Abstract
Background: This study aimed to investigate diurnal variations in copper-induced hepatic toxicity and the molecular mechanisms underlying this chronotoxicity.
Methods: Male C57BL/6J mice were intraperitoneally injected with copper chloride (CuCl2) at zeitgeber time 2 (ZT2) or 14 (ZT14), twice per week for 5 or 8 weeks. Seventy-two hours after the final CuCl2 injection, the mice were euthanized, and plasma samples were collected. The livers and kidneys were collected and weighed. In vitro experiments were performed to assess cell viability and fluctuations in clock gene expression levels in Hepa1-6 cells after CuCl2 treatment. We examined copper homeostasis- and apoptosis-related genes under clock genes overexpression.
Results: Repeated CuCl2 administration for 8 weeks resulted in more severe toxicity at ZT14 compared to ZT2. CuCl2 administration at ZT14 elevated plasma aspartate aminotransferase, hepatic tumor necrosis factor-α, and interleukin-6 for 5 weeks, whereas the toxic effects of CuCl2 administration at ZT2 were weaker. Moreover, CuCl2 treatment inhibited Hepa1-6 cell viability in a dose-dependent manner. We observed increased expression of three clock genes (Ciart, Cry2, and Per1) after CuCl2 treatment. Among them, overexpression of Cry2 and Per1 accelerated CuCl2-induced inhibition of Hepa1-6 cell viability. Moreover, we found that the overexpression of Cry2 and Per1 regulates cleaved caspase-3 by modulating the copper transporter genes ATP7B and CTR1.
Conclusion: These results suggest that CuCl2-induced diurnal toxicity is associated with Cry2 and Per1 expression through the regulation of copper transporter genes in mice.
Background
Shift work is a common practice in various industries and services, including steel factories, power plants, nursing, and police forces, in which professionals work during both day and night shifts [1]. In the United States of America, approximately 20–30% of employees are categorized as shift workers [2]. However, shiftwork has been associated with various health issues, such as cancer, obesity, and cardiovascular disease, due to disruptions in circadian rhythms [3–6]. Factors contributing to circadian rhythm disruption include light exposure, specific diets (i.e., high-fat diet), chemical exposures, and radiation exposures [7–9]. Therefore, preventing unexpected circadian rhythm disorders is essential.
Copper (Cu) is an essential cofactor for key metabolic enzymes involved in various physiological processes, including radical detoxification, iron uptake, respiration, and neurotransmitter biosynthesis [10]. However, free Cu ions are potentially toxic to cells due to their redox activity [11]. Mutations in Cu-binding proteins or Cu overdose have been correlated with diseases such as Alzheimer disease, Wilson disease, and Menkes disease [12, 13]. Various studies have reported that accumulated Cu disrupts cellular homeostasis, leading to oxidative stress and apoptosis in the liver, brain, kidneys, and spleen [14, 15]. Since excessive Cu exposure can occur due to accidents or occupational hazards, proper Cu handling is crucial for workers in the steel industry.
We studied the relationship between the timing of medicine or chemical administration and the severity of toxicity as chronotoxicology [16–21]. For instance, we previously demonstrated that the industrial solvent bromobenzene induces more severe toxicity during the light phase than during the dark phase [17]. Bromobenzene metabolites, such as 4-bromocatechol, confirmed renal dysfunction during the dark phase [19]. Additionally, we evaluated the chronotoxicology of seven metals (Hg, Pb, Ni, Cr, Cu, Zn, and Fe) [18] in mice. The mice were susceptible to Cu and Zn toxicity when the light period changes to the dark period. Moreover, we recently reported that overexpression of Period2 (Per2) and neuronal PAS domain protein 2 (Npas2) attenuates Zn-induced toxicity in murine hepatoma Hepa1-6 cells [21]. However, limited information is available on the chronotoxicity of Hg, Pb, Ni, Cr, Cu, and Fe, as we only tested for their lethal toxicity [18]. Therefore, further research is necessary to elucidate the chronotoxicity related to metal exposure.
In this study, we examined diurnal variations in Cu-induced hepatic toxicity and investigated the biological factors involved in this chronotoxicity in mice.
Methods
Animal experimental protocol
Forty male 6-week-old C57BL/6J mice were purchased from CLEA Japan, Inc. (Tokyo, Japan) and housed in a controlled environment (temperature 24 ± 1 °C and humidity 55 ± 5%) with a standard 12-h light/dark cycle (8:00, zeitgeber time 0 [ZT0]; 20:00, ZT12) and provided with food and water ad libitum. This experiment was approved by the Institutional Animal Care and Experimentation Committee of Kinjo Gakuin University and Gifu University of Medical Science. After 1 week of acclimatization to laboratory conditions, the mice were randomly divided into four groups (10 mice each): the control group at ZT2, Cu-treated group at ZT2, control group at ZT14, and Cu-treated group at ZT14. Copper chloride (CuCl2: Fujifilm Wako Pure Chemical, Osaka, Japan) was intraperitoneally injected into the mice at 6.08 mg/kg (45 µmol Cu/kg) at 10:00 (ZT2) or 22:00 (ZT14) twice per week (Monday and Thursday) for 8 weeks, while the control group received intraperitoneal injections of saline solution as a placebo.
