luminal and myoepithelial cells surrounded by basement membrane; (upper right) diagram of the cell arrangement in a human sweat gland ductal portion consisting of luminal and basal cells surrounded by basement membrane. (B) Expressions of K8, αSMA, S100P, and S100A2 in human skin. K8 and αSMA are localized in luminal and myoepithelial cells, respectively, in the secretory portions of human sweat glands. S100P and S100A2 are expressed in luminal and basal cells, respectively, in the ductal portions of human sweat glands. Boxed areas in the left panels are shown at higher magnification in the right panels. Arrowheads indicate coiled ducts of sweat glands that are negative for K8 and αSMA. Arrows indicate secretory portions of sweat glands that are negative for S100P and S100A2. (C) Double-immunofluorescence detection of K8, αSMA, S100P, and S100A2 in sweat glands. Boxed areas in the upper panels are shown at higher magnification in the lower panels. Nuclei (blue) are counterstained with Hoechst 33342. Scale bars: 50 μm (B, C).
We attempted to isolate sweat gland cells from human skin using a protocol for isolation of mammary gland cells (Shackleton et al., 2006). Mammary glands are exocrine glands that evolved from sweat glands (Oftedal, 2002). While sweat glands include “clear” (serous) and “dark” (mucous) secretory cells and surrounding myoepithelial cells, mammary glands comprise three different cell types: myoepithelial cells and two types of luminal lineage cells (Dontu et al., 2003; Sato et al., 1989). The luminal lineage cells include ductal and alveolar epithelial cells. The former generates the ducts and the latter constitutes the alveolar units that expand during pregnancy. It has been reported that the myoepithelial and luminal cell subpopulations in mammary glands can be isolated based on expression of CD29, a stem cell marker in skin (Jones and Watt, 1993), integrin α6 (CD49f), an epidermal basal keratinocyte marker (Hertle et al., 1991), and CD24, a heat-stable antigen used to enrich neural stem cells (Rietze et al., 2001). The CD24+CD29hi and CD24+CD49f hi subpopulations, which are both comprised of mammary gland myoepithelial cells, show properties of mammary stem cells by possessing multipotency and self-renewal ability (Shackleton et al., 2006; Stingl et al., 2006).
In this study, we show that myoepithelial cell layers in human sweat glands express epidermal stem cell markers. Sweat gland myoepithelial cells isolated as CD29hiCD49f hi cells contain cells possessing self-renewal ability and multipotency. Furthermore, these cells can generate three-dimensional spheres, similar to the case for mammary stem cells, thereby corroborating their nature as sweat gland stem cells.
Frozen human skin tissues were obtained with informed consent from ILSbio (Chestertown, MD, USA). Fresh human skin tissues were obtained with informed consent from Osaka University Hospital (Osaka, Japan) and Biopredic International (Rennes, France). Experiments using human skin were approved by the Ethics Committee of Osaka University.
AntibodiesAntibodies for immunohistochemical and immunofluorescence staining were as follows: anti-Keratin 8 (K8) (1:100, Progen, Heidelberg, Germany), anti-α-smooth muscle actin (αSMA) (1:100, Abcam, Cambridge, MA), anti-S100P (1:100, Novus Biologicals, Littleton, CO), anti-S100A2 (1:100, Novus Biologicals), anti-Notch1 (1:100, Cell Signaling, Beverly, MA), anti-p63 (1:100, Santa Cruz, California, USA), anti-Aquaporin 5 (AQP5) (1:100, in house), anti-CD29 (1:100, Abcam), and anti-CD49f (1:100, Millipore, Milford, MA), anti-ATP1a1 (1:100, Abcam), horseradish peroxidase (HRP)-conjugated sheep anti-mouse (1:500, Amersham, San Francisco, CA), Envision+System/HRP rabbit (Dako Cytomation, Glostrup, Denmark), HRP-conjugated donkey anti-goat (1:400, Santa Cruz), HRP-conjugated goat anti-chicken (1/1000, Abcam), HRP-conjugated goat anti-rat (1:400, American Qualex, San Clemente, CA), and species-specific fluorescent secondary antibodies (Invitrogen, Carlsbad, CA, USA). Antibodies for flow flow cytometry and cell sorting were as follows: Allophycocyanin-conjugated anti-CD29 (1:6, BD Pharmingen, San Diego, CA), Brilliant Violet 421-conjugated anti-CD49f (1:20, Biolegend, San Diego, CA), anti-AQP5 (1:100, in house) and Alexa Fluor 488-conjugated donkey anti-chicken (1:100, Jackson ImmunoResearch Laboratories, West Grove, PA).
