2024 年 89 巻 1 号 p. 39-46
2024 年 89 巻 1 号 p. 39-46
To analyze radiation-induced chromosomal aberrations in cultured human peripheral blood lymphocytes, a modified protocol for replication banding in combination with fluorescence in situ hybridization (FISH) using whole-chromosome-specific DNA probes was examined. The aim of the major modification of the experimental procedure was to efficiently generate clear and consistent replication bands along metaphase chromosomes in the first division of lymphocytes after culture initiation. This technique enabled the mapping of the sites of rearrangements directly on banded chromosomes at 400–550 band levels. On the basis of the characteristics of replication banding, the procedure described in the present study demonstrated the applicability for the analysis of gamma-ray-induced chromosomal aberrations in early- and late-replicating X chromosomes in cultured human peripheral blood lymphocytes.
Human peripheral blood lymphocytes are the most validated material for the analysis of genomic alterations induced by ionizing radiation (IAEA 2011). In the study of cytogenetic dosimetry, the frequency of radiation-induced chromosomal aberrations in the peripheral blood lymphocytes of exposed individuals is a significant indicator for the estimation of radiation dose after accidents or incidents of overexposure to radiation. The culture protocol for lymphocytes has been internationally standardized in detail (IAEA 2011). Circulating blood lymphocytes are in their resting G0 phase of the cell cycle. Upon transfer to in vitro culture conditions, the G0 lymphocytes proceed to cell cycle phases of replication after adding phytohemagglutinin (PHA), which stimulates the mitotic division of lymphocytes (mostly T-cells). The primary damage induced in DNA (double-strand breaks) can be detected as the yield of chromosome-type aberrations in the first metaphase (M1) cells after culture initiation.
The first in vitro mitotic wave of lymphocytes appears after about 40–48 h in culture. In the subsequent mitotic divisions, the proliferating cell populations may not faithfully reflect the original makeup of the cells owing to the prolonged in vitro culture conditions. For instance, unlike cells with stable-type chromosomal aberrations such as translocations, which have a greater likelihood of being successfully passed through mitosis and onto viable daughter cells, those with unstable-type chromosomal aberrations (dicentrics, centric rings, and acentric fragments) are prone to be eliminated owing mainly to cellular kinetic defects and genomic imbalances (Pala et al. 2001, Krishnaja and Sharma 2004, IAEA 2011, Kaddour et al. 2017). Therefore, to examine the initial DNA damage, it is necessary to deal with M1 cells after culture initiation for chromosomal analyses.
Unstable-type chromosomal aberrations are detectable from the morphological features of chromosomes and are used as a sensitive and reliable measure in biodosimetry (IAEA 2011). Regarding the detection of stable-type chromosomal aberrations (translocations), molecular cytogenetic methods such as fluorescence in situ hybridization (FISH) using whole-chromosome-specific DNA probes have been applied. FISH methods using fluorophore-labeled DNA probes specific for individual chromosomes (chromosome painting) and the entire human chromosome complement (multiplex FISH, M-FISH) enable the detection of chromosomal rearrangements. DNA probe kits for these molecular cytogenetic methods are commercially available. The precise localization of structural chromosomal alterations provides a significant indicator to analyze genomic imbalances induced by ionizing radiation. To localize rearranged sites revealed by FISH, counterstaining with 4′,6-diamidino-2-phenylindole (DAPI) is mostly carried out to generate a banding pattern as a chromosomal landmark (Heng and Tsui 1993, Knapp et al. 1995, Burde et al. 1996, Barros e Silva and Guerra 2010). DAPI has an affinity to chromosomal regions with high contents of adenine and thymine residues (AT-rich regions) that correspond to late-replicating bands (Costantini and Bernardi 2008). According to the internationally standardized nomenclature (ISCN 2020), the banding pattern produced by DAPI in combination with nonfluorescent distamycin A is coded as DA-DAPI (Schweizer 1980). However, with DA-DAPI staining, it is not easy to obtain microscopy images with apparent light and dark bands. Furthermore, with DAPI staining alone, the fluorescent bands are much less clear.
Here, we describe a modified method of replication banding (R-banding) using 5-bromo-2′-deoxyuridine (BrdU) (Dutrillaux et al. 1973, Latt 1973) followed by differential staining (fluorescence plus Giemsa, FPG) (Perry and Wolff 1974) to reveal chromosomal bands in cultured M1 lymphocytes. The combination of R-banding and FISH has already been utilized since early studies of genetic mapping and chromosomal abnormalities (e.g., Takahashi et al. 1990). In accordance with the conventional R-banding protocol, blood lymphocytes were cultured for three days and treated with excess thymidine to synchronize the cell cycle and to obtain a sufficient number of dividing cells, which were mostly in the second or third division. We modified the conventional method to widen the application range for efficiently localizing radiation-induced break/rearrangement sites on chromosomes by the two-day (48 h) culture of lymphocytes so as to deal with M1 lymphocytes.