The mice’s body weight was measured weekly during the study period. After the final CuCl2 injection (Monday), the mice were euthanized 72 h later (Thursday), and plasma samples were obtained by cardiocentesis and stored at −80 °C. The livers and kidneys were weighed, and liver samples were either snap-frozen in liquid nitrogen and stored at −80 °C or fixed in 15% neutral-buffered formalin (pH 7.4; Fujifilm Wako Pure Chemical).
Plasma biochemical analysis
Plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were measured using the Transaminase CII Test (Fujifilm Wako Pure Chemical) according to the manufacturer’s instructions and our previous studies [22–24]. Calibration curves were prepared using standard solutions for relative quantification.
Quantitative RT-PCR
Total RNA from liver sections (80 mg) and Hepa1-6 cells were extracted using ISOGEN II (Nippon Gene, Tokyo, Japan), or RNA Basic Kit (NIPPON Genetics, Tokyo, Japan), respectively, following the manufacturer’s protocol (n = 3–6). The levels of each target mRNA were normalized to those of β-actin. Oligonucleotide sequences of the primers are listed in Supplementary Table S1.
Histopathological analysis
A portion of the left lobe of the liver from each animal was perfused with phosphate-buffered saline (PBS) (pH 7.4) and neutral buffered formalin, dehydrated, and embedded in paraffin. Tissues were sliced into 4 µm-thick sections and stained with hematoxylin and eosin (H&E), following previous protocols [25, 26].
Cell viability assay
Murine hepatoma Hepa1-6 cells obtained from the RIKEN Cell Bank (Tsukuba, Japan) were cultured in Dulbecco’s modified Eagle’s medium (high glucose) (DMEM; Fujifilm Wako Pure Chemical) supplemented with 10% fetal bovine serum and penicillin/streptomycin (Fujifilm Wako Pure Chemical) at 37 °C in a humidified atmosphere with 5% CO2. Hepa1-6 cells were seeded into 96-well plates at a density of 10 000 cells/well and treated with various concentrations of CuCl2 (Fujifilm Wako Pure Chemical) 24 h after seeding. After 24 h of Cu treatment, the cell viability was assessed using Alamar Blue (Bio-Rad Laboratories, Hercules, CA). For the transfection experiment, Hepa1-6 cells were seeded into 96-well plates at a density of 10 000 cells/well and transfected with 50 ng of pcDNA3.1-neo, pcDNA3.1-neo-circadian associated repressor of transcription (Ciart), pcDNA3.1-neo-cryptochrome 2 (Cry2), or pcDNA3.1-neo-Per1 using the TransIT-LT1 Transfection Reagent (Takara Bio) after 3 h of seeding. After 24 h of transfection, cells were treated with 500 or 1000 µM Cu, and cell numbers were evaluated after 24 h of Cu treatment. The progress of plasmid construction is described in the Supplementary Fig. S1.
Western blot analysis
Hepa1-6 cells were plated at a density of 500 000 cells per 35 mm dish and transfected with 1 µg pcDNA3.1-neo, pcDNA3.1-neo-Cry2, or pcDNA3.1-neo-Per1 using the TransIT-LT1 Transfection Reagent after 24 h of seeding. Twenty-four hours after transfection, the cells were treated with 500 µM Cu or MilliQ water (0.1%). After 24 h, the cells were homogenized in 100 µL of ice-cold RIPA buffer (Nacalai Tesque) containing a protease inhibitor and centrifuged (18000 × g for 20 min at 4 °C). The supernatant from each sample was collected and protein was extracted using a BCA protein kit (Nacalai Tesque). The concentration of each protein samples was normalized to 1.0 µg/µL by adding 6x sample buffer (Nacalai Tesque) and 2-mercaptoethanol (355 mM: Fujifilm Wako Pure Chemical). After heat denaturation (100 °C, 3 min), protein samples (10 µg) were separated by 10% SDS-PAGE and transferred to a polyvinylidene fluoride membrane. The primary antibodies used were as follows: mouse ATPase copper-transporting beta (ATP7B) monoclonal antibody (1:1000; Santa Cruz Biotechnology, Dallas, TX), mouse β-actin monoclonal antibody (1:2500; MBL, Aichi, Japan), rabbit cleaved caspase-3 polyclonal antibody (1:3000; Cell Signaling Technology, Beverly, MA) and rabbit copper uptake protein 1 (CTR1) polyclonal antibody (1:3000; Sigma-Aldrich). Peroxidase-conjugated anti-mouse IgG or anti-rabbit mouse IgG were used as the secondary antibody (1:5000; Cell Signaling Technology).
