Abstract
Background: Alcohol consumption is a modifiable lifestyle, but its role in heart failure (HF) development is controversial. Herein, we investigated the prospective association between alcohol consumption and HF risk.
Methods: A total of 2,712 participants (1,149 men and 1,563 women) from the Suita Study were followed up every two years. Cox regression was applied to calculate the hazard ratios (HRs) and 95% confidence intervals (95% CIs) of HF risk for heavy drinking (≥46 g/day in men or ≥23 g/day in women) and never drinking compared to light drinking (<23 g/day in men or <11.5 g/day in women). Then, we combined the results of the Suita Study with those from other eligible prospective cohort studies in a meta-analysis using the random-effects model.
Results: In the Suita Study, within a median follow-up period of 8 years, 319 HF cases (162 in men and 157 in women) were detected. In men, but not women, never and heavy drinking carried a higher risk of HF than light drinking: HRs (95% CIs) = 1.65 (1.00, 2.73) and 2.14 (1.26, 3.66), respectively. Alike, the meta-analysis showed a higher risk of HF among heavy drinkers: HR (95% CI) = 1.37 (1.15, 1.62) and abstainers: HR (95% CI) = 1.18 (1.02, 1.37).
Conclusion: We indicated a J-shaped association between alcohol consumption and HF risk among Japanese men. The results of the meta-analysis came in line with the Suita Study. Heavy-drinking men should be targeted for lifestyle modification interventions.
1. Introduction
Heart failure (HF) is a major public health concern in Japan and worldwide. In addition to its grave consequences, HF is estimated to seize 1–2% of total healthcare budgets [1–3]. Since HF is potentially preventable, identifying at-risk individuals with the aim of lifestyle modification interventions is promoted [4, 5].
Among modifiable lifestyle factors, alcohol consumption appears to play a role in the development of cardiovascular disease (CVD), including HF [6–9]. Heavy alcohol consumption could induce myocardial damage and impaired left ventricular function, factors that eventually result in alcoholic cardiomyopathy and HF [10–12].
Several studies have examined the association between alcohol consumption and HF risk [13–31]. Apart from their inconsistent findings, many of these studies were limited by 1) the use of alcohol abstinence as a reference group [13–23] that could hide the hazardous impact of heavy drinking on HF [32], 2) involving former drinkers who posed unfavorable clinical profile in the alcohol abstinence group [14, 16, 22, 25], 3) applying low cut-offs of alcohol consumption to define heavy drinking which was insufficient to detect a significant association and carried high risks of misclassification [14–16, 19–22, 25], and 4) underrepresenting Asian populations although the contribution of alcohol consumption to CVD risk may vary across races [33].
We, therefore, used data from the Suita Study, a prospective cohort study conducted in urban Japan, to examine the association between alcohol consumption and HF risk. We avoided the main limitation of previous studies by assigning light drinking as a reference group to avoid false negative results. We also stratified the results by several personal and clinical factors to help personalize future preventive measures. Then, we performed a meta-analysis combining the results of the Suita Study with those from other eligible cohort studies to confirm our findings.
2. Methods
2.1. The Suita Study
2.1.1. Study population and design
The Suita Study is a population-based study with a prospective cohort design. Middle-aged and older adults residing in the urban city of Suita in Southwest Japan were randomly recruited by age category and sex. A baseline assessment, in the form of an interview, clinical examination, blood sample collection, and ECG, was performed at the National Cerebral and Cardiovascular Center (NCVC) in Suita. Then, participants were asked to attend follow-ups every couple of years at the same place [34–39].
In this study, a total of 3,573 participants (1,590 men and 1,983 women), aged 40–92 years, were included. However, we excluded participants with critical missing data (n = 5), participants who quit drinking (n = 140), participants with a previous history of HF or brain natriuretic peptide (BNP) levels ≥ 100 pg/mL (n = 181), or participants who were lost to follow-up (n = 535), leaving 2,712 participants (1,149 men and 1,563 women) for analysis. In both sexes, those who were lost to follow-up were significantly older and included higher proportions of CVD risk factors than participants in the analysis group (Supplementary file 1).
2.1.2. Assessment of alcohol consumption
Alcohol consumption was assessed using a baseline questionnaire administered by an interviewer. Participants were asked about the frequency of alcohol consumption during a typical week and the amount of alcohol consumed on each occasion. Alcohol consumption was measured by gou, a traditional Japanese measurement unit expressing an amount of alcohol equivalent to two drinks or 23 g of pure alcohol [39]. In the current study, light, moderate, and heavy alcohol consumption was defined as daily drinking equivalent to <one gou in men or <0.5 gou in women, ≥one but <two gou in men or ≥0.5 but <one gou in women, and ≥two gou in men or ≥one gou in women, respectively.
2.1.3. Diagnosis of heart failure
As described elsewhere [38], HF was identified if participants developed BNP levels ≥ 100 pg/mL during the follow-ups that were conducted at the NCVC every two years or were diagnosed with HF by their physicians as shown in their medical records. The Japanese Heart Failure Society (JHFS) suggested the applied BNP cut-off as a likely indicator for HF [40]. The diagnostic accuracy of BNP in HF was confirmed in a previous meta-analysis [41]. BNP, in this study, was measured using the Chemiluminescent Enzyme Immunoassay (CLEIA) as described elsewhere [42].
2.1.4. Statistical analyses
We used the SAS version 9.4 software (SAS Institute Inc, Cary, NC) for statistical analyses. The differences in baseline characteristics of participants by their alcohol consumption (never, light, moderate, and heavy) were assessed by the Chi-squared test for categorical variables and one-way ANOVA for continuous variables. Then, we performed the Cox proportional hazards models to calculate the age-adjusted and the multivariable-adjusted hazard ratios (HRs) and their 95% confidence intervals (95% CIs) of HF risk for never, moderate, and heavy drinkers compared to light drinkers in the overall population and population with no preceding stroke or coronary heart disease (CHD). Person-years of follow-up were calculated from baseline examination (between 2005 and 2016) to the date of HF diagnosis, death, censoring, or the last health examination (up to 2020), whichever came first.
We further stratified the results by age group (<70 and ≥70 years), body mass index (BMI) (<25 and ≥25 kg/m2), smoking behavior (never and ever), blood pressure (<140/90 and ≥140/90 mmHg), hypertension medication (yes and no), and high-density lipoprotein (HDL) (<50 and ≥50 mg/dL in men and <60 and ≥60 mg/dL in women), and the corresponding p-interactions were computed. All analyses were stratified by sex.
The following baseline characteristics were included in the Cox regression models: age (continuous), BMI (continuous), smoking (never, former, or current), systolic and diastolic blood pressure (continuous), hypertension medication (yes or no), fasting blood glucose (FBG) (continuous), glomerular filtration rate (GFR) (continuous), HDL (continuous), lipid-lowering agent use (yes or no), arrhythmia including atrial fibrillation (yes or no), cardiac murmurs or valvular diseases (yes or no), and preceding stroke or CHD (yes or no).
