2024 年 89 巻 1 号 p. 29-37
2024 年 89 巻 1 号 p. 29-37
Cashmere quality represents a crucial economic trait in cashmere goats. Investigating methods to enhance cashmere yield and quality is essential for the sustainable development of the cashmere industry. Although melatonin (MT) has been shown to promote cashmere wool growth, the underlying molecular mechanisms, particularly those involving melatonin-mediated long non-coding RNA (lncRNA), remain poorly understood. In this study, cashmere growth-related lncRNA (LncRNA MTC) was used as the starting point. LncRNA MTC overexpression and interference lentivirus vectors were constructed and transfected into Liaoning cashmere goat skin fibroblasts. Results from the cholecystokinin-octapeptide (CCK-8) assay demonstrated that LncRNA MTC overexpression significantly enhanced cell proliferation, while LncRNA MTC interference notably suppressed it. RNA pull-down coupled with mass spectrometry revealed a significant increase in LncRNA MTC’s binding to glutathione S-transferase M1 (GSTM1) in Liaoning cashmere goat skin fibroblasts following melatonin treatment. RNA immunoprecipitation (RIP) technology confirmed that the intracellular GSTM1 protein indeed interacts with LncRNA MTC. CCK-8 results showed that interference with GSTM1 could reverse the promotion of MT on the proliferation of skin fibroblasts of Liaoning cashmere goats. Co-immunoprecipitation (Co-IP) results demonstrated an association between GSTM1 and apoptosis signal-regulating kinase 1 (ASK1) proteins in Liaoning cashmere goat skin fibroblasts. The findings indicate that melatonin-mediated LncRNA MTC, which is related to cashmere growth, binds to the GSTM1 protein. This interaction affects the GSTM1-ASK1 protein complex formation, consequently inhibiting the apoptosis of Liaoning cashmere goat skin fibroblasts.
Liaoning cashmere goat holds the top position in cashmere production in China and serves as a vital genetic conservation resource for the country’s livestock and poultry (Chen 2016). Cashmere is a non-medullary hair fiber generated by secondary hair follicles in the skin of cashmere goats. Increasing the number of secondary hair follicles can enhance cashmere fineness and production. Long non-coding RNA (lncRNA), while lacking protein-coding potential, plays essential roles in processes such as chromosome modification, gene imprinting, regulation of cell proliferation and migration, hair follicle regeneration, and more (Yang et al. 2014, Shang et al. 2019, Chen et al. 2020, He et al. 2021). LncRNA is also crucial for hair follicle development and skin homeostasis maintenance (Wang et al. 2017, Feng et al. 2022). Studies have identified that lncRNAs XLOC_297809 and XLOC_764219 are localized to the substrate and dermis (Nie et al. 2018), and that RP11-766N7.3 is abnormally expressed in dermal papilla cells, playing a key role in regulating Wnt signal transduction (Lin et al. 2014). Research has also found that the relative expression of lncRNA-H19/lncRNA-HOTAIR in the early stages of secondary hair follicle growth in cashmere goats is significantly higher than in catagen and telogen phases, suggesting that lncRNA-H19/lncRNA-HOTAIR may be involved in stimulating hair follicle reconstruction and cashmere fiber formation and growth (Zhu et al. 2018, Jiao et al. 2019).
It has been demonstrated that RNA frequently binds to proteins, functioning as RNA-protein complexes (RNP) (Mitchell and Parker 2014, Yang et al. 2020, Koliński et al. 2022). The direct binding of annexin A2 to lncRNA FOXF1 adjacent noncoding developmental regulatory RNA (Fendrr) contributes to the apoptosis of human pancreatic cells in an acute pancreatitis cell model (Zhao et al. 2018). Lnc-DC is specifically expressed in human dendritic cells (DCs) and targets cytoplasmic STAT3 to promote its phosphorylation. Knocking down lnc-DC can inhibit the differentiation process of DC cells (Wang et al. 2014). NF-κB interaction lncRNA (NKILA) directly targets IκB, preventing IKK from inducing IκB phosphorylation and inhibiting the activation of NF-κB (Liu et al. 2015). The highly coordinated interaction between lncRNA and proteins is crucial for normal cellular physiology, as they regulate the activity of corresponding proteins by stabilizing or accelerating protein complex depolymerization. However, current research on cashmere growth has scarcely involved lncRNA–protein interactions, warranting further exploration of how proteins play a role in regulating cashmere growth-related lncRNA to promote cashmere growth and development.