Determination of liver copper concentration
Individual liver specimens (40–100 mg each) were digested in 0.5 mL of concentrated nitric acid in glass test tubes. The temperature was held at 90 °C for 1 h, then gradually increased (at 10 °C per h) to 130 °C. When the acid-digested specimens became transparent, volumes of the digests were raised to 5 mL with distilled water, and copper concentrations were determined by atomic absorption using a Z-2300 (Hitachi, Tokyo, Japan).
Statistical analysis
Multiple comparisons were performed using a one-way analysis of variance (ANOVA) with Tukey’s test. All statistical analyses were performed using SPSS Statistics for Windows (version 24.0; IBM Corp., Armonk, NY). Differences were considered statistically significant at P < 0.05.
Results
Lethal toxicity test
To investigate Cu-induced chronotoxicity, we administered a single injection of CuCl2 (9.0 mg/kg) to C57BL/6J mice at six different time points (ZT2, ZT6, ZT10, ZT14, ZT18, or ZT22). Mice injected at ZT10 and ZT14 experienced 100% mortality within 5 days (Supplementary Fig. S2A). At ZT2, ZT6, and ZT22, 40% [2] of the mice died within 14 days after the injection. The ZT18 injection group, had an 80% mortality rate (Supplementary Fig. S2B). This result indicated that the mice were tolerant to Cu-induced toxicity at ZT2, ZT6, and ZT22, whereas highly susceptible at ZT10 and ZT14. This result aligns with our previous study in ICR mice [18].
Subsequently, we examined the repeated effects of Cu-induced chronotoxicity using a lower dose of CuCl2 (6.08 mg/kg). Based on the previous results, we selected ZT2 and ZT14 as representative injection times (Supplementary Fig. S2). Cu administration at ZT2 resulted in a gradual increase in mortality, with a 30% mortality rate (3/10) after 8 weeks (Fig. 1). In contrast, although a similar pattern was observed until 5 weeks, sudden death occurred at 6 weeks, leading to a 90% (9/10) mortality rate after 8 weeks. These data confirmed the conservation of Cu-induced chronotoxicity after single and repeated administrations.
Body weight change and organ weight
We examined the effect of injection timing on the toxicity severity under non-lethal conditions during a 5-week administration, since mortality ratio between ZT2 and ZT14 remained the same after 5 weeks (Fig. 1). Throughout the experiment, exposure to Cu tended decrease body weight compared to the control group (Supplementary Fig. S3). Following Cu administration, the change in body weight was almost the same until 4 weeks. To examine the effects of Cu on organ weight, we measured the weights of liver and kidney samples (Table 1). The liver/body weight ratio of the control group at ZT2 was significantly higher than that at ZT14 (P < 0.01). Because mice tend to eat at midnight, the liver weight at ZT2 decreased by glycogenolysis [27]. After Cu administration, no changes in the liver/body weight ratio were observed at ZT2, whereas a significant increase was observed at ZT14 (P < 0.05) (Table 1, Supplementary Fig. S3). Similar results were observed in the kidneys, indicating that Cu exposure at ZT14 affected the Cu-induced increase in relative liver weight.
Table 1 Body weight, liver and kidney ratio in the mouse administrated with Cu for 5 weeks at ZT2 or ZT14
| |
BW (g) |
Liver/BW (%) |
Kidney/BW (%) |
| ZT2 control |
25.82 ± 1.50 |
5.14 ± 0.17 |
1.21 ± 0.07 |
| ZT2 Cu |
23.84 ± 1.00 |
5.15 ± 0.36 |
1.22 ± 0.08 |
| ZT14 control |
25.59 ± 1.55 |
4.00 ± 0.13a |
1.09 ± 0.03c |
| ZT14 Cu |
23.06 ± 2.46 |
4.44 ± 0.46b |
1.16 ± 0.06b |
a: P < 0.01 versus ZT2 control, b: P < 0.05 versus ZT14 control, c: P < 0.05 versus ZT2 control.