Immunohistochemical analysisHuman skin tissues were embedded in OCT compound (Sakura Finetechnical Co., Tokyo, Japan) and frozen in 2-methylbutane chilled by liquid nitrogen. Cryosections were prepared, fixed in 4% formaldehyde in phosphate-buffered saline (PBS), cold methanol, or cold acetone, and blocked with 1% goat serum (DAKO, Carpinteria, CA, USA) in PBS, followed by incubation with primary antibodies at 4°C overnight. After three washes with PBS, the sections were treated with horseradish peroxidase-conjugated secondary antibodies, followed by color development using 3,3-diaminobenzidine. After counterstaining with hematoxylin, the sections were examined under an Eclipse E800 microscope (Nikon, Melville, NY). For double-immunofluorescence staining, sections were prepared, fixed in 4% formaldehyde in PBS, cold methanol, or cold acetone, and incubated with primary antibodies at 4°C overnight. After washing in PBS, the sections were treated with secondary antibodies for 1 h, washed with PBS, and stained with Hoechst 33342 (Molecular Probes, Eugene, OR) to visualize the nuclei. All procedures were performed at room temperature. Immunofluorescence images were recorded under an LSM5 confocal microscope (Carl Zeiss, Oberkochen, Germany).
Tissue dissociation and cell preparationThe connective tissues encasing the secretory regions of sweat glands were surgically separated from the epidermal side of each human skin tissue using fine scissors. The resulting dermis was chopped with sharp scissors. The sweat glands in the dermis were visualized using 10 μM neutral red (Sigma, St. Louis, MO, USA) in PBS and picked out with fine forceps (Brayden and Fitzpatrick, 1995). The isolated sweat glands were enzymatically disaggregated for 6 h at 37°C in Complete MammoCult Human Medium (Stem Cell Technologies, Vancouver, BC, Canada) with 300 U/ml collagenase type II (Worthington Biochemicals, Freehold, NJ, USA) and 100 U/ml hyaluronidase (Sigma) on a tube rotator. After centrifugation at 200×g for 10 min, red blood cells were removed by treatment with 0.16% NH4Cl/0.02 mM EDTA. A single-cell suspension was obtained by repeated pipetting for 3 min in prewarmed 0.5% trypsin/1 mM EDTA, followed by repeated pipetting for 1 min in prewarmed 5 mg/ml dispase (Gibco, Paisley, UK)/0.1 mg/ml DNase I (Sigma). The resulting suspension was filtered through a 40-μm mesh (BD Biosciences, San Jose, CA, USA). The surgically separated epidermis was minced and enzymatically disaggregated for 16 h at 4°C in 0.25% trypsin. The resulting suspension was filtered through a 40-μm mesh.
Cell labeling, flow cytometry, and cell sortingImmunolabeling of cells was performed at 4°C for 20 min in PBS containing 2% bovine serum albumin (BSA) (Sigma). After two washes in PBS, the cells were resuspended in PBS containing 2% BSA, filtered through a 40-μm mesh, and kept on ice until sorting. Flow cytometric analysis and cell sorting were performed with a FACSAria (BD Biosciences).
Quantitative RT-PCR (qRT-PCR)Total RNA was isolated using ISOGEN-LS (Nippon Gene, Tokyo, Japan) and Ethachinmate (Wako Pure Chemical Industries, Osaka, Japan). cDNA was synthesized using SuperScript III with random hexamers (Invitrogen). Real-time monitored quantitative PCR was performed using SYBR Green Super Mix and an ABI PRISM 7000 (Applied Biosystems, Foster City, CA, USA). The expression levels of the target genes were normalized by the corresponding expression levels of GAPDH using a standard curve method. Primer sets for qRT-PCR were the following: αSMA (Forward primer: 5'-ATAGAACATGGCATCATCACCAAC-3', Reverse primer: 5'-GGGCAACACGAAGCTCATTGTA-3'), Keratin 18 (K18) (Forward primer: 5'-CCCTGCTGAACATCAAGGTCAA-3', Reverse primer: 5'-GCTGTCCAAGGCATCACCAA-3'), S100A2 (Forward primer: 5'-GCCAAGAGGGCGACAAGTT-3', Reverse primer: 5'-AGGAAAACAGCATACTCCTGGA-3'), S100P (Forward primer: 5'-AAGGATGCCGTGGATAAATTGC-3', Reverse primer: 5'-ACACGATGAACTCACTGAAGTC-3'), and GAPDH (Forward primer: 5'-GCACCGTCAAGGCTGAGAAC-3', Reverse primer: 5'-TGGTGAAGACGCCAGTGGA-3'), produced by Custom Primer Greiner Bio-One Co., Japan.
