2024 年 89 巻 1 号 p. 47-51
2024 年 89 巻 1 号 p. 47-51
Turkey is very rich in terms of spined loaches. The identification of Cobitis species is usually made by morphological and meristic characteristics. It has recently been supported by molecular studies. Although studies on the cytogenetic analysis of European and Asian loaches are sufficient, these studies are not sufficient in Turkey. Banded cytogenetic features of Cobitis biseli specimens in Beyşehir were revealed for the first time in this study. Its diploid chromosome number (2n) is 50, and its karyotype consists of 4 pairs of metacentric, 3 pairs of submetacentric, 3 pairs of subtelocentric, and 15 pairs of acrocentric chromosomes (NF=70). A secondary constriction was observed in the telomeric region of the short arm of the largest metacentric chromosome pair. An active nucleolar organizer region (NOR) by Ag-staining was detected on the same chromosome. Except for one pair, all the chromosomes had a centromeric C-band. It was determined that the cytogenetic characteristics of this species were different from other species studied in Turkey.
Considering the number and diversity of species, Turkey shows the characteristics of a continent on its own. Therefore, it is rich in freshwater fish fauna and endemic species. Species belonging to the Cobitidae family are widely distributed in Asia, Europe, and Africa. It is known that 27 Cobitis Linnaeus, 1,758 species are distributed in Turkey, and 22 are endemic (Freyhof et al. 2018, Çiçek et al. 2020, Eagderi et al. 2022). Cobitis species in Turkey are distributed in Central, Western, and Southeastern Anatolia. Cobitis bilseli, which was first identified in Lake Beyşehir, also lives in Sarıöz and Sarıçay streams in the Lake Beyşehir basin. It is also located in the river flowing from Lake Beyşehir to Lake Suğla (Freyhof et al. 2018, Bayçelebi et al. 2020).
Due to the morphological similarity of different Cobitis species and the presence of sibling species, their taxonomic differentiation is complex, and their systematics still need to be fully resolved. Recent descriptions of new species and the discovery of significant diversity among spiny loaches have been made possible by combining detailed morphological studies and karyological analysis (Schneider et al. 2000, Vasil’eva 2000, Freyhof and Stelbrink 2007). Remarkable hybridization is known to occur between some of the Cobitis species. After this hybridization, a few female-only gynogenetic lineages attracted the attention of researchers (Bohlen and Ráb 2001). The differences in karyotype and chromosome structure between parents have been suggested as the causes of gynogenetic reproduction in these hybrids. Great variability in karyotypes and chromosomal markers of Cobitis species has been observed (Janko et al. 2007).
Chromosome studies have shown that most Cobitis species have a diploid chromosome number (2n) of 50 but have highly diverse karyotypes (Ráb and Slavík 1996, Arai 2011). The chromosome number of C. taenia in Europe is 48. Hybrid forms (triploid, tetraploid) with at least one copy of the genome of diploid C. taenia, C. tanaitica, C. taurica, and C. elongatoides have been identified in Europe (Janko et al. 2007). The chromosomal characteristics of only C. phrygica and C. simplicispina from 27 species in Turkey were determined (Ayata et al. 2018). This study aims to determine the detailed chromosomal characteristics of Beyşehir spined loach, C. bilseli, and to reveal the similarities and differences by comparing them with the previously published results.
Four C. bilseli specimens were collected from Eylik Stream in the Beyşehir district of Konya province. The specimens were collected with the permission of the TR Ministry of Forestry and Water Affairs (Permit No: E-21264211-288.04-3435924). The caught fish specimens were transported live to the laboratory under suitable conditions and kept in a well-ventilated aquarium until the analysis. Karyological analysis was performed according to the method of Bertollo et al. (2015). 0.1% colchicine was injected (1 mL 100 g−1) into each specimen for which chromosome preparations were to be prepared and kept in the aquarium for 50 min. After anaesthetization with 10% benzocaine solution, the cell suspension from the head kidney was kept in 0.075 M KCl solution at 37°C in an incubator for 20 min. Then, fixation steps (methanol : acetic acid, 3 : 1) were repeated at least three times, and at least 10 metaphase slides were prepared from each individual. Some obtained slides were preserved by staining with 10% Giemsa for standard karyotype. Some other slides were applied C-banding (CBG banding) (Sumner 1972), C-banding and stained with 4′,6-diamidino-2-phenylindole (CB-DAPI banding), and Ag-NOR staining (Howell and Black 1980). Well-spread metaphases of each staining were photographed under the microscope and karyotyped. Chromosomes were defined according to Levan et al. (1964). The fundamental number of chromosomal arms (NF) was calculated by considering the number of two-armed and acrocentric chromosomes.