In the early stage, a lncRNA exhibiting a significantly increased expression level during the process of promoting cashmere growth by 0.2 g L−1 melatonin (MT) was identified through high-throughput sequencing screening and was named LncRNA MTC (Jin et al. 2018). The strategy involves using RNA pull-down mass spectrometry technology to construct a protein database that binds to LncRNA MTC and selecting proteins that bind to LncRNA MTC from it. RNA pull-down is a key technology for studying RNA-protein interactions; the experimental principle involves using known RNA to pull down proteins and identifying protein types by mass spectrometry. Subsequently, RNA immunoprecipitation (RIP) technology will be employed, with protein as the starting point, to confirm the relationship between them. Ultimately, cholecystokinin-octapeptide (CCK-8) and co-immunoprecipitation (Co-IP) techniques will be used to explore the molecular mechanism of MT-mediated LncRNA MTC promoting the proliferation of Liaoning cashmere goat skin fibroblasts.
Liaoning cashmere goat skin fibroblasts, previously preserved in the laboratory, were cultured in DMEM medium containing 20% FBS (Gemini, USA) and placed in a 37°C, 5% CO2 incubator. Transfection experiments were conducted when cells reached 70–80% confluence.
Construction of LncRNA MTC overexpression virus(1) Determining the MOI value of cellular lentivirus infectionLenti-GFP virus solution with a titer of 1.0×108 TU mL−1 was used to infect Liaoning cashmere goat skin cells. Target cells in the logarithmic growth phase were digested with trypsin to prepare a cell suspension, which was inoculated into a well plate. The culture conditions were 37°C, 5% CO2 incubator, and cell density confluence was approximately 40–60%. MOI gradient was set to 0, 10, 20, 30, 50, and 100. Corresponding doses of negative viral fluid were added according to the viral titer. Polybrene was added with a final concentration of 8 µg mL−1 to enhance infection. Green fluorescent protein (GFP) expression in each well was observed using a fluorescence microscope 48 h after virus infection.
(2) Construction of LncRNA MTC overexpression vectorPrimers were designed and synthesized for PCR based on the target gene sequence. Primer LncRNA MTC-F sequence is GCTGATTTTGTTCGGGACTG, and primer LncRNA MTC-R sequence is GAGATTTTGATGCCCAGTCG. PCR products were separated by agarose gel electrophoresis, and target fragments were recovered according to the instructions using a recovery kit (Axygen, USA). The PCR product of the target gene and the target vector were digested separately to connect the two. The ligation product (10 µL) was transformed into DH5α competent cells, plated on LB plates containing ampicillin (Amp) resistance, and cultured overnight at 37°C. PCR-positive clones were inoculated into LB culture medium and shaken overnight to extract the plasmid for cell transfection. The vector sequence of plenti6.3-MCS-IRES2-EGFP (Research Science, China) is confidential, and the sequence map and polyclonal restriction site are shown in supplementary file 1.