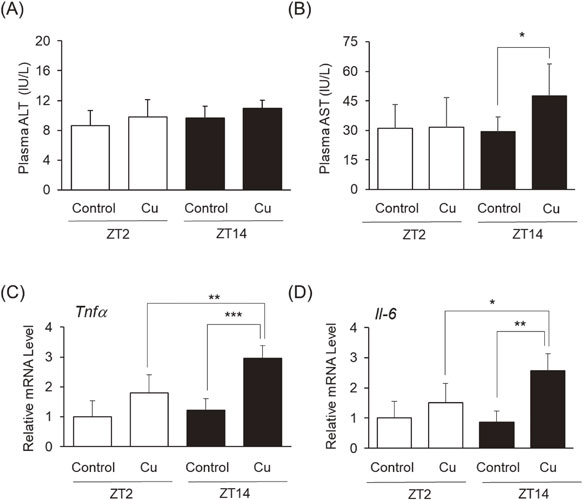
Chronic exposure to Cu induces toxicity in the liver through oxidative stress, cell death, and inflammatory responses [28]. As illustrated in Fig. 2, we measured plasma levels of ALT and AST, indicators of hepatic injury. ALT levels remained unchanged after Cu administration at both ZT2 and ZT14 (Fig. 2A). In contrast, Cu exposure at ZT14 significantly increased AST levels (Fig. 2B). In addition, we measured hepatic tumor necrosis factor-α (Tnfα) and Interleukin-6 (Il-6), markers of inflammatory cytokines (Fig. 2C and 2D). Cu administration at ZT2 tended to increase each level, whereas Cu administration at ZT14 significantly upregulated Tnfα and Il-6 in the liver compared to the control group at ZT14 and Cu at ZT2, respectively.
Histopathology
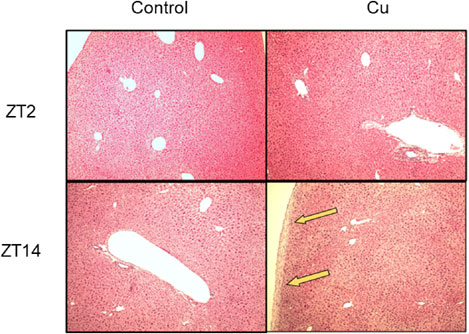
Along with the measurement of plasma biochemical parameters and inflammatory cytokines, we conducted histopathological studies on liver tissues (Fig. 3). Liver sections of the control (at ZT2 and ZT14) and Cu-treated groups (at ZT2), stained with H&E, exhibited a normal hepatic architecture. In contrast, the livers of Cu-treated mice at ZT14 evidenced thickening of the liver capsules.
Inhibition of cell viability and contribution of clock genes
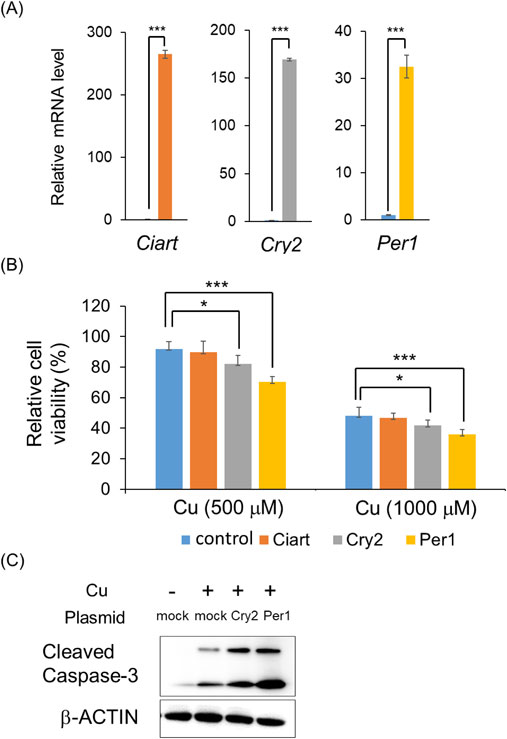
To investigate the mechanism of Cu-induced diurnal toxicity, we performed in vitro experiments using murine hepatoma Hepa1-6 cells. CuCl2 treatment resulted in a dose-dependent inhibition of Hepa1-6 cells viability (Fig. 4A). To investigate the involvement of clock genes, we measured their expression levels after Cu treatment. We observed an upregulation of Ciart, Cry2, and Per1 following treatment of Hepa1-6 cells with 500 µM Cu (Fig. 4B). Recent studies reported that clock genes, such as Per1 and Per2, play a role in hepatic injury in mice [29, 30]. Therefore, we hypothesized that the clock genes Ciart, Cry2, and Per1 may influence cell viability in response to Cu toxicity in Hepa1-6 cells.