2.2. The meta-analysis
2.2.1. Literature search
The first-and second-place authors searched MEDLINE (PubMed) and Scopus for potential studies published in English before the 15th of June 2022 (the last day of the data search) using the following terms: (Alcohol) AND (Heart failure). The search strategy was provided (Supplementary file 2). In addition, we manually searched the reference lists of the selected articles to obtain additional relevant studies. We reported this meta-analysis per the checklists of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [43] and Meta-analyses Of Observational Studies in Epidemiology (MOOSE) [44].
2.2.2. Study selection
Studies abiding by the following criteria were selected for the meta-analysis: 1) heavy alcohol drinking and alcohol abstinence were the exposures, 2) light or moderate drinking was the reference group, 3) HF was the outcome, and 4) the study had a prospective cohort design. No limits were set regarding the year of publication, but we did not manage to obtain unpublished data. The following relevant information was extracted from the selected studies: study ID (last name of the first author, study name and place, and publication year), frequency, age, and sex of the study populations, alcohol consumption cut-offs for heavy drinking and the reference group, follow-up years, number of HF incident cases, and covariates included in the most adjusted regression models. We assessed the quality of studies per the modified Newcastle-Ottawa Quality Assessment Scale (NOS) based on studies’ selection (representativeness, selection of the non-exposed, ascertainment of the exposure, and demonstration of the outcome), comparability, and outcome (assessment, follow-up length, and adequacy) [45].
2.2.3. Statistical analysis
We applied the random-effects model to compute the pooled HR with 95% CI of HF risk for heavy drinking and alcohol abstinence compared to light or moderate drinking [46], the I2 statistic to measure heterogeneity across studies [47], and the regression test for funnel plot asymmetry to assess publication bias [48]. To test the impact of each study, we removed studies one by one and combined the remaining studies in separate meta-analyses. We used the R-3.2.0 statistical package (Metafor: Meta-Analysis Package for R) for meta-analysis [49].
3. Results
3.1. The Suita Study
Among the 1,149 included men, 340 (29.6%) were never drinkers, 212 (18.5%) were light drinkers, 291 (25.3%) were moderate drinkers, and 306 (26.6%) were heavy drinkers. Among the 1,563 included women, 1,162 (74.4%) were never drinkers, 94 (6.0%) were light drinkers, 155 (9.9%) were moderate drinkers, and 152 (9.7%) were heavy drinkers. Men and women who reported heavy drinking were more likely to be current smokers, but they were significantly younger and had higher HDL levels (p < 0.05) (Table 1).
Table 1 Characteristics of participating men and women by alcohol consumption
| Characteristics |
Alcohol consumption |
P-value |
| Never |
Light |
Moderate |
Heavy |
| Men |
| Frequency |
340 |
212 |
291 |
306 |
— |
| Age, years* |
68.4 ± 10.5 |
69.3 ± 10.8 |
68.0 ± 9.7 |
63.3 ± 8.8 |
<0.001 |
| Body mass index, kg/m2 * |
23.3 ± 3.1 |
23.5 ± 2.7 |
23.3 ± 2.5 |
23.5 ± 3.0 |
0.055 |
| Smoking, % |
Never |
35.9 |
28.8 |
21.3 |
17.3 |
<0.001 |
| Former |
44.1 |
54.7 |
61.5 |
44.5 |
| Current |
20.0 |
16.5 |
17.2 |
38.2 |
| Systolic blood pressure, mmHg* |
128.6 ± 18.2 |
129.1 ± 17.9 |
130.2 ± 18.0 |
131.9 ± 18.3 |
0.975 |
| Diastolic blood pressure, mmHg* |
78.8 ± 10.5 |
79.4 ± 10.1 |
79.9 ± 9.7 |
83.1 ± 10.4 |
0.525 |
| Hypertension medication, % |
30.6 |
26.9 |
36.8 |
32.7 |
0.113 |
| Fasting blood glucose, mg/dL* |
107.6 ± 26.2 |
106.0 ± 23.2 |
108.6 ± 23.4 |
109.5 ± 22.8 |
0.871 |
| Glomerular filtration rate, ml/min/1.73 m2 * |
72.7 ± 16.8 |
73.0 ± 15.1 |
72.8 ± 14.7 |
78.3 ± 14.8 |
0.232 |
| High-density lipoprotein-cholesterol, mg/dL* |
50.8 ± 12.5 |
54.2 ± 13.5 |
57.3 ± 14.3 |
61.5 ± 16.4 |
0.006 |
| Lipid-lowering agent, % |
17.4 |
12.7 |
16.5 |
10.1 |
0.037 |
| Cardiac murmur or valvular disease, % |
5.9 |
8.5 |
7.6 |
4.6 |
0.259 |
| Arrhythmia including atrial fibrillation, % |
16.8 |
18.9 |
14.4 |
12.8 |
0.233 |
| Preceding stroke or coronary heart disease, % |
9.4 |
7.6 |
8.9 |
7.5 |
0.786 |
| Women |
| Frequency |
1,162 |
94 |
155 |
152 |
— |
| Age, years* |
66.7 ± 10.0 |
66.1 ± 9.9 |
61.3 ± 8.2 |
60.4 ± 9.1 |
<0.001 |
| Body mass index, kg/m2 * |
22.3 ± 3.4 |
21.8 ± 2.6 |
22.0 ± 2.8 |
22.4 ± 3.1 |
0.199 |
| Smoking, % |
Never |
92.4 |
93.6 |
82.6 |
71.1 |
<0.001 |
| Former |
4.6 |
4.3 |
11.6 |
10.5 |
| Current |
3.0 |
2.1 |
5.8 |
18.4 |
| Systolic blood pressure, mmHg* |
125.9 ± 20.6 |
124.5 ± 17.9 |
121.0 ± 19.8 |
123.9 ± 19.2 |
0.032 |
| Diastolic blood pressure, mmHg* |
74.8 ± 11.0 |
75.9 ± 10.4 |
73.6 ± 11.6 |
76.2 ± 11.6 |
0.644 |
| Hypertension medication, % |
26.8 |
23.4 |
18.1 |
27.6 |
0.114 |
| Fasting blood glucose, mg/dL* |
99.4 ± 14.8 |
97.6 ± 9.0 |
96.8 ± 10.2 |
98.6 ± 12.3 |
0.031 |
| Glomerular filtration rate, ml/min/1.73 m2 * |
76.4 ± 16.1 |
76.5 ± 15.1 |
79.9 ± 13.0 |
80.6 ± 14.8 |
0.001 |
| High-density lipoprotein-cholesterol, mg/dL* |
64.4 ± 14.5 |
66.2 ± 12.9 |
69.5 ± 15.6 |
72.5 ± 15.8 |