The diploid chromosome number detected in all four specimens caught from Eylik Stream was 2n=50. The karyotype consisted of 4 pairs of metacentric (nos. 1–4), 3 pairs of submetacentric (nos. 5–7), 3 pairs of subtelocentric (nos. 8–10) to 15 pairs of acrocentric (nos. 11–25) chromosomes. The NF was 70. Secondary constriction was observed in the short arm of one of the homologs of autosome pair 1 (Fig. 1A). Heteromorphic sex chromosomes were not detected in males and females. C-bands of C. bilseli with both CBG and CB-DAPI karyotypes are shown in Fig. 1B and C. The C-bands were observed in the centromeric regions of the other bi-armed and acrocentric chromosomes, except for chromosome no. 7. These C-bands were detected slightly in some chromosomes and dark in others. Ag-NOR was detected in a pair of the largest metacentric chromosomes (no. 1) in all samples examined. This Ag-NOR is homozygous and not associated with the heterochromatin region (Fig. 1D).
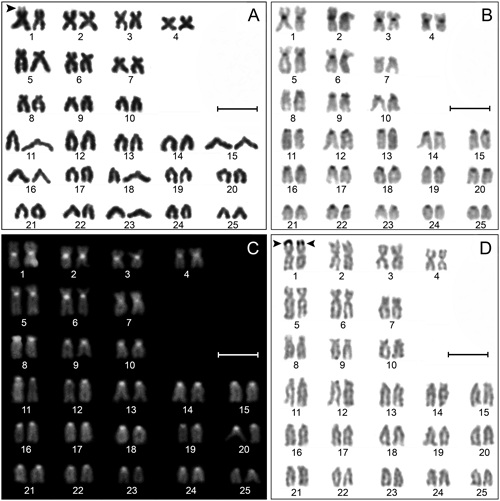
(A) Standard Giemsa staining karyotype, (B) C-banded (CBG) karyotype, (C) C-banded (CB-DAPI) karyotype, and (D) Silver-stained karyotype. Arrows indicates the position of secondary constrictions in (A), and the Ag-NOR in (D). Scale bars=10 µm.
The diploid chromosome number of Cobitis species worldwide and in Turkey is generally 50, except for C. taenia and some hybrid, triploid, and tetraploid forms (Table 1). So far, only two Cobitis species have been studied in Turkey (Ayata et al. 2018). Triploid and tetraploid individuals have not been detected in C. bilseli or other studied species. Triploid and tetraploid female forms have been determined in European countries and Russia (Vasil’ev et al. 1989, Boroń 2003, Janko et al. 2005, Vasil’ev and Vasil’eva 2022). C. taenia (2n=48) and its hybrid females (2n=74) have been detected in Poland and the Czech Republic (Ráb et al. 2000, Boroń 2003, Janko et al. 2007). This hybrid form consists of a haploid set of C. taenia and a diploid set of an unidentified species (Boroń 1999). The number of metacentric, submetacentric, subtelocentric, and acrocentric chromosomes in the chromosome set of different Cobitis species are different (Table 1). In karyological studies, it has been determined that the chromosome morphologies of different species populations are stable. Chromosome morphologies of Czech and Poland populations of C. taenia are similar (Ráb et al. 2000, Boroń 2003, Janko et al. 2007). Likewise, different populations of C. elongatoides in Czechia have similar karyotypes (Ráb et al. 2000). This study determined that all the studied individuals of C. bilseli had the same chromosome morphology. The fact that each Cobitis species has a fixed karyotype formula may contribute to their differentiation. This karyotype stability can also be associated with the gynogenetic reproduction of females (Schneider et al. 2000, Vasil’eva 2000, Freyhof and Stelbrink 2007).