Contribution of Ciart, Cry2, and Per1 against Cu toxicity
To evaluate the effects of Ciart, Cry2, and Per1 against Cu-induced toxicity in Hepa1-6 cells, we conducted transfection experiments. Transfection with Ciart, Cry2, and Per1 upregulated the expression of each gene (Fig. 5A). Overexpression of Cry2 and Per1 accelerated Cu-induced toxicity in Hepa1-6 cells, whereas overexpression of Ciart did not modulate Cu-induced cell viability (Fig. 5B). As Cu is known to induce apoptosis, we additionally evaluated this effect [15, 31]. Treatment with Cu induced the expression of cleaved caspase-3, an indicator of apoptosis, in Hepa1-6 cells (Fig. 5C). Overexpression of Cry2 and Per1 accelerated apoptosis. These results indicate that Cry2 and Per1 are associated with apoptosis-induced cell death.
Influence of Cu homeostasis
We measured the expression of genes related to Cu homeostasis, including transporters, chaperones, and metallothionein (MT), to determine their association with Cry2 and Per1. Treatment with Cu significantly downregulated the expression levels of Atp7b (Cu exporter) and Ctr1 (Cu importer) in Hepa1-6 cells (Fig. 6A). Moreover, the overexpression of Cry2 and Per1 significantly increased the expression level of Ctr1 whereas the overexpression of Per1 significantly decreased the expression of Atp7b (Fig. 6A). This result was confirmed at the protein level using immunoblotting (Fig. 6D). Although Cu treatment regulated Cu chaperones (Antioxidant 1 Copper Chaperone [Atox1] and copper chaperone for superoxide dismutase [Ccs]) and Mt1/2, no significant change was observed upon overexpression of Cry2 and Per1 (Fig. 6B and 6C). These results suggest that Cry2 and Per1 may accelerate Cu accumulation in the liver.

We evaluated expression levels of clock gene (Ciart, Cry2, and Per1) and Cu transporter (Atp7b and Ctr1) by administration with Cu in mice (Fig. 7). We found that expression levels of Ciart, Cry2, and Per1 (Fig. 7A, 7B, and 7C) were higher in the dark phase (ZT14) than that of light phase (ZT2). Cu administration at ZT2 tended to increase each level, whereas Cu administration at ZT14 significantly upregulated Cry2, and Per1 in the liver compared to the control group at ZT14 (Fig. 7B and 7C). Along with the clock genes, we measured Cu transporter (Atp7b and Ctr1) (Fig. 7D and 7E). We found that Atp7b expression level tend to decrease in the dark phase than that of light phase (Fig. 7D, P = 0.123). Cu administration at ZT2 and ZT14 significantly downregulated Atp7b in the liver (Fig. 7D). In addition, we demonstrated that Ctr1 expression level increased in the dark phase than that of light phase (Fig. 7E). Cu administration at ZT2 tended to decrease Ctr1 level, whereas Cu administration at ZT14 significantly downregulated Ctr1 in the liver compared to the control group at ZT14 (Fig. 7E). Finally, we measured Cu accumulations in the liver (Fig. 7F). We demonstrated that most of the Cu was excreted after 72 h at ZT2 and ZT14, respectively. On the other hand, Cu accumulation was still left in the group administrated with Cu at ZT14. These results suggest that Cu administration at ZT14 potentiated Cu-induced toxicity through increasing Cu accumulation time in the liver.

Discussion
Cu homeostasis is known to be controlled by multiple genes [32]. The majority of Cu is imported into mammalian cells through Ctr1, and Cu is passed to intracellular chaperone proteins, such as Ccs, which carry Cu through the cytoplasm. Cox17 is a key mitochondrial Cu chaperone protein responsible for the delivery of Cu ions to the mitochondria. Another Cu chaperone protein, Atox1, delivers Cu to the Cu-transporting ATPases Atp7a and Atp7b, which pump Cu into both the secretory and export pathways. MT is a major Cu-binding protein that protects against Cu-induced toxicity [33]. Disruption of Cu homeostasis, whether by Cu overdose or suppression of Cu-related genes, can lead to Cu-induced toxicity, characterized by apoptosis, cell cycle arrest, inflammation, and oxidative stress, in several tissues such as the liver, kidney, brain, and spleen [34–36]. Since the liver is the first site where Cu deposits after entering the bloodstream, and Cu regulation is controlled mainly by the liver, chronic Cu toxicity mainly damages this organ [28].
We have previously demonstrated that Cu-induced sensitivity differed with changing administration times in ICR mice [18]. In this study, we confirmed that the diurnal susceptibility to Cu was conserved between ICR and C57BL/6J mice (Supplementary Fig. S2). This result aligns with our previous findings, which evidenced no difference in Cu chronotoxicity among the three strains (C57BL/6J, ICR, and Balb/c) [16]. Moreover, the diurnal susceptibility to Cu toxicity was observed both with a single and a repeated administration (Fig. 1). Therefore, there was no strain or administration time difference in the diurnal variation in Cu toxicity. Moreover, we demonstrated that diurnal variation of Cu-induced lethal toxicity (8-week administration) and hepatic injury (5-week administration) was confirmed. However, since hepatic injury was mild (Fig. 2 and Fig. 3), it is unclear whether Cu-induced lethal toxicity is occurred through hepatic injury or not. We also evaluated the Cu-induced renal toxicity in mice and found that Cu-induced renal toxicity at ZT14 was stronger than that of ZT2 measured by malondialdehyde, Il-6 expression level, and morphology (Supplementary Fig. S4). These results suggest that Cu-induced lethal toxicity was associated with multi tissues-induced damage. Further investigation is needed to focus on other tissues such as brain and spleen in the future.