Construction of LncRNA MTC interfering lentivirusThree pairs of miRNA oligos were designed and synthesized based on the sequence of LncRNA MTC (Table 1). The interfering vector was digested with BsaI according to the digestion system. The linearized miRNA vector pcDNA6.2 plasmid was ligated to the oligo double strand following a ligation system, and the ligation product was transformed into competent Escherichia coli DH5α. The next day, the plasmid was extracted to obtain the interfering vector. Using the interfering vector as a template, F and R primers were designed, the EGFP miR fragment was amplified by PCR, the product was isolated, and the target fragment was recovered. The PCR product of the target gene was ligated to the target vector and the ligation product was transformed into DH5α competent cells, which were incubated overnight at 37°C. The following day, a single colony was selected for colony PCR. Plasmids positive for colony PCR were digested for identification. Clones positive for colony PCR were sent for sequencing, sequence alignment, and cell transfection. See supplementary file 2 for the structure of the interference vector pcDNA6.2-EmGFP. Snapgene Viewer download site is https://www.snapgene.com/snapgene-viewer.
| Name | Interference sequence (5′→3′) |
|---|---|
| LncRNA MTC-1F | TGCTGTACAATGACAGACCCAGAATAGTTTTGGCCACTGACTGACTATTCTGGCTGTCATTGTA |
| LncRNA MTC-1R | CCTGTACAATGACAGCCAGAATAGTCAGTCAGTGGCCAAAACTATTCTGGGTCTGTCATTGTAC |
| LncRNA MTC-2F | TGCTGTTATAGGCAACCATACTTCTGGTTTTGGCCACTGACTGACCAGAAGTAGTTGCCTATAA |
| LncRNA MTC-2R | CCTGTTATAGGCAACTACTTCTGGTCAGTCAGTGGCCAAAACCAGAAGTATGGTTGCCTATAAC |
| LncRNA MTC-3F | TGCTGTAATAAGGAAGGGAATGTTCCGTTTTGGCCACTGACTGACGGAACATTCTTCCTTATTA |
| LncRNA MTC-3R | CCTGTAATAAGGAAGAATGTTCCGTCAGTCAGTGGCCAAAACGGAACATTCCCTTCCTTATTAC |
Liaoning cashmere goat cell suspension was inoculated into 96-well plates (3,000 cells per well) and cultured in an incubator. After adding CCK-8 reagent and incubating it in an incubator for 2 h, the absorbance value at 450 nm was measured.
RT-qPCRRNA was extracted from the experimental group and the control group using a Trizol kit (TaKaRa, Japan). After DNase I treatment, the RNA was reverse-transcribed into cDNA, and the expression of the target gene was detected by qPCR. PCR Mix was prepared with 2×SYBR Green Mix, and SYBR Green fluorescence quantitative PCR was used to analyze the expression of various genes. The 2−△△Ct method was employed for the relative quantification of gene expression.
Detection of apoptosis by flow cytometryCultured skin fibroblasts of Liaoning cashmere goats were digested with trypsin, washed twice with PBS, and collected after centrifugation. Each sample’s cells were resuspended in 300 µL buffer and mixed evenly. AnnexinV-APC (5 µL) and PI (5 µL) were added, incubated at approximately 20°C for 30 min, and then examined using a flow cytometer (NovoCyte, Agilent).
RNA Pull-down and mass spectrometry analysisProbe primers were designed based on the LncRNA MTC sequence, and PCR amplification was performed. The PCR product was isolated, and the target fragment was recovered. The MEGAscript™ T7 Transcription Kit (Invitrogen, USA) was employed to transcribe the target DNA into RNA and purify the recovered RNA. Biotin labeling was added to the target RNA using the Pierce RNA 3′ End Desthiobiotinylation Kit (Invitrogen, USA). Protein extraction was carried out on both the experimental group (treated with 0.2 g L−1 MT for 72 h) and the control group. The labeled RNA was coupled to magnetic beads, which were washed twice with 50 µL 20 mM Tris (pH=7.5), placed on a magnetic rack, and the supernatant was discarded. 10×protein-RNA binding buffer was diluted to 1× with water, and add 100 µL of protein-RNA binding buffer to the EP tube. Put the EP tube on the magnetic frame and discard the binding buffer. Add 100 µL of reaction MIX to each tube and mix well. Incubate at 4°C for 60 min. Wash the magnetic beads twice with 1×washing solution and discard the supernatant. Following the addition of 50 µL of eluent, the samples were incubated at 37°C for 25 min, and then collect the supernatant. Then, moldi-tof/TOF-MS mass spectrometry was used to detect the pull-down protein with LncRNA MTC, and the mass spectrometry data was searched in the uniprot database with mascot software.