ImmunocytochemistryCell suspensions were fixed in 4% formaldehyde in PBS, and placed on Matsunami adhesive silane-coated slides (Matsunami Glass, Osaka, Japan). After incubation for 8 h at 37°C, the cells were incubated with primary antibodies, followed by incubation with secondary antibodies. Nuclei were visualized with Hoechst 33342. Fluorescence images were recorded under an LSM 5 PASCAL confocal microscope (Carl Zeiss).
In vitro sphere culturesSuspension sphere cultures were performed according to Spike et al. (2012) with slight modification. Freshly sorted cells were plated on ultralow-adherence plates (Corning, Corning, NY) at 1000 cells/cm2 in sphere culture medium (Complete MammoCult Human Medium containing 0.5 μg/ml hydrocortisone 21-hemisuccinate (Sigma), 10 ng/ml recombinant human epidermal growth factor (PeproTech, Rocky Hill, NJ), 10 ng/ml recombinant human basic fibroblast growth factor (Stem Cell Technologies), 4 μg/ml heparin (Stem Cell Technologies), and 100 μg/ml penicillin/streptomycin (Gibco)) with or without 2% Matrigel (growth factor-reduced; BD Biosciences). For long-term serial passages, spheres grown on plates as described above were recovered from Matrigel using Cell Recovery Solution (BD Biosciences), and then treated with trypsin followed by dispase. Dissociated cells were collected by passing through a 40-μm mesh prior to plating on ultra-low adherence plates at 1000 cells/cm2 in sphere culture medium containing 2% Matrigel. For clonal sphere cultures, freshly sorted single cells were seeded into separate wells of 96-well ultralow-adherence plates at one cell/well in sphere culture medium with 2% Matrigel.
Sweat glands mainly consist of secretory and ductal portions, the latter of which comprises epidermal, straight, and coiled ducts. The secretory portion consists of secretory luminal cells and encompassing myoepithelial cells, while the ductal portion consists of luminal cells and basal cells (Fig. 1A). We confirmed the localizations of these cells by immunohistology using selectively expressed marker proteins. Secretory luminal cells were characterized by expression of K8, whereas myoepithelial cells were identified by expression of αSMA (Fig. 1B) (Moll and Moll, 1992; Schon et al., 1999). Luminal and basal cells in the ductal portions were characterized by expressions of S100P and S100A2, respectively (Fig. 1B) (Zhu et al., 2013). Selective expressions of K8 and αSMA in luminal and myoepithelial cells in the secretory portion was further confirmed by double-immunofluorescence detection (Fig. 1C). Expressions of S100P and S100A2, markers for duct cells, were hardly detected in K8- and αSMA-positive secretory portions (Fig. 1C).
To investigate the stem cell properties of human sweat gland cells, we examined whether human sweat glands contain cells expressing epidermal stem cell markers. CD29 and Notch are cell surface receptors expressed in stem cells that were shown to be involved in niche recognition through cell-extracellular matrix and cell-cell interactions, respectively (Campos et al., 2006). The expression levels of CD29 and Notch were more pronounced in sweat glands than in the epidermis (Fig. 2A). Double-immunofluorescence staining demonstrated that CD29 and Notch were expressed in the secretory portion and coexpressed with αSMA, but not K8 (Fig. 2B). p63 is another epidermal stem cell marker and is induced by Notch signaling. Its cross-talk with Notch is involved in the balance between keratinocyte self-renewal and differentiation (Nguyen et al., 2006). p63 was strongly expressed in the epidermis and moderately expressed in sweat glands (Fig. 2A). Double-immunofluorescence staining demonstrated that the p63 expression levels were diminished in the αSMA- and K8-positive secretory portions, compared with the αSMA- and K8-negative ductal portions (Fig. 2C). These findings indicate that cells showing positive staining for epidermal stem cell markers reside among the αSMA-positive myoepithelial cells of human sweat glands, with the exception of cells positive for p63.