One possible explanation for the diurnal variation in Cu toxicity is the involvement of clock genes. Circadian rhythms are endogenous oscillators that regulate 24-h behavioral and physiological processes, and clock genes play a crucial role in this regulation [37]. At the molecular level, clock gene expression is regulated by BMAL1, CLOCK, and NPAS2. These proteins form heterodimers and function as transcriptional activators. Activation of BMAL1/NPAS2 or BMAL1/CLOCK increases the protein levels of repressors, such as CIART, CRY1/2, and PER1/2/3, which in turn inhibit their own transcription by binding to the BMAL1/NPAS2 or BMAL1/CLOCK heterodimer. Diurnal variation in these clock genes was confirmed in the livers of C57BL/6J male mice (Supplementary Fig. S5). Various studies have suggested that clock genes are involved in the production toxic factors. For example, Cry1a, Cry2a, and Per2 regulate oxidative status in zebrafish [38]. Per1 and Per2 regulate radiosensitivity in mice [39]. Clock knockout mice were found to accelerate acetaminophen-induced hepatic injury [40]. Therefore, it is reasonable to focus on clock genes as potential regulators of Cu-induced toxicity. We found that Cu treatment of Hepa1-6 cells upregulated the expression levels of Ciart, Cry2, and Per1, with well-known high levels in the dark phase (Supplementary Fig. S5 and Fig. 7). These results suggest that the three clock genes act as toxic factors induced by Cu treatment. Overexpression of Cry2 and Per1 potentiated the Cu-induced inhibition of Hepa1-6 cells viability, whereas overexpression of Ciart did not affect cell viability (Fig. 5B). Although it is unclear how Ciart is upregulated by treatment with Cu, Cry2 and Per1 are key clock genes associated with Cu-induced diurnal variation. This hypothesis was supported by our in vivo experiment, since Cry2 and Per1 were also induced by the administration of Cu at ZT14 (Fig. 7).
Elevated levels of cleaved caspase-3 are important markers of apoptosis. Oxidative stress and inflammation disturb mitochondrial homeostasis by increasing its membrane permeability. As a result, cytochrome C is released and, by activating cleaved caspase-3, the apoptotic pathway is initiated [41]. Elevated levels of cleaved caspase-3 have been reported following Cu administration [42]. Moreover, the apoptotic events induced by Cu promote inflammation in mice [42]. We found that the expression levels of Tnfα and Il-6 after Cu injection were significantly higher at ZT14 than at ZT2 (Fig. 2C and 2D); additionally, Cu treatment increased the level of cleaved caspase-3, with the overexpression of Cry2 and Per1 in Hepa1-6 cells accelerating this effect (Fig. 5C). Therefore, Cu-induced diurnal toxicity may be attributed to a difference in apoptosis.
To investigate the molecular mechanisms by which Cry2 and Per1 regulate Cu-induced toxicity, we measured the mRNA expression of seven genes (Atp7b, Ctr1, Atox1, Ccs, Cox17, Mt1, and Mt2) (Fig. 6A, 6B, 6C). We found that Atp7b was significantly downregulated by the overexpression of Per1. In the liver, Atp7b transports Cu from the cytosol into the lumen of the Golgi network for incorporation into ceruloplasmin [43]. Atp7b is also required to export Cu excess from the liver. A knock out mice model of Atp7b was authorized for the Wilson disease model, as Cu accumulates [44]. Cu accumulation induces marked changes in the liver structure and function. Moreover, we found that Ctr1 was significantly upregulated by the overexpression of Cry2 and Per1. Ctr1 is an ATP-dependent transported to the cell membrane. Ctr1 expression in the liver is regulated at both transcriptional and translational levels, with a lack of Cu inducing Ctr1 gene expression, whereas Cu overdose downregulates Ctr1 expression [45–47]. As Atp7b and Ctr1 play important roles in Cu transport and are regulated by the overexpression of Cry2 and Per1, we concluded that Cry2 and Per1 regulate Cu-induced apoptosis through the modulation of Cu transporters. In addition, our present data demonstrated that overexpression of Per1 with Cu downregulated Atp7b levels compared to Cu, while overexpression of Cry2 was comparable to Cu. Moreover, although overexpression of Cry2 and Per1 with Cu upregulated Ctr1 level compared to Cu, upregulation level between overexpression of Cry2 and Per1 was different. These data suggest that the potentiation of Cu toxicity by Cry2 and Per1 is different. To support this hypothesis, we found that overexpression of Per1, not Cry2, downregulated Atp7b expression level (around 35%) in Hepa1-6 cells (Data not shown). Since Atp7b knock out mice was reported to accumulate Cu and induce hepatic injury [48], related phenomenon may occur by overexpression of Per1. Moreover, we found that overexpression of Cry2, not Per1, upregulated Ctr1 expression level (around 20%). Since Ctr1 was associated with Cu incorporation, overexpression of Cry2 may contribute to enhance Cu uptake. To elucidate the association with Cu transporters and Cry2/Per1 in detail, future work should be conducted to use knock out mice and/or knock out cell line of Cry2 and Per1.