Cell transfectionThe interference sequence of Glutathione S-transferase M1 (si-GSTM1) is designed. The interference sequence of GSTM1-F is CCAGAGCAACGCCAUCCUUTT, and the interference sequence of GSTM1-R is AAGGAUGGCGUUGCUCUGGTT. The sequence was provided by Jin Tuosi (China). Liaoning cashmere goat skin fibroblasts were inoculated in antibiotic-free culture medium, and the cells were transfected to 30–50% fusion. Trans-Mate transfection reagent (Jin Tuosi, China) was used to complete the cell transfection.
RNA Immunoprecipitation (RIP)The vector plenti6.3-MCS-IRES2-EGFP was constructed, see supplementary file 3 for its sequence map. A single colony was selected for colony PCR. Target cells were infected with the target virus and negative control lentivirus, and infected cells were expanded. The cell fusion rate was approximately 90%. RIP lysis buffer (400 µL) was added, and samples were incubated on ice for 30 min. Antibodies were added to protein A/G magnetic beads, using homologous IgG (Abcam, UK) as a control. EDTA, RNA inhibitor, and RIP wash buffer were combined to create a precipitation buffer, which was added to the pretreated magnetic beads and the pretreated cell lysate. The magnetic bead protein complex was added to PBS as an “IP/IgG,” stored at −80°C, and then added to a loading buffer to detect the IP effect. A protease K solution was prepared using RIP wash buffer, 10% sodium dodecyl sulfate (SDS), and 10 mg mL−1, and the magnetic bead antibody–protein complex was resuspended in the protease K solution. The input was added to the protease K, and the samples were incubated at 55°C. RNA extraction was performed using the Trizol method, and the concentration and RNA integrity were determined. RNA was reverse-transcribed into cDNA for qPCR experiments. The sequence of primer LncRNA MTC-F is GGTGACATGTCAGGGGCTTTAAC, and the sequence of primer LncRNA MTC-R is GCCCATGATCTGGACTCCAATC. Relative quantification of gene expression was performed using the 2−△△Ct method.
KEGG pathway analysisTo explore the key pathways of LncRNA MTC-mediated related proteins, KEGG pathway annotation analysis was conducted on proteins that interact with and differentially express LncRNA MTC. Gene lists were submitted to KOBAS 3.0 for pathway enrichment analysis.
Co-IP detection of protein interactionsFollowing successful transfection of the target vector and the control vector, cells were lysed on ice with RIPA neutral lysate at 4°C and collected by centrifugation. The protein lysis solution was mixed with the target antibody and corresponding IgG, and incubated at 4°C. Pretreated Protein A/G magnetic beads/agarose beads were added to the protein–antibody complex tube and mixed well. Agarose beads were centrifuged to the bottom of the tube, the supernatant was removed, and the agarose beads were rinsed with lysis buffer. The supernatant was discarded by centrifugation. Finally, SDS sample loading buffer was added and boiled. Proteins were transferred onto a PVDF membrane. The membrane was sealed at room temperature with a sealing solution (TBST solution containing 5% skimmed milk) for 1 h. Anti-ASK (Abclonal, China) was diluted with a freshly prepared blocking solution and incubated with the primary antibody overnight at 4°C. The membrane was washed with TBST. The corresponding secondary antibody for each protein primary antibody was diluted with a blocking solution (rabbit secondary antibody 1 : 3,000) (Abmart, China), and the membrane was incubated at room temperature for 1 h, followed by washing with TBST. The mixed solution was applied to the membrane in a 1 : 1 ratio and allowed to react for 10 s, placed in a chemiluminescence imager, and exposed. Photographic records were obtained.
Statistical analysisData were statistically analyzed using the t-test method. A p-value of less than 0.05 indicates that the difference is statistically significant. All experiments were independently repeated three times.