In this article, we show examples of M1 chromosome images with clear and consistent replication bands obtained by the modified method with an optimized experimental procedure. Then, as an application model of this method, we describe the comparative analysis of the yield of chromosomal aberrations in two homologs of the X chromosome. In mammalian female chromosomes, one of two X chromosomes is genetically inactive. X-chromosome inactivation is a form of dosage compensation used by female cells to balance X-linked gene expression levels between two sexes in mammals (Lyon 1961). The inactive X chromosome forms compact chromatin (facultative heterochromatin) in interphase cells. There is a delay of DNA replication in the inactive X chromosome during the synthesis phase (S phase), such that the DNA replicates asynchronously relative to that in the active X chromosome. This provides the possibility of studying the role of chromatin structure in the induction, processing, and persistence of radiation-induced chromosomal aberrations. On the basis of current knowledge about the phenomenon of X-chromosome inactivation, especially in terms of the replication-related state of facultative heterochromatin and response to DNA damage (Richer and Drouin 1990, Puerto et al. 2000, 2001, Falk et al. 2008, Cremer and Cremer 2010, Chiolo et al. 2013, Jäger et al. 2013, Müller et al. 2013, Fortuny and Polo 2019), we examined the frequencies of aberrations induced by 4.0 Gy gamma ray irradiation in two homologs of the X chromosome.
Peripheral blood specimens from two healthy donors (one female and one male) were used after obtaining their informed consent (protocol code 18-023, approved by the Ethics Committee of the National Institutes for Quantum Science and Technology). Blood specimens irradiated with 4.0 Gy gamma rays (dose rate, 0.5 Gy min−1) were kept at 37°C for 2.5 h (post-irradiation repair) (Suto et al. 2013). Unirradiated control specimens were also prepared. The fraction of mononuclear cells was separated by the gradient separation method using Ficoll Paque (Cytiva). The cells were cultured in 5 mL of a medium (5×105 mL−1) composed of RPMI1640 supplemented with 10% fetal bovine serum and 3% PHA for 48 h. For the final 7 h of culture, the cells were treated with BrdU (25 µg mL−1) and cultured in the dark. After adding colcemid solution (200 ng mL−1) for 30 min to arrest metaphase cells, the cells were treated with a hypotonic solution (0.075 M KCl) for 25 min and fixed with an acetic acid/methanol (1 : 3) fixative three times. Chromosome preparations made by the standard air-dry method were kept at room temperature for 2–4 days for aging, stained with Hoechst 33258 (Sigma, 2 µg mL−1) for 7–8 min, washed in distilled water, air-dried, mounted in 2×SSC (sodium saline citrate) solution under coverslips, and finally exposed to blacklight (a source of long-wave UV light) for 7–15 min at a distance of 1.5 cm above the slide. To check the appropriate UV-exposure time, one or two slides from each batch of slides were stained with 3% Giemsa stain solution for 12 min. Then, the remaining unstained slides were kept in a freezer at −25°C until use.
FISHThe following two sets of FISH experiments using commercially available probe kits (MetaSystems, Altlussheim, Germany) were performed: (1) M-FISH for the whole chromosome complement (24-color FISH), and (2) painting for the X chromosome and chromosome 7. An active X chromosome replicates early, whereas an inactive X chromosome replicates late. Chromosome 7 was selected as a control autosome, since the DNA content and CpG-island density of this chromosome are equivalent to those of the X chromosome (Reney et al. 2024). FISH was performed following the manufacturer’s protocol with modifications as described previously (Suto et al. 2012, 2015, 2021). In brief, slides were kept in a Coplin staining jar containing 2×SSC solution, incubated at 70°C for 30 min, left in the jar at room temperature for about 20 min, and rinsed in 70% ethanol solution. Then, the chromosomes were denatured in an alkaline solution (0.1 M sodium hydroxide 70%; ethanol 30%) for 1 min and dehydrated in a graded alcohol series (70, 95, and 99.5% ethanol, 1–3 min each). The denaturation and hybridization of probe solutions were performed following the manufacturer’s protocol. After one-day (painting) or two-day (M-FISH) incubation at 37°C in a humid chamber, the slides were incubated at 72°C in 0.4×SSC solution for 2 min, and then washed in 2×SSC solution containing 0.05% Tween-20 at room temperature for 1 min. After rinsing in distilled water, the chromosomes were counterstained with DAPI (painting, 125 ng mL−1; M-FISH, 42 ng mL−1).