<0.001 |
| Lipid-lowering agent, % |
24.4 |
21.3 |
12.3 |
11.8 |
<0.001 |
| Cardiac murmur or valvular disease, % |
8.4 |
8.5 |
3.9 |
4.6 |
0.108 |
| Arrhythmia including atrial fibrillation, % |
13.3 |
16.0 |
9.7 |
5.9 |
0.032 |
| Preceding stroke or coronary heart disease, % |
5.3 |
2.1 |
0.0 |
1.3 |
0.003 |
*Mean ± standard deviation for all such variables
In men, within 8,619 person-years (median follow-up = 8 years), 162 HF cases were detected. The incidence of HF / 1,000 person-years was distributed as follows: 20.5 in never drinkers, 16.1 in light drinkers, 19.0 in moderate drinkers, and 18.6 in heavy drinkers. Compared to light drinkers, heavy and never drinkers showed a higher risk of HF in the multivariable-adjusted models: HRs (95% CIs) = 2.14 (1.26, 3.66) and 1.65 (1.00, 2.73), respectively. Moderate drinkers showed a slightly higher HF risk than light drinkers, but this rise was statistically insignificant. However, in current drinkers, the amount of alcohol consumed was not linearly associated with HF risk. Among participants with no preceding stroke or CHD, the risk of HF in heavy and never drinkers, like the overall population, was elevated: HRs (95% CIs) = 2.41 (1.33, 4.37) and 1.74 (1.01, 3.00), respectively. Yet, unlike the overall population, every 23 g/day increase in alcohol consumption was associated with a 19% increase in HF risk. In women, within 12,059 person-years (median follow-up = 8 years), 157 HF cases were detected. The incidence of HF / 1,000 person-years was distributed as follows: 13.8 in never drinkers, 13.6 in light drinkers, 12.1 in moderate drinkers, and 8.6 in heavy drinkers. Unlike men, women who reported heavy and never drinking showed no excess HF risk (Table 2).
Table 2 Association between alcohol consumption and heart failure risk in Japanese men and women
| |
Alcohol consumption |
P for trend |
Per 23 g/day increase* |
| Never |
Light |
Moderate |
Heavy |
| All men |
| Incident cases |
50 |
25 |
42 |
45 |
— |
— |
| Incidence / 1,000 person-years |
20.5 |
16.1 |
19.0 |
18.6 |
— |
— |
| Model I, HR (95% CI) |
1.48 (0.91, 2.41) |
1 (Ref) |
1.38 (0.84, 2.29) |
2.40 (1.44, 4.02) |
0.008 |
1.03 (0.97, 1.09) |
| Model II, HR (95% CI) |
1.62 (0.99, 2.67) |
1 (Ref) |
1.31 (0.79, 2.19) |
2.24 (1.31, 3.83) |
0.026 |
1.02 (0.96, 1.09) |
| Model III, HR (95% CI) |
1.65 (1.00, 2.73) |
1 (Ref) |
1.27 (0.76, 2.12) |
2.14 (1.26, 3.66) |
0.019 |
1.01 (0.94, 1.08) |
| Men with no preceding stroke or coronary heart disease |
| Incident cases |
43 |
21 |
32 |
39 |
— |
— |
| Incidence / 1,000 person-years |
19.1 |
14.5 |
15.7 |
17.1 |
— |
— |
| Model I, HR (95% CI) |
1.55 (0.91, 2.65) |
1 (Ref) |
1.31 (0.75, 2.30) |
2.60 (1.48, 4.57) |
0.011 |
1.24 (1.06, 1.45) |
| Model II, HR (95% CI) |
1.72 (1.00, 2.97) |
1 (Ref) |
1.20 (0.68, 2.13) |
2.22 (1.23, 4.00) |
0.032 |
1.19 (0.99, 1.43) |
| Model III, HR (95% CI) |
1.74 (1.01, 3.00) |
1 (Ref) |
1.27 (0.72, 2.26) |
2.41 (1.33, 4.37) |
0.018 |
1.19 (1.00, 1.42) |
| All women |
| Incident cases |
120 |
10 |
16 |
11 |
— |
— |
| Incidence / 1,000 person-years |
13.8 |
13.6 |
12.1 |
8.6 |
— |
— |
| Model I, HR (95% CI) |
0.78 (0.41, 1.50) |
1 (Ref) |
1.22 (0.55, 2.70) |
1.01 (0.43, 2.40) |
0.931 |
1.06 (0.94, 1.19) |
| Model II, HR (95% CI) |
0.79 (0.42, 1.52) |
1 (Ref) |
1.24 (0.56, 2.76) |
0.98 (0.41, 2.35) |
0.952 |
1.06 (0.93, 1.20) |
| Model III, HR (95% CI) |
0.73 (0.38, 1.41) |
1 (Ref) |
1.24 (0.56, 2.76) |
0.95 (0.40, 2.30) |
0.987 |
1.06 (0.94, 1.19) |
| Women with no preceding stroke or coronary heart disease |
| Incident cases |
106 |
9 |
16 |
11 |
— |
— |
| Incidence / 1,000 person-years |
12.7 |
12.3 |
12.1 |
8.7 |
— |
— |
| Model I, HR (95% CI) |
0.81 (0.41, 1.60) |
1 (Ref) |
1.34 (0.59, 3.04) |
1.17 (0.48, 2.83) |
0.715 |
1.07 (0.96, 1.18) |
| Model II, HR (95% CI) |
0.83 (0.42, 1.65) |
1 (Ref) |
1.34 (0.59, 3.08) |
1.11 (0.45, 2.75) |
0.750 |
1.06 (0.94, 1.20) |
| Model III, HR (95% CI) |
0.81 (0.41, 1.61) |
1 (Ref) |
1.37 (0.59, 3.14) |
1.14 (0.46, 2.83) |
0.724 |
1.06 (0.95, 1.19) |
*In current drinkers only (23 g of alcohol is equivalent to 1 gou or approximately 2 drinks)
Model I: Adjusted for age
Model II: Adjusted further for body mass index, smoking, systolic and diastolic blood pressure, hypertension medication, fasting blood glucose, glomerular filtration rate, high-density lipoprotein-cholesterol, and lipid-lowering agent
Model III: Adjusted further for cardiac murmur or valvular disease, arrhythmia, and preceding stroke or coronary heart disease
P-interaction for preceding stroke or coronary heart disease = 0.398 in men and 0.985 in women
In the stratified analysis, the risk of HF was more prominent among men with BMI ≥ 25 kg/m2 than their counterparts with BMI < 25 kg/m2; HRs (95% CIs) = 2.27 (0.68, 7.54) versus 1.69 (0.96, 2.99) in never drinkers, 3.01 (0.95, 9.53) versus 1.05 (0.58, 1.90) in moderate drinkers, and 3.73 (1.14, 12.22) versus 2.03 (1.08, 3.84) in heavy drinkers, respectively (p-interaction = 0.014). Besides, the association between heavy drinking and HF risk was more robust among ever-smoking than never-smoking men; HRs (95% CIs) = 2.33 (1.27, 4.27) and 1.69 (0.54, 5.26), respectively (p-interaction = 0.106) (Table 3).