Conclusion
The present study demonstrated that Cu-induced chronotoxicity is conserved across multiple factors, including mouse strain, administration times, and toxicity levels. Moreover, Cu treatment disrupted specific clock genes such as Ciart, Cry2, and Per1 both in vivo and in vitro. Additionally, we established that overexpression of Cry2 and Per1 exacerbates Cu-induced hepatotoxicity through the regulation of the Cu transporters (Atp7b and Ctr1) in Hepa1-6 cells. These results suggest that clock genes induction by Cu exposure modulate Cu toxicity. The Cu concentration we used are high concentration compared to workplace in human except major accident. However, our present findings are particularly relevant for shift workers in the steel industry, who may handle Cu in both light and dark phases of the day. The impacts of our results are significant for the safety and self-protection of these workers, given that Cu exposure can alter clock gene levels, during the light and dark phases of the day. While further investigation is needed to fully elucidate the mechanisms by which Cry2 and Per1 modulate Cu transport, our present study highlights the importance of considering injection timing in basic research and underscore the need for careful self-protection measures for people working in the steel industry.
Abbreviations
ALT
alanine aminotransferase
ANOVA
analysis of variance
AST
aspartate aminotransferase
Atox1
antioxidant 1 Copper Chaperone
ATP7B
ATPase Copper Transporting Beta
Ccs
copper chaperone for superoxide dismutase
Ciart
circadian associated repressor of transcription
Ctr1
copper uptake protein 1
Cu
copper
Cry
cryptochrome
DMEM
dulbecco’s modified Eagle’s medium
H&E
hematoxylin and eosin
Il-6
Interleukin-6
Tnfα
tumor necrosis factor alpha
MT
metallothionein
Npas2
neuronal PAS domain protein 2
Per2
period2
ZT
zeitgeber time
Declarations
Ethics approval and consent to participate
All experiments were approved by the Institutional Animal Care and Experimentation Committee of Kinjo Gakuin University and Gifu University of Medical Science.
Consent for publication
Not applicable.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Funding
This research was financially supported by The Uehara Memorial Foundation and JSPS KAKENHI Grant Numbers 22K15328 and 20K21733.
Author’s contribution
ST performed experiments such as animal experiments and wrote a draft manuscript.
HY performed experiments such as in vitro experiment and edited draft manuscript. HY designed of the work.
YT analyzed the data and interpretation of data.
SY discussed the interpretation of the results and wrote the paper in part.
MS performed the experiments such as histological analysis.
MN performed the experiments such as histological analysis and supported statistical analysis.
HH contributed in writing the manuscript.
TM designed of the work and was a major contributor in writing the manuscript.
NM designed of the work and was a major contributor in writing the manuscript.
All authors read and approved the final manuscript.
Acknowledgments
The authors thank Dr. Tsunemasa Nonogaki (Kinjo Gakuin University, Japan) for his kind suggestions.
References
- 1. Ghiasvand M, Heshmat R, Golpira R, Haghpanah V, Soleimani A, Shoushtarizadeh P, et al. Shift working and risk of lipid disorders: a cross-sectional study. Lipids Health Dis. 2006;5:9. https://doi.org/10.1186/1476-511X-5-9.
- 2. Burch JB, Alexander M, Balte P, Sofge J, Winstead J, Kothandaraman V, et al. Shift Work and Heart Rate Variability Coherence: Pilot Study Among Nurses. Appl Psychophysiol Biofeedback. 2019;44:21–30. https://doi.org/10.1007/s10484-018-9419-z.
- 3. Crnko S, Du Pre BC, Sluijter JPG, Van Laake LW. Circadian rhythms and the molecular clock in cardiovascular biology and disease. Nat Rev Cardiol. 2019;16:437–47. https://doi.org/10.1038/s41569-019-0167-4.