To construct an LncRNA MTC lentivirus overexpression vector, the MOI value of cell lentivirus infection was first explored. The MOI gradient was set to 0, 10, 20, 30, 50, and 100, ultimately determining the optimal MOI value for cell lentivirus infection to be 20. The addition of 8 µg mL−1 polybrene was necessary to promote infection (Fig. 1A). A stable LncRNA MTC overexpression lentivirus vector was successfully constructed, and qRT-PCR detected that the expression of LncRNA MTC was significantly higher than that of the NC (negative control) group (Fig. 1B). Three pairs of shRNAs targeting LncRNA MTC were designed, and the interfering lentiviruses at the three LncRNA MTC targets were transfected into 293T cells, respectively. The green fluorescence of GFP was observed in microscopic photos, demonstrating the successful construction of three interfering lentivirus vectors (Fig. S1). After transfecting the interfering lentivirus into cashmere goat skin fibroblasts, qRT-PCR detection showed that the interfering effect of both target 1 and target 3 was 54%, p<0.01. Subsequently, target 1 was selected for the experiment (Fig. 1C). CCK-8 results showed that, compared with the NC group, overexpression of LncRNA MTC promoted the proliferation of Liaoning cashmere goat skin fibroblasts, and interference with LncRNA MTC inhibited the proliferation of Liaoning cashmere goat skin fibroblasts (Fig. 1D). Overexpression of LncRNA MTC reduced the apoptosis rate of Liaoning cashmere goat skin fibroblasts and promoted apoptosis of skin fibroblasts after interfering with LncRNA MTC (Fig. 1E).
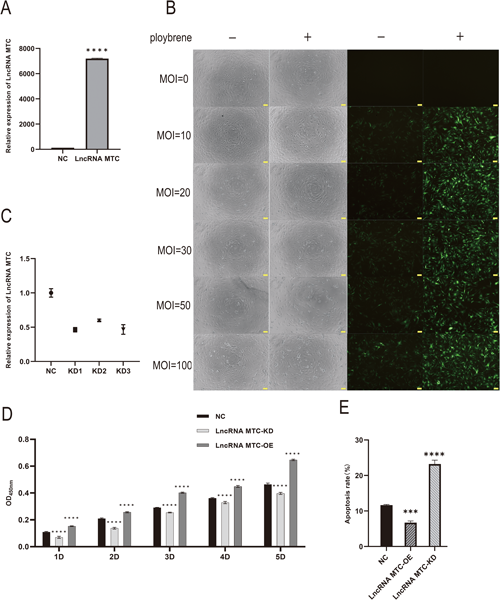
(A) Expression of target gene LncRNA MTC. (B) Photos of sheep primary skin cells infected with Lenti GFP virus with different MOI gradients. The left side is bright field and the right side is dark field. Scale bars=50 µm. (C) Knockdown efficiency of three interfering targets of LncRNA MTC in skin cells. (D) Determination of the viability of skin fibroblasts after overexpression or interference with LncRNA MTC using CCK-8. (E) Apoptosis rate of fibroblasts after overexpression or interference with LncRNA MTC. The result are presented as mean±SD. Significant results are presented as *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 in comparison with control group (Con). NC is negative control; LncRNA MTC-KD is knocking down LncRNA MTC; LncRNA MTC-OE is the overexpression of LncRNA MTC group.