Table 3 Multivariable-adjusted associations between alcohol consumption and heart failure risk by several factors
| Variables |
Alcohol consumption |
P-interaction |
| Never |
Light |
Moderate |
Heavy |
| Men |
| Age (years) |
<70 |
1.25 (0.42, 3.69) |
1 (Ref) |
1.66 (0.58, 4.80) |
2.00 (0.71, 5.60) |
0.698 |
| ≥70 |
1.87 (1.04, 3.39) |
1 (Ref) |
1.14 (0.62, 2.08) |
2.17 (1.12, 4.20) |
Body mass index
(kg/m2) |
<25 |
1.69 (0.96, 2.99) |
1 (Ref) |
1.05 (0.58, 1.90) |
2.03 (1.08, 3.84) |
0.014 |
| ≥25 |
2.27 (0.68, 7.54) |
1 (Ref) |
3.01 (0.95, 9.53) |
3.73 (1.14, 12.22) |
| Smoking |
Never |
2.16 (0.85, 5.49) |
1 (Ref) |
1.14 (0.37, 3.54) |
1.69 (0.54, 5.26) |
0.106 |
| Ever |
1.50 (0.81, 2.77) |
1 (Ref) |
1.33 (0.74, 2.37) |
2.33 (1.27, 4.27) |
Blood pressure
(mmHg) |
<140/90 |
1.67 (0.85, 3.31) |
1 (Ref) |
1.70 (0.85, 3.43) |
3.02 (1.47, 6.22) |
0.678 |
| ≥140/90 |
1.62 (0.74, 3.54) |
1 (Ref) |
0.97 (0.46, 2.06) |
1.56 (0.69, 3.53) |
Hypertension
medication |
Yes |
2.05 (0.88, 4.77) |
1 (Ref) |
1.38 (0.59, 3.25) |
1.74 (0.70, 4.34) |
0.196 |
| No |
1.27 (0.67, 2.41) |
1 (Ref) |
1.10 (0.58, 2.10) |
2.51 (1.27, 4.97) |
High-density
lipoprotein (mg/dL) |
≥50 |
1.61 (0.82, 3.17) |
1 (Ref) |
1.66 (0.86, 3.19) |
2.40 (1.24, 4.64) |
0.706 |
| <50 |
1.55 (0.73, 3.29) |
1 (Ref) |
0.91 (0.37, 2.23) |
2.07 (0.77, 5.60) |
| Women |
| Age (years) |
<70 |
1.74 (0.41, 7.35) |
1 (Ref) |
2.54 (0.54, 12.05) |
1.95 (0.37, 10.14) |
0.604 |
| ≥70 |
0.52 (0.25, 1.10) |
1 (Ref) |
1.07 (0.38, 3.01) |
0.88 (0.28, 2.83) |
Body mass index
(kg/m2) |
<25 |
0.77 (0.37, 1.60) |
1 (Ref) |
1.63 (0.68, 3.90) |
0.78 (0.28, 2.21) |
0.588 |
| ≥25 |
0.20 (0.04, 1.01) |
1 (Ref) |
0.09 (0.01, 1.15) |
0.59 (0.08, 4.15) |
| Smoking |
Never |
0.76 (0.38, 1.51) |
1 (Ref) |
1.10 (0.45, 2.67) |
0.92 (0.34, 2.50) |
0.018 |
| Ever |
0.14 (0.01, 3.78) |
1 (Ref) |
1.05 (0.04, 25.25) |
0.53 (0.02, 13.94) |
Blood pressure
(mmHg) |
<140/90 |
1.38 (0.50, 3.79) |
1 (Ref) |
2.45 (0.77, 7.79) |
2.10 (0.61, 7.22) |
0.631 |
| ≥140/90 |
0.28 (0.11, 0.72) |
1 (Ref) |
0.47 (0.13, 1.70) |
0.29 (0.07, 1.23) |
Hypertension
medication |
Yes |
0.36 (0.14, 0.89) |
1 (Ref) |
0.47 (0.12, 1.80) |
1.25 (0.39, 4.02) |
0.062 |
| No |
1.18 (0.42, 3.30) |
1 (Ref) |
2.07 (0.66, 6.53) |
0.65 (0.14, 3.05) |
High-density
lipoprotein (mg/dL) |
≥60 |
0.66 (0.31, 1.40) |
1 (Ref) |
1.37 (0.56, 3.39) |
0.91 (0.34, 2.43) |
0.309 |
| <60 |
0.82 (0.19, 3.56) |
1 (Ref) |
0.87 (0.14, 5.58) |
1.09 (0.13, 9.26) |
After removing duplicates, review articles, and irrelevant studies, a shortlist of 183 studies was made. We further removed 179 studies for not investigating HF (n = 164), assigning alcohol abstinence as a reference group (n = 13), and performing a Mendelian randomization analysis (n = 2). Eventually, four studies (five cohorts) were found eligible [24, 27–29], and their results were combined with those from the Suita Study (two cohorts) in this meta-analysis (Fig. 1). The included studies used data from the British Regional Heart Study (BRHS) [24], the British ClinicAl research using LInked Bespoke studies and Electronic health Records (CALIBER) [27], the Cohort of Swedish Men (COSM) [28], the Swedish Mammography Cohort (SMC) [28], 83 cohorts from 19 high-income countries [29], and the Japanese Suita Study (Table 4). All studies were of good quality per the modified NOS (Supplementary file 3).