| Species | Locality | 2n | Karyotype | NF | Reference |
|---|---|---|---|---|---|
| C. taenia | Czechia | 48 | 28M−Sm+20St-A | 76 | Ráb et al. (2000) |
| C. taenia | Czechia | 48 | 10M+18Sm+20St-A | 76 | Boroń (2003) |
| C. taenia | Poland | 48 | 12M+18Sm+18St | 78 | Boroń (1999), Boroń (2003) |
| C. taenia | Poland | 48 | 10M+20Sm+18St | 78 | Janko et al. (2007) |
| C. elongatoides | Czechia | 50 | 28M+18Sm+4St-A | 96 | Boroń (2003) |
| C. elongatoides | Czechia | 50 | 30M+16Sm+2St+2A | 96 | Ráb et al. (2000) |
| C. vardarensis | Greece | 50 | 26M+20Sm+4St | 96 | Rábová et al. (2001) |
| C. taurica | Bulgaria, Ukraine | 50 | 10M+30Sm+8St+2A | 90 | Janko et al. (2005) |
| C. pontica | Bulgaria | 50 | 10M+30Sm+8St+2A | 90 | Vasil’eva and Vasil’ev (2006) |
| C. tanaitica | Russia | 50 | 8M+28Sm+14St-A | 86 | Vasil’eva and Vasil’ev (1998), Vasil’ev et al. (2007) |
| C. tanaitica | Russia | 49 | 9M+28Sm+12St-A | 88 | Vasil’eva and Vasil’ev (1998), Vasil’ev et al. (2007) |
| C. lutheri | Russia | 50 | 12M+8Sm+30St-A | 70 | Kim et al. (1999), Vasil’ev and Vasil’eva (2008) |
| C. choii | Russia | 50 | 8M+10Sm+8St+24A | 68 | |
| C. melanoleuca | Russia | 50 | 8M+18Sm+24St-A | 76 | |
| C. calderoni | Portugal | 50 | 6M+14Sm+30A | 70 | Madeira et al. (1992) |
| C. maroccana | Portugal | 50 | 6M+12Sm+32A | 68 | Madeira et al. (1992) |
| C. longicorpus | Korea | 50 | 12M+8Sm+30A | 70 | Kim and Lee (1986) |
| C. koreensis | Korea | 50 | 10M+12Sm+28A | 72 | Kim and Lee (1986) |
| C. tetralineata | — | 50 | 10M+6Sm+34St-A | 66 | Kim et al. (1999) |
| C. rotundicaudata | Korea | 50 | 10M+4Sm+36St-A | 64 | Ueno et al. (1985) |
| C. striata | Korea | 50 | 10M+6Sm+34A | 66 | Kim and Lee (1986) |
| C. takatsuensis | Japan | 50 | 12M+18Sm+20St-A | 80 | Kimizuka et al. (1982) |
| C. linea | Iran | 50 | 4M+40Sm+6St | 100 | Esmaeili et al. (2015) |
| C. phrygica | Türkiye | 50 | 8M+8Sm+34St-A | 66 | Ayata et al. (2018) |
| C. simplicispina | Türkiye | 50 | 16M+16Sm+18St-A | 82 | Ayata et al. (2018) |
| C. bilseli | Türkiye | 50 | 8M+6Sm+6St+30A | 70 | This study |
NF: fundamental number of chromosome arms, M: metacentric, Sm: submetacentric, St: subtelocentric, A: acrocentric.
The chromosome morphology of C. phrygica from Lake Salda in Burdur province (Ayata et al. 2018) is similar to that of C. bilseli regarding metacentric chromosome number. However, the submetacentric chromosome number is less in C. bilseli than in C. phrygica. Although the morphology of subtelocentric chromosomes varies by the researcher, they have been evaluated together in most studies. The number of subtelocentric and acrocentric chromosomes is higher in C. phrygica than in C. bilseli. The morphology of the chromosomes of C. bilseli is quite different from that of C. simplicispina from Denizli (Ayata et al. 2018). This species has more metacentric and submetacentric chromosome numbers than C. bilseli. There is no secondary constriction in any chromosome of C. phrygica and C. simplicispina (Ayata et al. 2018). However, in this study, secondary constrictions associated with NOR were detected in the telomeric region of the short arm of the first chromosome of C. bilseli. Morphologically, it is not easy to distinguish Cobitis species (Freyhof et al. 2018). However, C. bilseli is larger than the species in the C. simplicispina group (C. battalgilae, C. dorademiri, C. joergbohleni, C. phrygica, C. pirii, C. simplicispina, C. sipahilerae, and C. turcica). In addition, males of C. bilseli have one ‘lamina circularis’ compared to males of other species (Freyhof et al. 2018). We think that the standard karyotype characteristics of the Beyşehir spined loach determined in this study are different from those of other studied spiny loaches (C. phrygica and C. simplicispina), and will contribute to morphological discrimination.