Expression patterns of epidermal stem cell markers in human sweat glands. (A) Expression patterns of CD29, Notch, and p63 in human skin. Boxed areas in the left panels are shown at higher magnification in the right panels. (B) Colocalization of epidermal stem cell markers (CD29 and Notch) with sweat gland cell markers (αSMA and K8) in human sweat glands. Boxed areas in the left panels are magnified in the right panels. Arrowheads indicate colocalization of αSMA and epidermal stem cell markers (Notch and CD29). (C) Colocalization of p63 and sweat gland cell markers (αSMA and K8) in human sweat glands. Boxed areas in the left panels are magnified in the right panels. p63 is expressed in K8- and αSMA-negative cells (arrowheads). Nuclei (blue) are counterstained with Hoechst 33342 (B, C). Scale bars: 50 μm (A–C).
To verify the stemness of the myoepithelial cells in human sweat glands, we first established protocols for isolation of sweat gland myoepithelial cells from human skin tissue. Because of the anatomical similarities between sweat glands and mammary glands, we employed the cell surface markers CD29 and CD49f used for isolation of mammary cells (Dontu et al., 2003; Sato et al., 1989; Shackleton et al., 2006; Stingl et al., 2006), and investigated their expression patterns in sweat glands. As shown in Fig. 2A, CD29 was expressed in sweat glands. Similar to mammary glands, CD49f was strongly expressed in sweat glands (Fig. 3A). We then examined their expression patterns with the expression pattern of a sweat gland cell surface marker, AQP5, which was almost exclusively detected in sweat glands (Fig. 3A). Double-immunofluorescence staining showed that CD29 and CD49f were strongly expressed in the basal regions of sweat glands, whereas AQP5 was expressed in the luminal regions (Fig. 3B). Furthermore, CD29 was strongly expressed in the secretory portions expressing K8 and αSMA and diminished in the ductal portions expressing S100P and S100A2 (Fig. 2B, Fig. 3C). CD49f was expressed in the secretory portions, and was also detected to lesser extents in the ductal portions (Fig. 3D). AQP5 was specifically expressed in the secretory portions, but not in the ductal portions (Fig. 3E). These findings suggest that CD29 and CD49f are useful cell surface markers for the isolation of sweat gland myoepithelial cells.

Expression patterns of cell surface markers for isolation of human sweat gland cells. (A) Expression patterns of CD49f and AQP5 in human sweat glands. Boxed areas in the left panels are shown at higher magnification in the right panels. (B) Double-immunofluorescence detection of CD29, CD49f, and AQP5 in sweat glands. (C) Colocalization of CD29 and sweat gland duct markers (S100A2 and S100P) in human sweat glands. (D) Colocalization of CD49f and sweat gland cell markers (αSMA, K8, S100A2, and S100P) in human sweat glands. Arrowheads indicate colocalization of CD49f with αSMA. (E) Colocalization of AQP5 and sweat gland cell markers (αSMA, K8, S100A2, and S100P) in human sweat glands. Arrowheads indicate colocalization of AQP5 with K8. (F) Expression patterns of CD49f and AQP5 in the human epidermis. (G) Expression patterns of CD29, CD49f, and AQP5 in human hair follicles. (H) Schematic representation of the experimental design for isolation of sweat gland cells and epidermal basal keratinocytes. The dermis was separated from the epidermis using surgical scissors. The sweat glands in the dermis were visualized using neutral red and collected with fine forceps. Single-cell suspensions were prepared from the resulting sweat glands and epidermis, respectively, by enzymatic disaggregation as indicated. Sweat gland cells and basal keratinocytes were isolated from single-cell suspensions by cell sorting. (B, G) Boxed areas in the upper panels are shown at higher magnification in the lower panels. (C–F) Boxed areas in the left panels are shown at higher magnification in the right panels. Nuclei (blue) are counterstained with Hoechst 33342 (B–E). Scale bars: 50 μm (A–G).
Based on these observations, we sought to isolate sweat gland myoepithelial cells from human skin by flow cytometry. Since CD29 and CD49f were also expressed in the epidermis and hair follicles of human skin, sweat gland cells cannot be directly isolated from skin using these cell surface markers (Fig. 3F, G). To prevent the inclusion of epidermal keratinocytes and hair follicle cells, we microsurgically collected individual sweat gland organs from human skin tissue after staining with neutral red, a dye that distinctively stains sweat glands (Fig. 3H). The collected organs were disaggregated into single-cell suspensions by a three-step treatment with collagenase/hyaluronidase, trypsin/EDTA, and dispase, and subjected to flow cytometry.