Table 4 Summary of the studies included in the meta-analysis
| Study ID |
Population |
Drinking |
Follow-up |
Incident HF |
Covariates |
Wannamethee et al. (2015)
BRHS, UK |
n = 3,530
60–79 years
Men |
Heavy: ≥35 drinks/week
Ref: 1–6 drinks/week |
11 years
Mean |
n = 120 |
1, 3, 4, 5, 6,
7, 8, 9, 10 |
Bell et al. (2017)
CALIBER, UK |
n = 1,937,360
≥30 years
Men and women
No cardiovascular disease |
Heavy: ≥21 drinks/week in men
and ≥14 drinks/week in women
Ref: <21 drinks/week in men
and <14 drinks/week in women |
6 years
Median |
n = 14,359 |
1, 2, 3, 4, 5,
6, 7 |
Larsson et al. (2017)
COSM, Sweden |
n = 40,590
45–79 years
Men |
Heavy: >28 drinks/week
Ref: <1 drink/week |
11.9 years
Mean |
n = 1,905 |
1, 3, 4, 5, 6,
7, 8, 9, 10 |
Larsson et al. (2017)
SMC, Sweden |
n = 34,022
49–83 years
Women |
Heavy: >21 drinks/week
Ref: <1 drink/week |
12.2 years
Mean |
n = 1,328 |
1, 3, 4, 5, 6,
7, 8, 9, 10 |
Wood et al. (2018)
83 cohorts, 19 countries |
n = 670,923
Mean age: 50s
Men and women
No cardiovascular disease |
Heavy: 300 g/week
Ref: <25 g/week |
7.5 years
Mean |
n = 2,711 |
1, 2, 4, 6 |
Arafa et al. (2022)
Suita Study, Japan |
n = 2,712
40–92 years
Men and women |
Heavy: ≥2 gou/day in men
and ≥1 gou/day in women
Ref: <1 gou/day in men
and <0.5 gou/day in women |
8 years
Median |
n = 319 |
1, 3, 4, 5, 6,
7, 8, 9, 10 |
Covariates: 1- age, 2- sex, 3- body mass index, 4- smoking, 5- hypertension, 6- diabetes, 7- dyslipidemia, 8- chronic kidney disease, 9- arrhythmia, 10- preceding or incident stroke or coronary heart disease
The meta-analysis that assessed the relationship between heavy drinking and HF risk included seven cohorts with the following weights: BRHS (5.8%), CALIBER (29.5%), COSM (19.0%), SMC (4.0%), 83 Cohort (30.2%), and the Suita Study in men (8.1%) and in women (3.4%). Heavy drinking was associated with the increased risk of HF: HR (95% CI) = 1.37 (1.15, 1.62). A moderate heterogeneity across studies was detected (I2 = 43.16% and p-heterogeneity = 0.103) (Fig. 2). Removing the CALIBER study significantly minimized the heterogeneity (I2 = 18.72%) and modestly strengthened the association: HR (95% CI) = 1.44 (1.21, 1.72) (Supplementary file 4). We detected no publication bias (Z = −0.392, p-publication bias = 0.695) (Supplementary file 5).

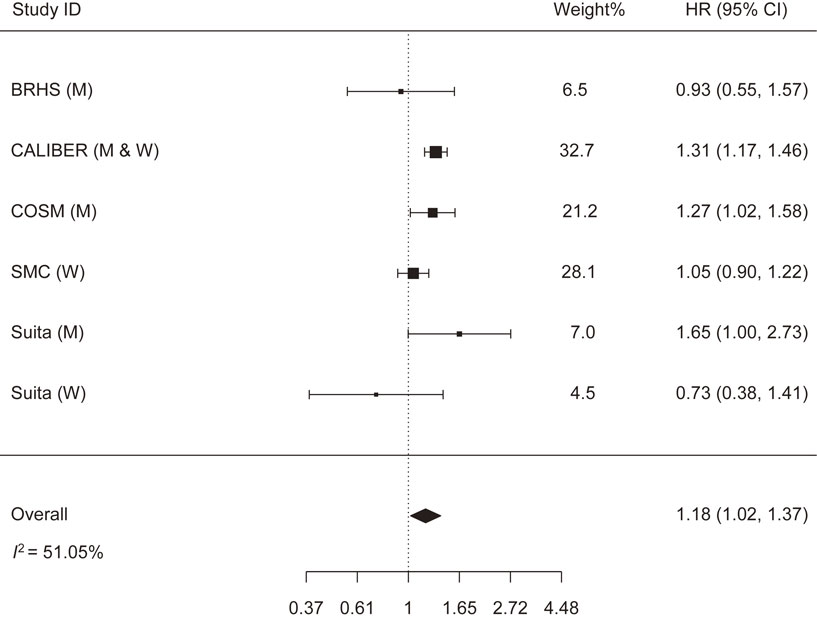
The meta-analysis that assessed the relationship between alcohol abstinence and HF risk included six cohorts with the following weights: BRHS (6.5%), CALIBER (32.7%), COSM (21.2%), SMC (28.1%), and the Suita Study in men (7.0%) and in women (4.5%). It should be noted that the studies of CALIBER, COSM, SMC, and Suita confined their alcohol abstinence group to never-drinkers, while the BRHS study included non-drinkers who might have included former drinkers. Alcohol abstinence was significantly associated with increased HF risk: HR (95% CI) = 1.18 (1.02, 1.37). The heterogeneity across studies was moderate (I2 = 51.05% and p-heterogeneity = 0.069) (Fig. 3). Removing the SMC study almost halved the heterogeneity (I2 = 25.88%), while the associations remained significant: HR (95% CI) = 1.26 (1.08, 1.45) (Supplementary file 4). No publication bias was identified (Z = −0.743, p-publication bias = 0.457) (Supplementary file 6).
4. Discussion
The Suita Study indicated higher HF risk in heavy and never drinking men than light drinkers by 114% and 65%, respectively. A similar pattern was detected in the meta-analysis which included the prospective cohort studies that assigned light or moderate drinkers as a reference group; the HF risk increased by 37% in heavy drinkers and 18% in abstainers. Our findings reflect the need to consider alcohol consumption in future HF risk scores and target heavy drinkers for screening and intervention.
Previous studies assessing the relationship between alcohol consumption and HF risk investigated Western populations and reached inconsistent findings. The Established Populations for the Epidemiologic Study on the Elderly program (EPESE) [14], the Framingham Heart Study [15], the Cardiovascular Health Study (CHS) [18], the Physicians’ Health Study I (PHS I) [19, 20], the Stockholm Heart Epidemiology Program (SHEEP) [21], the Atherosclerosis Risk in Communities Study (ARIC) [23], and the Nord-Trøndelag Health Study (HUNT) [25] indicated that drinking in moderation could carry protective effects against HF risk, while heavy drinking was not associated with the increased risk of HF. However, these studies assigned abstainers as a reference group [14, 15, 18–21, 23, 25], and many of them used low cut-offs to define the highest category of alcohol consumption [14, 19–21, 25]. A meta-analysis of eight prospective cohort studies reported that compared to abstainers, light-to-moderate drinking led to a 15% reduction in HF risk, but heavy drinking did not elevate HF risk [50]. On the other hand, heavy drinking was positively associated with HF risk in the BRHS [24], CALIBER [27], and COSM [28] studies. The increased HF risk with alcohol abstinence was reported in the studies of CALIBER [27] and COSM [28] but not in BRHS [24]. The three studies assigned light or moderate drinkers as a reference group and used high cut-offs of alcohol consumption to define heavy drinking. Importantly, when we repeated the analysis in the Suita Study with never-drinkers assigned as a reference group, the association between heavy drinking and HF risk disappeared (data not shown). When we conducted a meta-analysis on studies that assigned alcohol abstinence as a reference group, no association was detected between heavy drinking and HF risk (data not shown). Therefore, we believe that the main reason for not detecting the impact of heavy drinking on HF risk in previous studies was assigning abstainers, who are at higher risk of HF than light drinkers, as a reference group.