To explore the proteins binding to LncRNA MTC, a database of LncRNA MTC interacting proteins was constructed using RNA pull-down mass spectrometry. Protein electrophoresis separation was performed on RNA pull-down products, and the results of positive control electrophoresis for total protein in cells are shown in the figure below (Fig. 2A). Before and after melatonin treatment, LncRNA MTC could bind to different proteins (Fig. 2B). A total of 2,449 proteins were identified by RNA pull-down mass spectrometry. Changes in the expression of LncRNA MTC binding proteins before and after melatonin addition were analyzed. After melatonin addition, a total of 394 proteins were upregulated by LncRNA MTC binding, and 47 proteins were downregulated by LncRNA MTC binding (Fig. 2C, D). See the supplementary file 4 for the details of upregulated and downregulated protein types. Subsequent KEGG enrichment analysis of these differentially expressed proteins that bind to LncRNA MTC found that the upregulated proteins were significantly enriched in ribosomes, protein processing in the endoplasmic reticulum, focal adhesion, regulation of actin cytoskeleton, glycolysis/gluconeogenesis, pyruvate metabolism, and tight junction. Among the 25 signal pathways, focal adhesion, tight junction adhesion nodes, and phagosomes were associated with cashmere growth (Fig. 2E). The downregulated proteins significantly enriched 18 signal pathways such as lupus erythematosus, necroptosis, alcoholism, ribosomes, carbon metabolism, endocytosis, and phagosomes. Among these pathways, endocytosis and phagosomes were associated with cashmere growth, with 12 proteins enriched for endocytosis and 7 proteins enriched for exocytosis (Fig. 2F).
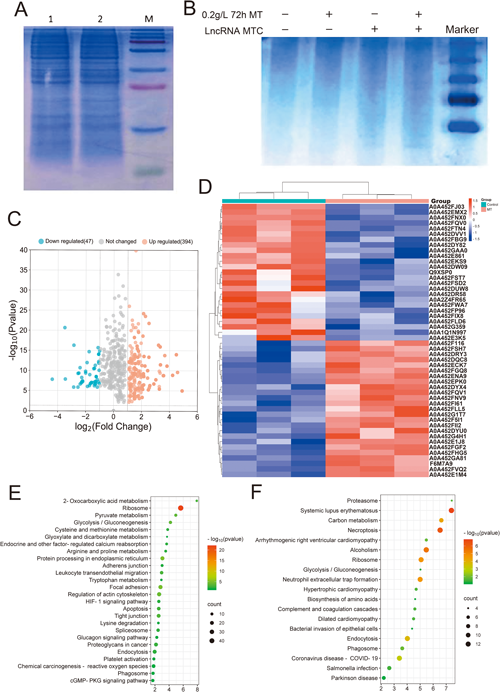
(A) Electrophoresis results of positive control for total protein in cells (lane 1 is the control group; lane 2 is the 0.2 g L−1 72 h MT treatment group). (B) Western Blot Test Results of Interaction Proteins (Coomassie Brilliant Blue Staining). (C) Scatter chart of upregulated and downregulated proteins after adding melatonin. (D) Hierarchical clustering heat map shows the top 25 up-regulated and down-regulated proteins in expression. (E) KEGG annotation analysis of upregulated proteins in differential expression. (F) KEGG annotation analysis of downregulated proteins in differential expression.
The experiment set the screening conditions for proteins as follows: after adding melatonin, the binding amount with LncRNA MTC significantly increased, and the coverage >2, unique peptides >2. A total of 152 proteins were screened, with 26 proteins having interactions. Using String software to construct a protein interaction network, it was discovered that
Glutathione S-transferase Mu 1-like (LOC108633298, XP_017901368.1)
Glutathione S-transferase Mu 1 (LOC102190481, XP_005678001.1)
Glutathione S-transferase Mu 1 (LOC100861222, XP_017901958)
Glutathione S-transferase Mu 1 (LOC102189813, XP_017901364.1)
They were involved in drug metabolism-cytochrome P450, drug metabolism-other enzymes, cytochrome P450 metabolism of exotic organisms, glutathione metabolism, and other pathways (Fig. 3A). Combining RNA pulldown data, the protein glutathione S-transferase Mu 1 (GSTM1, XP_005678001.1) that binds to LncRNA MTC was selected.

(A) Protein interaction network diagram (B) The expression of target gene LncRNA MTC. Compared with IgG, ***represents 0.0001<p≤0.001. (C) CCK-8 detected the activity of skin fibroblasts. Compared with the control group, *represents 0.01<p≤0.05.