Using a combination of CD29, CD49f, and AQP5, four distinct subpopulations were isolated: P1, CD29hiCD49f hi; P2, CD29midCD49f midAQP5–; P3, CD29midCD49f midAQP5+; and P4, CD29loCD49f lo (Fig. 4A). We examined the expressions of the sweat gland marker genes in these subpopulations by qRT-PCR (Fig. 4B). The epidermal CD29midCD49f hi subpopulation was also collected as a control for epidermal basal keratinocytes. The expression of αSMA, a myoepithelial cell marker, was restricted to P1 (CD29hiCD49f hi), while the expression of K18, a secretory luminal cell marker, was preferentially detected in P3 (CD29midCD49f midAQP5+). S100A2, a basal duct cell marker, was highly expressed in P2 (CD29midCD49f midAQP5–), while the expression of S100P, a luminal duct cell marker, was preferentially detected in P4 (CD29loCD49f lo). These results indicated that myoepithelial cells and secretory luminal cells were enriched in the CD29hiCD49f hi and CD29midCD49f midAQP5+ subpopulations, respectively. In support of these findings, immunohistochemistry revealed that the P1 (CD29hiCD49f hi) and P3 (CD29midCD49f midAQP5+) subpopulations were enriched with αSMA- and K8-positive cells, respectively (Fig. 4C). Quantification of the αSMA- and K8-positive cells revealed that 95% of the P1 (CD29hiCD49f hi) subpopulation was αSMA-positive, while 90% of the P3 (CD29midCD49f midAQP5+) subpopulation was K8-positive (Fig. 4D), confirming that the P1 (CD29hiCD49f hi) and P3 (CD29midCD49f midAQP5+) subpopulations represented myoepithelial and luminal cells, respectively, in sweat glands. These findings indicate that myoepithelial cells in human sweat glands can be isolated as a CD29hiCD49f hi subpopulation.

Myoepithelial cells in human sweat glands are enriched in the CD29hiCD49f hi subpopulation. (A) Flow cytometric analysis, gating, and sorting of dissociated sweat gland cells from human skin. Cells were analyzed for CD29, CD49f, and AQP5 expressions. The following gates were used to define the four subpopulations: P1, CD29hiCD49f hi; P2, CD29midCD49f midAQP5–; P3, CD29midCD49f midAQP5+; and P4, CD29loCD49f lo cells. (B) qRT-PCR analysis of the expressions of sweat gland marker genes in the four subpopulations. The expression levels were normalized by the corresponding expression levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (C) The CD29hiCD49f hi and CD29midCD49f midAQP5+ subpopulations were plated on MAS-coated slides immediately after sorting, and stained for αSMA and K8. The lower panels show high-magnification photomicrographs of the upper panels. Nuclei (blue) were counterstained with Hoechst 33342. (D) Quantification of αSMA- and K8-positive cells in the CD29hiCD49f hi and CD29midCD49f midAQP5+ subpopulations in five representative fields.
Next, we investigated whether the myoepithelial cells possess the ability to regenerate the secretory portion of sweat glands. The P1 (CD29hiCD49f hi) subpopulation was cultured on ultralow-adherence plates at 1000 cells/cm2 in sphere culture medium with or without 2% Matrigel. The P2 (CD29midCD49f midAQP5–), P3 (CD29midCD49f midAQP5+), and P4 (CD29loCD49f lo) subpopulations and the epidermal CD29midCD49f hi subpopulation were similarly seeded as controls. Similar to mammary gland cells, the myoepithelial cell-enriched P1 (CD29hiCD49f hi) subpopulation generated spheres in the presence, but not the absence, of Matrigel (Fig. 5A, B). Spheres were also generated from the P2 (CD29midCD49f midAQP5–) subpopulation in the presence of Matrigel, but were hardly generated from the P3 (CD29midCD49f midAQP5+), P4 (CD29loCD49f lo), and epidermal CD29midCD49f hi subpopulations irrespective of the presence or absence of Matrigel (Fig. 5A). The P1 (CD29hiCD49f hi) subpopulation-derived spheres exhibited exocrine gland-like hollow structures, in which the markers for luminal (K8) and myoepithelial (αSMA) cells were expressed in the apical and basal cell layers, respectively (Fig. 5C), reminiscent of their expressions in the secretory portion of human sweat glands (Fig. 1C). Furthermore, AQP5 and ATP1a1, sweat gland luminal markers, were expressed in the inner cells of the spheres (Fig. 5D). These findings suggest that human sweat gland myoepithelial cells possess the ability to differentiate into luminal cells and regenerate secretory portions.