In line with our findings, several Japanese epidemiological studies revealed J- or U-shaped associations between alcohol consumption and CVD among men. A study including 34,776 men (40–79 years) from the Japan Collaborative Cohort Study (JACC) showed increased CVD mortality among heavy drinkers and decreased CVD mortality among light-to-moderate drinkers [51]. Another study including 2,890 men (40–69 years) from three rural communities in Japan showed a J-shaped relationship between alcohol consumption and the risk of non-hemorrhagic stroke [52]. In 2,336 men (30–79 years) from the Suita Study, a U-shaped association between alcohol consumption and the risk of CVD and CHD was detected in hypertensives without hypertension medication [53].
While the pathophysiologic mechanisms illustrating the cardiotoxic effects of heavy drinking are well-described [10–12], those explaining the protective role of light drinking against the risk of HF are still obscure. Previous studies have demonstrated the beneficial effects of consuming alcohol in moderation on HDL levels [54] and insulin sensitivity [55]. Yet, adjusting for HDL and FBG in the current study did not affect the results. In addition, it was suggested that alcohol consumption might protect from incident HF by reducing CHD risk [14, 17, 19]. The US Kaiser Permanente study revealed a significant association between heavy drinking and increased risk of non-CHD-HF and apparent protection against CAD-HF risk [17]. Due to the limited number of participants with preceding stroke or CHD in the Suita Study, we could not stratify the results by this variable. Though, when we repeated the analysis after excluding those with preceding stroke or CHD, the association between heavy drinking and HF became stronger. Besides, a positive linear association between the amount of consumed alcohol and HF risk was noticed only among men with no preceding stroke or CHD; each 23 g increment in daily alcohol consumption was associated with a 19% increase in HF risk. However, the preceding stroke or CHD did not materially change the results and the preceding stroke or CHD variable did not interact with the association between alcohol consumption and HF risk in both sexes. Thus, we assume that cardiac ischemia did not solely explain the association between heavy drinking and HF risk. According to the sick quitter hypothesis, it could be speculated that some former drinkers who quit drinking for medical conditions might have inappropriately defined themselves as never-drinkers [32, 56]. Many studies reported an increased HF risk in former drinkers [17, 21, 22].
The association between heavy drinking and HF risk in the Suita Study was more pronounced among overweight/obese and ever-smoking men. Both obesity and smoking are major risk factors for CVD, including HF [57–59]. Our findings suggest a combined effect of alcohol consumption and other traditional CVD risk factors, such as obesity and smoking, on HF risk.
The strengths of the Suita Study are exploring the association between alcohol consumption and HF risk in a representative Asian population, applying a prospective design with frequent follow-ups, and controlling the results for most potential confounders. Still, some limitations should be addressed. First, although HF is typically diagnosed using a combination of clinical symptoms and echocardiography findings [41], we determined HF during follow-up using BNP levels or other physicians’ diagnoses. However, BNP has high diagnostic accuracy and BNP ≥ 100 pg/mL is likely to indicate HF [41]. Besides, previous population-based cross-sectional studies showed a strong association between heavy drinking and high BNP levels [60, 61]. Second, age, weight, and arrhythmia can affect BNP levels [62], suggesting misclassification. Though, adjusting for these variables did not materially change the results. Third, the absence of echocardiography findings did not allow us to investigate the relationship between alcohol consumption and HF subtypes. Fourth, alcohol consumption was self-reported; therefore, participants might have underreported the amount of their consumption. Though, since the baseline analysis showed a positive correlation between the amount of alcohol and HDL, a biological marker of alcohol consumption [63], this possibility is unlikely. Fifth, we did not stratify the results by the type of alcoholic beverage. However, the EPESE study showed similar associations with incident HF in beer, wine, and liquor [14]. Sixth, compared to the analysis group, those who lost to follow-up were significantly older and had higher proportions of hypertension, a cardiac murmur or valvular disease, arrhythmia, and preceding CVD, suggesting that they were more likely to develop HF.
Our meta-analysis had several strengths. First, we confined the meta-analysis to studies that assigned light or moderate drinkers as a reference group to avoid a potential spurious insignificant association between alcohol consumption and HF risk if never drinkers were assigned as a reference group. Second, all included studies used high cut-offs of alcohol consumption to define heavy drinking (at least three drinks/day). Further, the included studies were of good quality and had a prospective cohort design to avoid recall bias and imply a temporal association. Finally, our meta-analysis did not show publication bias, yet this possibility cannot be entirely excluded. It should be noted that the non-drinkers’ group in BRHS included former and never-drinkers; therefore, we used the term alcohol abstinence for the meta-analysis. Still, removing BRHS from the meta-analysis did not change the results.
The Suita Study and the meta-analysis reported a J-shaped association between alcohol consumption and HF risk. Alcohol consumption appears to have a complex relationship with incident HF. While heavy drinking may contribute to HF development, light drinking is not cardiotoxic and can even have a protective role against HF. Besides, the Suita Study showed that the impact of heavy drinking on HF was augmented among ever smokers and overweight/obese people, suggesting that these groups should be prioritized in screenings and other preventive approaches. Heavy drinking should be involved in clinical risk scores predicting HF.
Abbreviations
ARIC
Atherosclerosis Risk in Communities Study
BMI
Body mass index
BNP
Brain natriuretic peptide
BRHS
British Regional Heart Study
CALIBER
ClinicAl research using LInked Bespoke studies and Electronic health Records
CHD
Coronary heart disease
CHS
Cardiovascular Health Study
CI
Confidence interval
CLEIA
Chemiluminescent Enzyme Immunoassay
COSM
Cohort of Swedish Men
CVD
Cardiovascular disease
EPESE
Established Populations for the Epidemiologic Study on the Elderly program
FBG
Fasting blood glucose
HDL
High-density lipoprotein
HF
Heart failure
HR
Hazard ratio
HUNT
Nord-Trøndelag Health Study
JACC
Japan Collaborative Cohort Study
JHFS
Japanese Heart Failure Society
Metafor
Meta-Analysis Package for R
NCVC
National Cerebral and Cardiovascular Center
NOS
Newcastle-Ottawa Quality Assessment Scale
PHS I
Physicians’ Health Study I
SHEEP
Stockholm Heart Epidemiology Program
SMC
Swedish Mammography Cohort
Declarations
Ethical approval and consent to participate
The Suita Study protocol was approved by the Institutional Review Board of the NCVC (M19-005-8). We conducted the study according to the Declaration of Helsinki. All participants signed their informed consent forms before participation.