To confirm that LncRNA MTC indeed binds to GSTM1, a subsequent RIP experiment was conducted. The results showed that the content of LncRNA MTC in His precipitate was up to four times higher than that in the control group (p<0.001) (Fig. 3B). In order to explore the molecular mechanism of melatonin regulating GSTM1 to promote the proliferation of Liaoning cashmere goat skin fibroblasts, we observed the cell proliferation by interfering with GSTM1 after adding melatonin. The results showed that, compared with the control group, interference with GSTM1 significantly weakened the promotion of melatonin on cell proliferation (Fig. 3C). The above results suggest that after melatonin treatment, LncRNA MTC can promote the proliferation of Liaoning cashmere goat skin fibroblasts by combining with its target protein GSTM1.
Interaction between GSTM1 and ASK1 proteinIn order to verify the interaction between GSTM1 and apoptosis signal-regulating kinase 1 (ASK1) in Liaoning cashmere goat fibroblasts, a Lenti6.3-GSTM1-6Xhis-IRES2-EGFP vector was constructed, and transfected into Liaoning cashmere goat skin fibroblasts. After transfection, microscopic photos showed a uniform distribution of green fluorescence (Fig. 4A), indicating that the Lenti6.3-GSTM1-6Xhis-IRES2-EGFP lentivirus vector was successfully constructed. Immunoprecipitation results revealed bands in the input group, indicating that the lysate of skin fibroblasts used for immunoprecipitation contained target proteins GSTM1 and ASK1. No target band was found in the IgG treatment, proving no false positive. The ASK1 protein was detected after treatment with an anti-6xHis-tag antibody, demonstrating the interaction between GSTM1 and ASK1 protein in Liaoning cashmere goat skin fibroblasts (Fig. 4B).

(A) Cell fluorescence before and after transfection with the target vector a: pre transfection; b: pre transfection; c: Posttransfection dark field; d: Posttransfection bright field. Scale bars=50 µm. (B) Immunoprecipitation result chart.
In this study, the melatonin-mediated LncRNA MTC function in Liaoning cashmere goat skin fibroblasts was investigated. MT possesses potent antioxidant and anti-inflammatory effects (Reiter et al. 2016). Its mechanisms of action in secondary hair follicle development include enhancing the activity of antioxidant enzymes, upregulating the expression of anti-apoptotic protein Bcl-2, and downregulating the expression of pro-apoptotic proteins Bax and Caspase-3. It can also improve the yield and quality of cashmere by reducing fiber diameter (Yang et al. 2019). Previous studies have shown that melatonin affects hair growth, and high expression of LncRNA MTC in skin fibroblasts treated with 0.2 g L−1 MT was detected by high-throughput sequencing technology (Jin et al. 2018). To further explore the function and mechanism of melatonin-mediated LncRNA MTC, this experiment first investigated the function of LncRNA MTC. An overexpression/interference lentivirus vector of LncRNA MTC was successfully constructed and stably transfected into skin fibroblasts. The proliferation of cells was detected by CCK-8, revealing that under the condition of high expression of LncRNA MTC, the growth rate of cells accelerated. However, after LncRNA MTC interference, cell growth was inhibited. The same results were confirmed by flow cytometry experiments. These findings suggest that LncRNA MTC is involved in the process of melatonin regulating the development of cashmere follicles and the growth of cashmere.
Thousands of lncRNAs have been discovered in eukaryotes, but information about the lncRNA–protein regulatory network remains scarce. Therefore, identifying proteins related to lncRNA is crucial. In vitro RNA pull-down mass spectrometry was used to identify proteins that bind to LncRNA MTC, and it was found that the differential protein function was closely related to drug metabolism and glutathione transferase metabolism before and after melatonin treatment. Among these proteins, the glutathione transferase family plays a crucial role in mitochondrial function, plasma membrane stability, and mammalian oxidative regulation (Yu et al. 2013).