R-bandingSince R-banding patterns dynamically vary depending on the replication timing, we chose chromosome 13, a medium-sized acrocentric chromosome, as a reference chromosome for determining band levels; banding patterns of chromosome 13 were easy to determine in a wide range of band levels when compared with the internationally standardized karyogram (ISCN 2020).
Microscopy observation and automated image captureChromosome slides were examined using the following platforms: Axio Imager Z2 (Carl Zeiss Microscopy) and CoolCube 1, Metafer ver. 4, ISIS (MetaSystems). First, fluorescent images of DAPI-stained metaphase cells with 46 centromere-bearing chromosomes were selected. Then, chromosome images obtained from five different narrow-band filter sets were examined for structural aberrations. On the basis of the merged digital images, artificial intelligence (AI)-assisted karyograms were constructed in a coded pseudocolor mode. We used the following alternative platform when needed: fluorescence microscope (Olympus BX-50, Tokyo, Japan) equipped with a CCD camera (Spot, Diagnostic Instruments, Inc., MI, USA) coupled with a filter-wheel set (Ludl Electronic Products, NY, USA). By using filter sets specific for DAPI, fluorescein isothiocyanate (FITC), Cy3, and Cy5, we can merge metaphase images using image processing software (Diagnostic Instruments, Inc., MI, USA).
Scoring of aberrations in X chromosome and chromosome 7First, images of DAPI-stained metaphase cells with 46 centromere-bearing chromosomes were selected. Then, painted chromosomes were examined for structural aberrations. Owing to the high irradiation dose (4.0 Gy), complex structural aberrations generated through exchanges among more than two chromosomes were observed. This led to difficulties in accurately determining the number of breaks (Simpson and Savage 1995). In this study, we counted the number of the X chromosome or chromosome 7 showing structural aberrations. Since the statistical characteristics of these numerical data were currently unknown, only simple chi-square tests of contingency tables were performed to tentatively compare the frequencies of chromosomes with aberrations. Chromatid-type aberrations (chromatid breaks and gaps) were recorded but not scored as chromosomal aberrations.
Under the present experimental conditions, approximately 40% of metaphase cell images captured by Metafer exhibited R-banding patterns at the level of 400–550 bands when compared with the standardized nomenclature of banding patterns (ISCN 2020) (Fig. 1). The remaining metaphase cell images showed mostly highly contracted chromosomes with unclear banding patterns. DAPI has an affinity to AT-rich regions that correspond to late-replicating bands. Moreover, the replication banding procedure includes treatment with Hoechst 33258, which behaves very similarly to DAPI, although the fluorescence of Hoechst 33258 was significantly reduced after DNA denaturation, ethanol treatments, and post-hybridization washing. On the other hand, chromosomal DNA regions that incorporated BrdU during the late S phase were photodegraded by UV exposure after Hoechst 33258 staining; the affinities of DNA dyes to these degraded regions were expected to decrease. As demonstrated in Fig. 1, the actual staining results showed that the fluorescence intensity was significantly lower in late-replicated regions than in the remaining regions with the following exceptions: pericentromeric constitutive heterochromatin of chromosomes 1, 9, and 16, and the long arm of the Y chromosome, known as highly repeated AT-rich regions.
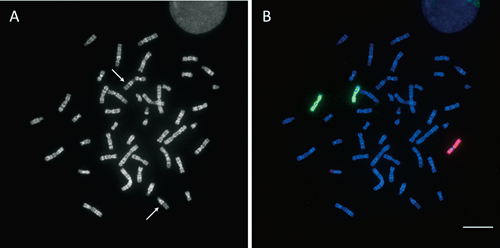
Lymphocytes obtained from an unirradiated blood specimen from a male donor were cultured for 48 h. BrdU (final concentration, 25 µg mL−1) was added to the culture in the final 7 h. (A) DAPI-stained chromosomes (gray scale). Arrows indicate chromosome 13 homologs. (B) Results of FISH using painting probes (chromosome 7, green; X chromosome, red) on the same metaphase cell shown in (A). Scale bar=10 µm.