Consent for publication
We obtained consent from patients to publish their findings while keeping their details anonymous and all authors accepted the final version of the manuscript.
Availability of data and materials
Available upon reasonable request.
Competing interests
None to declare.
Funding
This study was supported by Grants-in-Aid for Scientific Research in Japan (B, No. 16H05252), the Intramural Research Fund for the cardiovascular diseases of the NCVC (20-4-9), Japan Health Research Promotion Bureau (2019-(1)-1), Japan Science and Technology Agency (JPMJPF2018), the Health and Labour Sciences Research Grants of the Ministry of Health, Labour and Welfare of Japan (20FA1002), and the Meiji Yasuda Research Institute and Life Insurance Company.
Role of the funder
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgments
We would like to thank the Suita Medical Association, the members of Suita City Health Center, the Preventive Cardiology and Preventive Healthcare Departments staff, and all cohort members.
Code availability
Not applicable.
Authors’ contributions
AA (draft writing, review literature, and data analysis), RK (data check, review literature, and study coordination), YK (resources, funding acquisition, and supervision), and all authors (visualization, validation, critical revision, and editing).
References
- 1. Komuro I, Kaneko H, Morita H, Isobe M, Nakayama H, Minematsu K, et al. Nationwide actions against heart failure pandemic in Japan - what should we do from academia? Circ J. 2019;83:1819–21.
- 2. Kanaoka K, Okayama S, Nakai M, Sumita Y, Nishimura K, Kawakami R, et al. Hospitalization costs for patients with acute congestive heart failure in Japan. Circ J. 2019;83:1025–31.
- 3. Lesyuk W, Kriza C, Kolominsky-Rabas P. Cost-of-illness studies in heart failure: a systematic review 2004–2016. BMC Cardiovasc Disord. 2018;18:74.
- 4. Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1:4–25.
- 5. Butler J. Primary prevention of heart failure. ISRN Cardiol. 2012;2012:982417.
- 6. Mukamal K, Lazo M. Alcohol and cardiovascular disease. BMJ. 2017;356:j1340.
- 7. Piano MR. Alcohol’s effects on the cardiovascular system. Alcohol Res. 2017;38:219–41.
- 8. Goel S, Sharma A, Garg A. Effect of alcohol consumption on cardiovascular health. Curr Cardiol Rep. 2018;20:19.
- 9. Djoussé L, Gaziano JM. Alcohol consumption and heart failure: a systematic review. Curr Atheroscler Rep. 2008;10:117–20.
- 10. Guzzo-Merello G, Cobo-Marcos M, Gallego-Delgado M, Garcia-Pavia P. Alcoholic cardiomyopathy. World J Cardiol. 2014;6:771–81.
- 11. Piano MR, Phillips SA. Alcoholic cardiomyopathy: pathophysiologic insights. Cardiovasc Toxicol. 2014;14:291–308.
- 12. George A, Figueredo VM. Alcoholic cardiomyopathy: a review. J Card Fail. 2011;17:844–9.
- 13. Cooper HA, Exner DV, Domanski MJ. Light-to-moderate alcohol consumption and prognosis in patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:1753–9.
- 14. Abramson JL, Williams SA, Krumholz HM, Vaccarino V. Moderate alcohol consumption and risk of heart failure among older persons. JAMA. 2001;285:1971–7.
- 15. Walsh CR, Larson MG, Evans JC, Djousse L, Ellison RC, Vasan RS, et al. Alcohol consumption and risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2002;136:181–91.
- 16. Aguilar D, Skali H, Moyé LA, Lewis EF, Gaziano JM, Rutherford JD, et al. Alcohol consumption and prognosis in patients with left ventricular systolic dysfunction after a myocardial infarction. J Am Coll Cardiol. 2004;43:2015–21.
- 17. Klatsky AL, Chartier D, Udaltsova N, Gronningen S, Brar S, Friedman GD, et al. Alcohol drinking and risk of hospitalization for heart failure with and without associated coronary artery disease. Am J Cardiol. 2005;96:346–51.
- 18. Bryson CL, Mukamal KJ, Mittleman MA, Fried LP, Hirsch CH, Kitzman DW, et al. The association of alcohol consumption and incident heart failure: the Cardiovascular Health Study. J Am Coll Cardiol. 2006;48:305–11.
- 19. Djoussé L, Gaziano JM. Alcohol consumption and risk of heart failure in the Physicians’ Health Study I. Circulation. 2007;115:34–9.
- 20. Djoussé L, Gaziano JM. Alcohol consumption and heart failure in hypertensive US male physicians. Am J Cardiol. 2008;102:593–7.
- 21. Janszky I, Ljung R, Ahnve S, Hallqvist J, Bennet AM, Mukamal KJ. Alcohol and long-term prognosis after a first acute myocardial infarction: the SHEEP study. Eur Heart J. 2008;29:45–53.
- 22. Wang Y, Tuomilehto J, Jousilahti P, Antikainen R, Mähönen M, Katzmarzyk PT, et al. Lifestyle factors in relation to heart failure among Finnish men and women. Circ Heart Fail. 2011;4:607–12.
- 23. Gonçalves A, Claggett B, Jhund PS, Rosamond W, Deswal A, Aguilar D, et al. Alcohol consumption and risk of heart failure: the Atherosclerosis Risk in Communities Study. Eur Heart J. 2015;36:939–45.
- 24. Wannamethee SG, Whincup PH, Lennon L, Papacosta O, Shaper AG. Alcohol consumption and risk of incident heart failure in older men: a prospective cohort study. Open Heart. 2015;2:e000266.
- 25. Gémes K, Janszky I, Ahnve S, László KD, Laugsand LE, Vatten LJ, et al. Light-to-moderate drinking and incident heart failure--the Norwegian HUNT study. Int J Cardiol. 2016;203:553–60.
- 26. Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, Psaty BM, et al. Predicting heart failure with preserved and reduced ejection Fraction: the International Collaboration on Heart Failure Subtypes. Circ Heart Fail. 2016;9:10.
- 27. Bell S, Daskalopoulou M, Rapsomaniki E, George J, Britton A, Bobak M, et al. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: population based cohort study using linked health records. BMJ. 2017;356:j909.
- 28. Larsson SC, Wallin A, Wolk A. Contrasting association between alcohol consumption and risk of myocardial infarction and heart failure: two prospective cohorts. Int J Cardiol. 2017;231:207–10.
- 29. Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, et al. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet. 2018;391:1513–23.