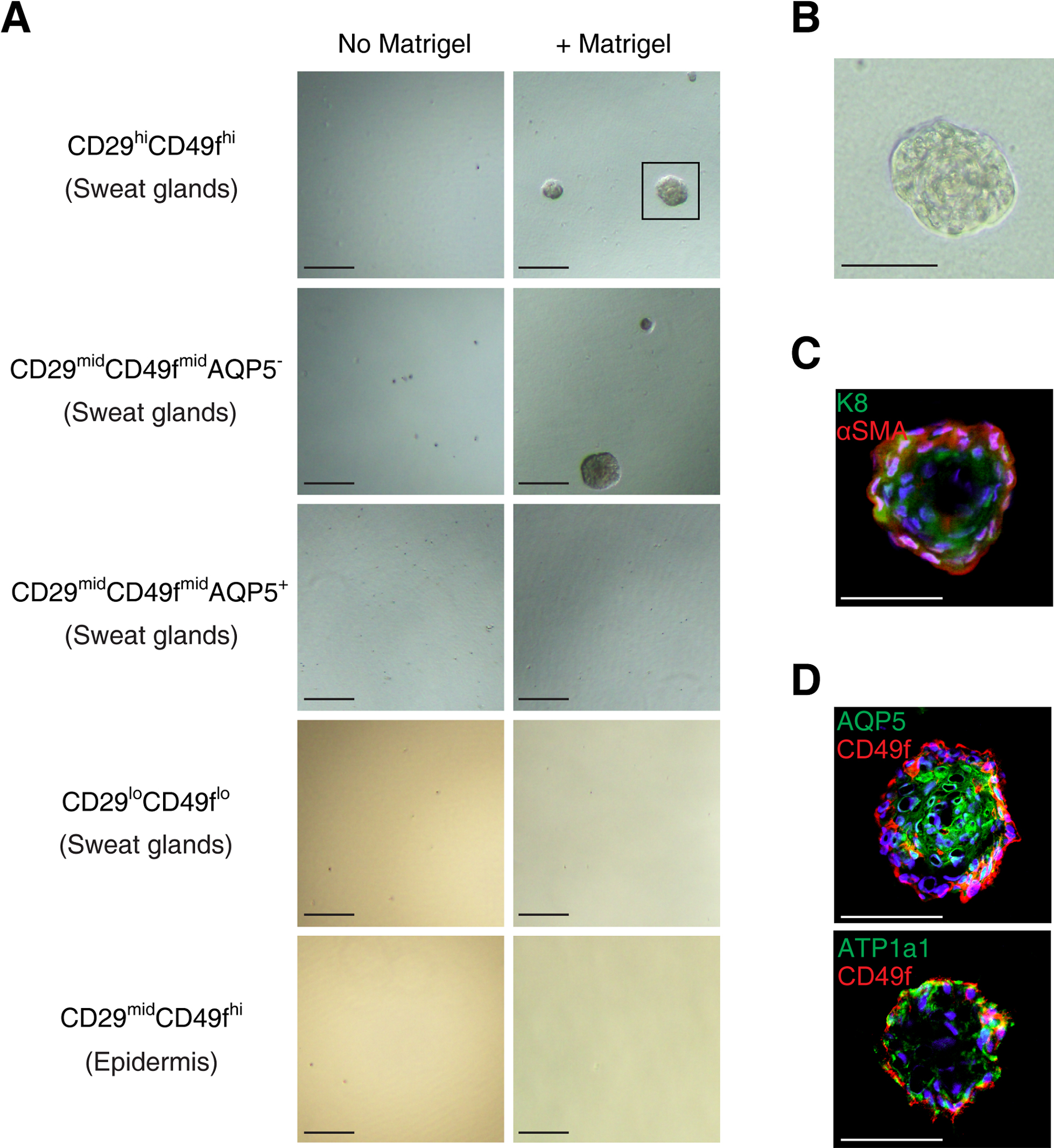
Myoepithelial cells in human sweat glands can differentiate into secretory luminal cells. (A) Morphologies of the CD29hiCD49f hi, CD29midCD49f midAQP5–, CD29midCD49f midAQP5+, and CD29loCD49f lo subpopulations and the epidermal CD29midCD49f hi subpopulation grown under non-adherent conditions in the presence or absence of Matrigel. (B) Enlarged view of the boxed area in (A). (C) Confocal immunofluorescence staining of spheres derived from the CD29hiCD49f hi subpopulation showing the expressions of K8 (green) and αSMA (red) with nuclear counterstaining (Hoechst 33342; blue). Outer cells of the sphere are positive for αSMA, while inner cells are positive for K8. (D) Double immunofluorescence staining of CD49f (red) and sweat gland cell markers (AQP5 and ATP1a1; green) in spheres derived from the CD29hiCD49f hi subpopulation with nuclear counterstaining (blue). Outer cells of the sphere are positive for CD49f, while inner cells are positive for AQP5 and ATP1a1. Scale bars: 100 μm (A); 50 μm (B, C, D).