The C-band distribution of C. bilseli differs from C. phrygica and C. simplicispina in Turkey. According to the CBG and CB-DAPI bandings of C. bilseli, except for the seventh chromosome, the others have centromeric C-bands. However, except for some chromosomes of C. phrygica and C. simplicispina, other chromosomes have pericentromeric C-bands (Ayata et al. 2018). According to them, some chromosomes of these species have interstitial C-bands on their long arms. However, these bands were not found in C. bilseli. Most of the chromosomes of both diploid (2n=48) individuals and triploid females of C. taenia have centromeric constitutive heterochromatin. There are also dark pericentromeric heterochromatin blocks in some metacentric chromosomes of this species (Boroń 1999). The chromosomes of the two Cobitis species in Greece all have pericentromeric C-bands. In contrast, there are larger and distinct C-positive blocks on the largest metacentric chromosome of C. vardarensis (Rábová et al. 2001) and two pairs of metacentric chromosomes of C. strumicae (Hnátková et al. 2018). As in the Greek species, no pericentromeric distinct C-bands were found in any chromosome of C. bilseli. These results show that the heterochromatin distribution of C. bilseli is different from the other two species studied in Turkey.
The active NOR position in C. bilseli differs from that of diploid male individuals of C. taenia in Poland. Boroń et al. (1999) detected the NORs of C. taenia in two subtelocentric chromosomes. According to them, in Chromomycin A3 application, one of the active NORs is on the short arm of the subtelocentric chromosome. At the same time, the other is on the long arm of another subtelocentric chromosome as a weak signal. The NOR distributions of C. simplicispina and C. phrygica in Anatolia (Ayata et al. 2018) are different from each other and C. bilseli. The NORs are localized on the short arm of two pairs of large metacentric chromosomes in C. simplicispina. In contrast, a NOR is localized on the short arm of one pair of submetacentric chromosomes in C. phrygica. Although the NOR phenotype of C. simplicispina is similar to that of C. bilseli, it is higher in number. There are also some European Cobitis species where the NOR phenotype of C. bilseli is similar or different. The NOR phenotypes of our specimens are similar to those of C. maroccana in the Iberian Peninsula and C. strumicae in Greece. The active NOR of C. maroccana is localized to the short arm of the largest metacentric chromosome pair, as in C. biseli (Madeira et al. 1992), while it was localized in the telomeric region of the short arm of the submetacentric chromosome pair in C. strumicae (Hnátková et al. 2018). However, active NORs of C. vardarensis in Greece were detected on three different metacentric chromosomes (Rábová et al. 2001). Similar NOR phenotypes were detected in C. elongatoides by Ráb et al. (2000). Based on the NOR results; we suggest evaluating the active NOR phenotype in the telomeric region of the short arm of the nearly largest metacentric or submetacentric chromosome as a plesiomorphic character for the genus Cobitis.
As a result, the genus Cobitis is one of the fish groups that show very little morphological variation. Many species are superficially very similar and have the same meristic characteristics. It is known that C. bilseli is different from other Turkish Cobitis species in size and ‘lamina circularis’. In addition, according to the phylogenetic relationship based on the mitochondrial COI barcode region, C. bilseli is located in a different clade than the clade containing C. phrygica and C. simplicispina, which are found in different streams (Freyhof et al. 2018). The distance in this phylogenetic relationship is also supported by the differences in some karyological features determined in this study. Therefore, we suggest that the differentiation of Cobitis species in Turkey should be evaluated together with cytogenetic features.
This article is based on Ahmed Sadeq Jaber Doori’s Ph.D. summarized from a part of his thesis. This study was financed with a grant from the Scientific Research Projects Coordination Board of Selçuk University (Project number: 21211009).