- 30. Larsson SC, Burgess S, Mason AM, Michaëlsson K. Alcohol consumption and cardiovascular disease: a Mendelian randomization study. Circ Genom Precis Med. 2020;13:e002814.
- 31. Biddinger KJ, Emdin CA, Haas ME, Wang M, Hindy G, Ellinor PT, et al. Association of habitual alcohol intake with risk of cardiovascular disease. JAMA Netw Open. 2022;5:e223849.
- 32. Fillmore KM, Stockwell T, Chikritzhs T, Bostrom A, Kerr W. Moderate alcohol use and reduced mortality risk: systematic error in prospective studies and new hypotheses. Ann Epidemiol. 2007;17:S16–23.
- 33. Fuchs FD, Chambless LE, Folsom AR, Eigenbrodt ML, Duncan BB, Gilbert A, et al. Association between alcoholic beverage consumption and incidence of coronary heart disease in whites and blacks: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2004;160:466–74.
- 34. Arafa A, Kokubo Y, Sheerah HA, Sakai Y, Watanabe E, Li J, et al. Weight change since age 20 and the risk of cardiovascular disease mortality: a prospective cohort study. J Atheroscler Thromb. 2022;29(10):1511–1521. https://doi.org/10.5551/jat.63191.
- 35. Arafa A, Kokubo Y, Sheerah HA, Sakai Y, Watanabe E, Li J, et al. Developing a stroke risk prediction model using cardiovascular risk factors: the Suita Study. Cerebrovasc Dis. 2022;51:323–30.
- 36. Arafa A, Kokubo Y, Shimamoto K, Kashima R, Watanabe E, Sakai Y, et al. Stair climbing and incident atrial fibrillation: a prospective cohort study. Environ Health Prev Med. 2022;27:10.
- 37. Arafa A, Kokubo Y, Shimamoto K, Kashima R, Watanabe E, Sakai Y, et al. Sleep duration and atrial fibrillation risk in the context of predictive, preventive, and personalized medicine: the Suita Study and meta-analysis of prospective cohort studies. EPMA J. 2022;13:77–86.
- 38. Arafa A, Kokubo Y, Teramoto M, Kashima R, Shimamoto K, Nakao YM, et al. Blood pressure per the 2017 ACC/AHA and 2018 ESC/ESH guidelines and heart failure risk: the Suita Study. Hypertens Res. 2023;46(3):575–582. https://doi.org/10.1038/s41440-022-01128-3.
- 39. Higashiyama A, Okamura T, Watanabe M, Kokubo Y, Wakabayashi I, Okayama A, et al. Alcohol consumption and cardiovascular disease incidence in men with and without hypertension: the Suita study. Hypertens Res. 2013;36:58–64.
- 40. Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, et al. JCS 2017/JHFS 2017 Guideline on Diagnosis and Treatment of Acute and Chronic Heart Failure- Digest Version. Circ J. 2019;83:2084–184.
- 41. Roberts E, Ludman AJ, Dworzynski K, Al-Mohammad A, Cowie MR, McMurray JJ, et al. The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta-analysis in the acute care setting. BMJ. 2015;350:h910.
- 42. Nishikimi T, Okamoto H, Nakamura M, Ogawa N, Horii K, Nagata K, et al. Direct immunochemiluminescent assay for proBNP and total BNP in human plasma proBNP and total BNP levels in normal and heart failure. PLoS One. 2013;8:e53233.
- 43. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097.
- 44. Brooke BS, Schwartz TA, Pawlik TM. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021;156(8):787–8.
- 45. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. Accessed on 16 June 2022.
- 46. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88.
- 47. Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
- 48. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
- 49. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48.
- 50. Larsson SC, Orsini N, Wolk A. Alcohol consumption and risk of heart failure: a dose-response meta-analysis of prospective studies. Eur J Heart Fail. 2015;17:367–73.
- 51. Ikehara S, Iso H, Toyoshima H, Date C, Yamamoto A, Kikuchi S, et al. Alcohol consumption and mortality from stroke and coronary heart disease among Japanese men and women: the Japan collaborative cohort study. Stroke. 2008;39:2936–42.
- 52. Iso H, Kitamura A, Shimamoto T, Sankai T, Naito Y, Sato S, et al. Alcohol intake and the risk of cardiovascular disease in middle-aged Japanese men. Stroke. 1995;26:767–73.
- 53. Higashiyama A, Okamura T, Watanabe M, Kokubo Y, Wakabayashi I, Okayama A, Miyamoto Y. Alcohol consumption and cardiovascular disease incidence in men with and without hypertension: the Suita study. Hypertens Res. 2013;36:58–64.
- 54. De Oliveira E Silva ER, Foster D, McGee Harper M, Seidman CE, Smith JD, Breslow JL, et al. Alcohol consumption raises HDL cholesterol levels by increasing the transport rate of apolipoproteins A-I and A-II. Circulation. 2000;102:2347–52.
- 55. Schrieks IC, Heil AL, Hendriks HF, Mukamal KJ, Beulens JW. The effect of alcohol consumption on insulin sensitivity and glycemic status: a systematic review and meta-analysis of intervention studies. Diabetes Care. 2015;38:723–32.
- 56. Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men: explaining the U-shaped curve. Lancet. 1988;2:1267–73.
- 57. Arafa A, Lee HH, Eshak ES, Shirai K, Liu K, Li J, et al. Modifiable risk factors for cardiovascular disease in Korea and Japan. Korean Circ J. 2021;51:643–55.
- 58. Arafa A, Kokubo Y, Kashima R, Teramoto M, Sakai Y, Nosaka S, et al. The Lifelong Health Support 10: a Japanese prescription for a long and healthy life. Environ Health Prev Med. 2022;27:23.
- 59. Kokubo Y, Padmanabhan S, Iwashima Y, Yamagishi K, Goto A. Gene and environmental interactions according to the components of lifestyle modifications in hypertension guidelines. Environ Health Prev Med. 2019;24:19.
- 60. Leon DA, Shkolnikov VM, Borinskaya S, Casas JP, Evans A, Gil A, et al. Hazardous alcohol consumption is associated with increased levels of B-type natriuretic peptide: evidence from two population-based studies. Eur J Epidemiol. 2013;28:393–404.
- 61. Britton A, O’Neill D, Kuh D, Bell S. Sustained heavy drinking over 25 years is associated with increased N-terminal-pro-B-type natriuretic peptides in early old age: Population-based cohort study. Drug Alcohol Depend. 2020;212:108048.
- 62. Fu S, Ping P, Zhu Q, Ye P, Luo L. Brain natriuretic peptide and its biochemical, analytical, and clinical issues in heart failure: a narrative review. Front Physiol. 2018;9:692.
- 63. Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ. 2011;342:d636.
 https://orcid.org/0000-0002-3335-2243
https://orcid.org/0000-0002-3335-2243