Stem cells have self-renewal ability that is typically assessed by sphere-forming assays. We addressed whether the CD29hiCD49f hi myoepithelial subpopulation in human sweat glands possesses self-renewal capability. First, to test the long-term proliferative potential of sweat gland myoepithelial cells, we evaluated the secondary and tertiary sphere-forming abilities of the P1 (CD29hiCD49f hi) subpopulation (Spike et al., 2012). The P1 (CD29hiCD49f hi)-derived spheres were dissociated and seeded on ultralow-adherence plates at 1000 cells/cm2 in sphere culture medium containing 2% Matrigel. The sphere-derived cells were capable of generating secondary and tertiary spheres (Fig. 6A). To verify the clonal growth of the myoepithelial cells, we examined whether single myoepithelial cells were capable of generating spheres. P1 (CD29hiCD49f hi) cells were cultured at clonal density on 96-well ultralow-adherence plates for 14 days in sphere culture medium containing 2% Matrigel. Single cells from the P1 (CD29hiCD49f hi) subpopulation formed spheres (sphere-forming efficiency = 2.3%), whereas single cells from the P3 (CD29midCD49f midAQP5+) subpopulation did not (Fig. 6B, C). These findings indicate that the myoepithelial CD29hiCD49f hi subpopulation contains stem cells with high proliferative potential.

Sphere-forming ability of myoepithelial cells in human sweat glands. (A) Morphologies of cell spheres generated from CD29hiCD49f hi cells. Secondary and tertiary spheres were generated from enzymatically disaggregated cell suspensions derived from primary and secondary spheres, respectively. (B) Clonal expansion of single cells within the CD29hiCD49f hi myoepithelial subpopulation yielded spheres. The right panel shows a high-magnification view of the left panel micrograph. (C) Single cells from the CD29hiCD49f hi subpopulation were seeded in 96-well plates at clonal density. The sphere-forming efficiency was assessed as the percentage of cells that formed a sphere. Scale bars: 100 μm (A, C).
The existence of human sweat gland stem cells was originally suggested by histological studies on skin wound repair. Lobitz et al. (1954) suggested that “partial” sweat glands, in which the epidermal sweat duct unit was removed after superficial epidermal injury, regenerated the epidermal duct of sweat glands (Lobitz et al., 1954). The ability of sweat glands to regenerate the straight duct was supported by the observation that the residues of human sweat glands following dermal trauma regenerated the dermal duct of sweat glands (Lobitz et al., 1956). Furthermore, Biedermann et al. (2010) suggested that human sweat glands contain multipotent cells. They demonstrated that cells derived from microsurgically isolated sweat gland organs were capable of reconstituting a multilayered epidermis-like structure in vitro (Biedermann et al., 2010). Although these studies suggested that sweat glands contain multipotent cells, it remains elusive which compartments of sweat glands contribute to the regeneration of the ductal portion and possess self-renewal ability. In this study, we identified human sweat gland stem cells residing among the myoepithelial cells of the secretory portion. The myoepithelial cells of human sweat glands exhibited high expressions of the stem cell markers CD29 and Notch, and were isolated as a CD29hiCD49f hi subpopulation. The freshly isolated cells in the myoepithelial subpopulation exhibited multipotency, as evidenced by the generation of sweat gland-like spheres composed of both cell types expressing secretory luminal and myoepithelial cell markers, respectively. Furthermore, the CD29hiCD49f hi myoepithelial subpopulation exhibited self-renewal ability and high proliferative potential in sphere-forming assays. Taken together, our findings indicate that the myoepithelial cell-enriched CD29hiCD49f hi subpopulation possesses the features of stem cells characterized by multipotency and self-renewal ability. However, it remains to be corroborated whether cell types other than myoepithelial cells also contribute to the stem cell characteristics of the CD29hiCD49f hi subpopulation.