GSTM1 can play a key role in protecting cells from oxidative stress by regulating oxidative stress (Mitchell et al. 1997), and it can also detoxify genotoxic metabolites into a more easily degradable water-soluble form. In a recent study, the absence of GSTM1 enhanced oxidative stress. It can be inferred that GSTM1 plays a protective role in oxidative stress, inflammatory reactions, and other processes. Glutathione S-transferase GST has a certain promoting effect on the maintenance of the hair growth cycle (Morisaki et al. 2013). Basak Kayhan designed a rat model to observe the effects of maternal exposure to artificial dietary pigment additives on rat skin and found that the expression of protein GSTM in sebaceous glands and hair follicles was upregulated (Başak et al. 2017). GSTM1 interacts with different protein partners to regulate cell proliferation, differentiation, and apoptosis (Singh 2015). GSTM1-1 can act as a negative regulator of MEKK1 to inhibit cell apoptosis (Ryoo et al. 2004).
In addition to its antioxidant effect, GSTM1 is an endogenous inhibitor of ASK1. Research has shown that ASK1’s function of promoting apoptosis depends on its own dimerization. GSTM1 inhibits ASK1 activity, prevents it from oligomerizing, and regulates stress or cytokine-induced apoptosis (Cho et al. 2001). To sum up, GSTM1 (XP_005678001.1) was selected as the research object for subsequent experiments.
ASK1 can induce apoptosis, and its activation requires its own homologous oligomerization (Psenakova et al. 2020). In normal cells, the activation of ASK1 is strictly controlled, such as threonine/serine phosphorylation and dephosphorylation, and protein–protein interactions (Jung et al. 2010). Serine threonine kinase receptor-associated protein (STRAP) regulates cell proliferation and death by interacting with a variety of target proteins (Manoharan et al. 2018). Studies have found that GSTM1 plays a role in drug metabolism by interacting with proteins such as GSTA4, GSTM2, and CYP2E1. GSTM1 can also interact with ASK1 proteins to regulate cell apoptosis induced by stress or cytokines. This suggests that LncRNA MTC-mediated GSTM1 proliferation in skin fibroblasts is achieved by influencing the binding of GSTM1 to the ASK1 complex.
LncRNA MTC is a lncRNA that is significantly upregulated in expression after melatonin treatment. There is a pairwise interaction between binding to lncRNA-GSTM1-ASK1, suggesting that melatonin mediates the binding of lncRNA to GSTM1, affecting the binding of GSTM1 to ASK1 protein complexes, to resist the apoptotic process of skin fibroblasts.
In this experiment, RNA pull-down/MS technology was used to analyze the targeted binding of LncRNA MTC to GSTM1 protein. In order to truly reflect the RNA–protein interaction, the interaction between LncRNA MTC and GSTM1 was further verified through RIP technology. To deeply explore how LncRNA MTC mediates GSTM1 to promote the proliferation of skin fibroblasts, we first treated fibroblasts with melatonin and then interfered with GSTM1. It was found that the promotion of melatonin on the proliferation of Liaoning cashmere goat fibroblasts was reversed. Immunoprecipitation technology was used to detect the target protein ASK1. The interaction between GSTM1 and ASK1 protein was determined in Liaoning cashmere goat skin fibroblasts. This study provides a new clue for further understanding the mechanism of LncRNA MTC promoting cashmere growth.
In conclusion, the LncRNA MTC related to cashmere growth mediated by melatonin binds to GSTM1 protein, affecting the interaction of GSTM1-ASK1 protein complexes and inhibiting cell apoptosis.
This study was supported by National Natural Science Foundation of China [grant number 31772557]; Dalian Science and Technology Innovation Fund Project [grant number 2019J12SN65]. We have no conflict of interest.
Mei Jin designed and performed the experiments, and drafted and edited the manuscript. Xinyue Han arranged the article, performed experiments, prepared the original draft, and surveyed the literature. Weiyu Fan performed the data curation and experiments. Yan Zhang performed the bioinformatics analysis. Xinyue Qiu performed the data analysis and arranged the article format.
Supplementary information including Fig. S1 and supplementary files 1–4 are available online.
The data in this study is not intended to be shared in the repository, but the results of this study can be obtained at the reasonable request of the corresponding author.