In our routine procedure for the diagnosis of chromosomal aberrations using the MetaSystems platform, digital images from five different fluorescence filter sets were merged. Subsequently, genomewide chromosomal aberrations could readily be visualized in a coded pseudocolor mode by the image processing software (MetaSystems). In some specific cases of analyses, images generated by merging image information from two or three fluorescence filters, instead of fully pseudocolored images, were used to corroborate the break sites on R-banded chromosomes. An example of M-FISH results generated by merging images from three band-pass filters specific for FITC, Cy-3, and Cy-5 using the Olympus platform is shown in Fig. 2. As indicated by arrows, the breakpoints of rearrangements can be distinctively seen as color junctions on banded chromosomes.

(A) Structural chromosomal aberrations detected by the combined method of M-FISH and replication banding (550-band level). Digital image data obtained from three different band-pass filters specific for FITC, Cy-3, and Cy-5 were merged to generate visually comprehensible chromosome images for the detection of rearrangements. Arrows indicate chromosomal breakpoints. (B) R-banded chromosomes of the same metaphase cell shown in (A) revealed by DAPI counterstaining (gray scale). Scale bar=10 µm.
The hybridization efficiencies of the chromosome-specific DNA probes for the late-replicated regions, and therefore the photodegraded regions, including the long arm of the late-replicating X chromosome, were not noticeably decreased (Figs. 3, 4). The banding pattern of the late-replicating X chromosome markedly varied, and the long arm was elongated and unclear owing to the high density of incorporated BrdU. Under the present hybridization and washing conditions, we were able to detect weak signals on the pseudo-autosomal region of the short arm of the Y chromosome.

Lymphocytes obtained from unirradiated blood specimen from a female donor were cultured for 48 h. BrdU (final concentration, 25 µg mL−1) was added to the culture in the final 7 h. (A) DAPI-stained chromosomes (gray scale). Arrow, early-replicating X chromosome. Arrowhead, late-replicating X chromosome. (B) Results of FISH using painting probes (chromosome 7, green; X chromosome, red) on the same metaphase cell shown in (A). Scale bar=10 µm.
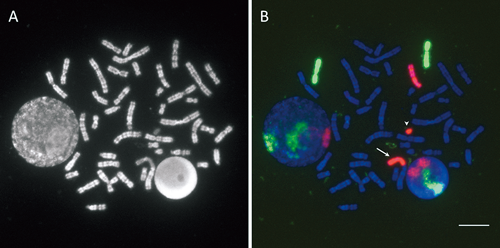
(A) R-banded chromosomes stained with DAPI (gray scale). Scale bar=10 µm. (B) R-banded metaphase cell showing chromosome 7 homologs (green) and X chromosome homologs (red). The late-replicating X chromosome is involved in the formation of a dicentric chromosome (arrow) and an associated fragment (arrowhead).
High frequencies of complex exchange-type aberrations were detected (Table 1). In this table, the early- and late-replicating X chromosomes are denoted tentatively as Xe and Xl, respectively. It was difficult to accurately count the number of breaks in metaphase cell images using a limited number of chromosome-specific painting probes (Simpson and Savage 1995, Sachs et al. 1999, Suto et al. 2021). In our present work, simply the frequency of aberrations in the X chromosome or chromosome 7 was used as a comprehensible indicator for comparative analyses. The results are summarized as follows: (1) for chromosome 7, no significant differences were observed between male and female cells (0.10<p<0.25); (2) chromosome 7 and the X chromosome in male cells showed no significant differences as expected from their genomic size similarity (0.75<p<0.90); (3) the X chromosome (Xe plus Xl) and chromosome 7 in female cells showed no significant differences (0.50<p<0.75); (4) Xe in female cells and the X chromosome in male cells showed no significant differences (0.50<p<0.75), (5) Xl (0.145 per chromosome) more frequently showed aberrations than Xe (0.097 per chromosome) in female cells, although the difference was not statistically significant (chi-square=3.56, 0.05<p<0.10).
| Donor | Sex | Radiation dose (Gy) | Number of cells | Chromosome | Number of chromosomes involved in structural aberrations | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dic | TL | CE | cR | Frg* | Total | Frequency (per chromosome) | |||||
| A | F | 4.0 | 351 | Xe | 7 | 17 | 6 | 2 | 2 | 34 | 0.097 |
| Xl | 18 | 20 | 9 | 2 | 2 | 51 | 0.145 | ||||
| Xe+Xl | 25 | 37 | 15 | 4 | 4 | 85 | 0.121 | ||||
| #7 | 35 | 37 | 15 | 6 | 1 | 94 | 0.134 | ||||
| 0.0 | 387 | Xe | 0 | 0 | 0 | 0 | 1 | 1 | 0.003 | ||
| Xl | 0 | 0 | 0 | 0 | 0 | 0 | — | ||||
| Xe+Xl | 0 | 0 | 0 | 0 | 1 | 1 | 0.001 | ||||
| #7 | 0 | 0 | 0 | 0 | 0 | 0 | — | ||||
| B | M | 4.0 | 425 | X | 20 | 20 | 1 | 1 | 3 | 50 | 0.118 |
| #7 | 42 | 42 | 1 | 1 | 4 | 95 | 0.112 | ||||
| 0.0 | 346 | X | 0 | 0 | 0 | 0 | 0 | 0 | — | ||
| #7 | 0 | 0 | 0 | 0 | 0 | 0 | — | ||||
Dic, dicentrics; TL, translocations; CE, complex exchanges; cR, centric rings; Frg, acentric fragments. Xe, early-replicating X chromosome; Xl, late-replicating X chromosome; #7, chromosome 7.