In mouse sweat glands, there are two multipotent stem cell populations, i.e., myoepithelial cells and basal duct cells (Lu et al., 2012). The basal duct cells form straight and coiled duct-like structures, while the myoepithelial cells generate a sweat gland-like structure that expresses ATP1a1, when transplanted into mammary fat pads. Consistent with these observations for mouse sweat glands, our results raise the possibility that the basal duct cells of human sweat glands also contain stem cells that are distinct from those among the myoepithelial cells. Thus, the basal duct cells of human sweat glands, isolated as the CD29midCD49f midAQP5– subpopulation, were capable of generating spheres, making it likely that not only the CD29hiCD49f hi myoepithelial cells in the secretory portion, but also the CD29midCD49f midAQP5– cells in the ductal portion contain stem cells harboring multipotency and proliferation ability. Together, these human sweat gland stem cells may contribute to the construction and maintenance of the glandular organization of sweat glands.
Similar to sweat glands, mammary glands are glandular organs consisting of myoepithelial and luminal cells. Several lines of evidence indicate that mammary glands have evolved from sweat glands (Jenness, 1974; Long, 1969; Oftedal, 2002). We found that sweat gland stem cells share many features with mammary stem cells (Shackleton et al., 2006; Spike et al., 2012; Stingl et al., 2006). Similar to mammary stem cells, sweat gland stem cells predominantly reside among myoepithelial cells and are capable of generating exocrine gland-like hollow spheres in vitro. Furthermore, both mammary and sweat gland stem cells require Matrigel, a reconstituted gel composed of basement membrane molecules, to form the spheres in vitro. These similarities between sweat gland and mammary stem cells suggest that the stem cells share common mechanisms operating in exocrine tissue organogenesis. Despite their similarities, there are some differences between sweat gland and mammary stem cells. Mammary stem cells contribute to mammary gland development during multiple cycles of pregnancy, whereas sweat glands hardly undergo periodic cycling (Shackleton et al., 2006). Morimoto and Saga (1995) reported that human sweat gland cells are in a dormant state and detected as label-retaining cells in 5‑bromo-2'-deoxyuridine pulse-chase experiments in vivo (Morimoto and Saga, 1995), suggesting that human sweat gland myoepithelial cells are generally quiescent. Another feature that distinguishes sweat gland and mammary stem cells is their expression patterns of Notch, a cell surface protein that controls the cell fate choice between self-renewal and differentiation. The myoepithelial cells predominantly express Notch in the sweat gland secretory portion, whereas Notch expression in the mammary gland secretory portion is predominantly detected in the luminal cells (Bouras et al., 2008). In mammary stem cells, constitutive Notch activation promotes luminal cell-fate commitment and eventually causes the development of tumors, whereas constitutive inhibition of Notch signaling leads to expansion of mammary stem cells (Bouras et al., 2008). Therefore, the opposing expression pattern of Notch in sweat glands may account, at least in part, for the infrequency of sweat gland tumors. Further comparative studies of sweat gland stem cells and other epithelial stem cells, e.g., hair follicle and mammary stem cells, may lead to better understanding of the mechanisms regulating epithelial morphogenesis and homeostasis of these stem cells.
In conclusion, we have provided evidence that human sweat gland stem cells reside among myoepithelial cells, similar to the case for mammary stem cells. Human sweat gland myoepithelial cells can be isolated as a CD29hiCD49f hi subpopulation. Sweat gland stem cells are normally in a quiescent state in secretory portions, but exhibit high proliferative potential and the ability to regenerate sweat gland-like spheres comprising both luminal and myoepithelial cells in vitro. The identification of human sweat gland stem cells provides new insights for the development of bioengineered glandular organs.
We thank Dr. Kenji Kusumoto (Institute for Protein Research, Osaka University) for his invaluable advice on flow cytometric experiments, and Dr. Fumitaka Fujita (Technical Development Center, Mandom Corporation) for his support and encouragement throughout this study.