*Fragments associated with dicentric or ring chromosome formation are excluded.
The major focus of this work was the modification of the R-banding procedure to consistently obtain clearly banded metaphase chromosomes in the first division of human peripheral blood lymphocytes after culture initiation. The results showed advantages as well as limitations from a technical perspective. Although the power of this combined method of R-banding and FISH was limited in terms of the size of chromosomal alterations, which were below the detection limits of molecular cytogenetic techniques (Cornforth and Durante 2018), this method enabled us to visualize radiation-induced genomic damage on a cell-by-cell basis. In view of the late effects of genomic alterations especially in cells at radiation-sensitive phases such as undifferentiated hematopoietic stem/progenitor cells (HSPCs), the fate of cells bearing chromosomal aberrations is of medical concern. Since it is difficult to directly examine HSPCs, early detection of the emergence and subsequent proliferation of clonal cell lines with chromosomal aberrations in differentiated blood cells is one of the important diagnostic issues in tumorigenesis. When the proportion of differentiated cells bearing chromosomal aberrations in circulating peripheral blood lymphocyte populations is small, it may be methodologically difficult to conduct, for example, single-cell genome sequencing at the DNA sequence level. Therefore, as an available tool for monitoring the presence of aberrant cells in circulating peripheral blood lymphocytes, chromosomal examinations using the combination of R-banding and M-FISH can provide information about genomic risk factors.
Comparative analysis of radiation-induced aberration yields in the X chromosome may provide an excellent model system for structure–function relationships in homologous chromosomes with different functional allocations (Cremer and Cremer 2010). The replication dynamics of the X chromosome in living cells have been actively studied (Eils et al. 1996, Goodarzi et al. 2010, Casas-Delucchi et al. 2011, Aladjam and Fu 2014, Koren and McCarroll 2014, Żylicz and Heard 2020). However, the timing correlation between heterochromatinization and DNA repair in human peripheral blood lymphocytes has not been fully understood (Wang et al. 2016). In an early study (Surrallés and Natarajan 1998), the frequencies of chromosomal aberrations induced by X-irradiations were analyzed using cytogenetic and immunogenetic methods. In that study, the early-replicating and late-replicating X chromosomes were shown to respond with similar radiosensitivity. The researchers in that study made further reference to the presence of heterogeneity in the processing of breakage and repair of radiation-induced chromosomal aberrations (Surrallés and Natarajan 1998). Interestingly, in our present study, the frequency of aberrant Xl was higher than that of aberrant Xe, although the difference was not statistically significant. Note that we dealt with a limited cell population, namely, cells that incorporated BrdU for the later part of the S phase (7 h) and were arrested by treatment with colcemid in the final 30 min of culture. This short-term treatment with colcemid in the present experimental procedure was indispensable for the generation of banding patterns with high resolution. Therefore, by further examining various sampling times in combination with various radiation doses, the combined method of R-banding and FISH can provide a better understanding of the complicated effects of dynamic replication processes on the yield of radiation-induced chromosomal aberrations especially in the late-replicating X chromosome.
We are grateful to Dr. Momoki Hirai for his helpful advice and discussions.
This work was partly supported by FY2021-Radiation Safety Research Promotion Fund (Nuclear Regulation Authority, Japan), JSPS KAKENHI Grant Number 17K07023, Industrial Disease Clinical Research Grants (Ministry of Health, Labour and Welfare, Japan), and FY2023-Research Project on the Health Effects of Radiation organized by Ministry of the Environment, Japan.
The authors declare no conflict of interest.
Informed consent and blood collection, T. T.; cell culture, chromosome image detection, data analysis and figure preparation, M. A., Y. S.; in vitro irradiation experiments and specimen management, Y. T., K. I., writing, review and editing of the paper, M. A., T. T., Y. T., K. I., Y. S.