- 4. Savvidis C, Koutsilieris M. Circadian rhythm disruption in cancer biology. Mol Med. 2012;18:1249–60. https://doi.org/10.2119/molmed.2012.00077.
- 5. Shi SQ, Ansari TS, McGuinness OP, Wasserman DH, Johnson CH. Circadian disruption leads to insulin resistance and obesity. Curr Biol. 2013;23:372–81. https://doi.org/10.1016/j.cub.2013.01.048.
- 6. Hasegawa M, Honjo K, Chiang C, Mita T, Watson BM, Ikerdeu E, et al. Sociodemographic and behavioral factors related to obesity among adults in the Republic of Palau based on the WHO STEPwise approach to NCD risk factor surveillance 2011–2013: A cross-sectional study. Environ Health Prev Med. 2023;28:39. https://doi.org/10.1265/ehpm.22-00309.
- 7. Honma K, Hikosaka M, Mochizuki K, Goda T. Loss of circadian rhythm of circulating insulin concentration induced by high-fat diet intake is associated with disrupted rhythmic expression of circadian clock genes in the liver. Metabolism. 2016;65:482–91. https://doi.org/10.1016/j.metabol.2015.12.003.
- 8. Jimenez-Ortega V, Cano Barquilla P, Fernandez-Mateos P, Cardinali DP, Esquifino AI. Cadmium as an endocrine disruptor: correlation with anterior pituitary redox and circadian clock mechanisms and prevention by melatonin. Free Radic Biol Med. 2012;53:2287–97. https://doi.org/10.1016/j.freeradbiomed.2012.10.533.
- 9. Qin F, Liu N, Nie J, Shen T, Xu Y, Pan S, et al. Circadian effects of ionizing radiation on reproductive function and clock genes expression in male mouse. Environ Health Prev Med. 2021;26:103. https://doi.org/10.1186/s12199-021-01021-4.
- 10. Pena MM, Lee J, Thiele DJ. A delicate balance: homeostatic control of copper uptake and distribution. J Nutr. 1999;129:1251–60. https://doi.org/10.1093/jn/129.7.1251.
- 11. Valko M, Morris H, Cronin MT. Metals, toxicity and oxidative stress. Curr Med Chem. 2005;12:1161–208. https://doi.org/10.2174/0929867053764635.
- 12. Bandmann O, Weiss KH, Kaler SG. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015;14:103–13. https://doi.org/10.1016/S1474-4422(14)70190-5.
- 13. Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet. 1993;3:7–13. https://doi.org/10.1038/ng0193-7.
- 14. Gaetke LM, Chow CK. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003;189:147–63. https://doi.org/10.1016/s0300-483x(03)00159-8.
- 15. Liu H, Deng H, Jian Z, Cui H, Guo H, Fang J, et al. Copper exposure induces hepatic G0/G1 cell-cycle arrest through suppressing the Ras/PI3K/Akt signaling pathway in mice. Ecotoxicol Environ Saf. 2021;222:112518. https://doi.org/10.1016/j.ecoenv.2021.112518.
- 16. Miura N, Yoshioka H, Ashimori A, Ohtani K, Hasegawa T, Hwang GW, et al. Multidirectional analyses of hepatic chronotoxicity induced by cadmium in mice. J Toxicol Sci. 2017;42:597–604. https://doi.org/10.2131/jts.42.597.
- 17. Yoshioka H, Nonogaki T, Fukuishi N, Shinohara Y, Hwang GW, Ohtani K, et al. Chronotoxicity of bromobenzene-induced hepatic injury in mice. J Toxicol Sci. 2017;42:251–8. https://doi.org/10.2131/jts.42.251.
- 18. Yoshioka H, Nonogaki T, Shinohara Y, Suzui M, Mori Y, Hwang GW, et al. Lethal chronotoxicity induced by seven metal compounds in mice. J Toxicol Sci. 2018;43:129–34. https://doi.org/10.2131/jts.43.129.
- 19. Yoshioka H, Tominaga S, Nishikawa M, Shinohara Y, Nakao M, Yoshikawa M, et al. Different Renal Chronotoxicity of Bromobenzene and Its Intermediate Metabolites in Mice. Biol Pharm Bull. 2021;44:150–3. https://doi.org/10.1248/bpb.b20-00694.
- 20. Yoshioka H, Tominaga S, Shinohara Y, Hwang GW, Maeda T, Miura N. Chronotoxicity of Streptomycin-Induced Renal Injury in Mice. Biol Pharm Bull. 2020;43:53–8. https://doi.org/10.1248/bpb.b19-00539.
- 21. Yoshioka H, Tominaga S, Suzui M, Shinohara Y, Maeda T, Miura N. Involvement of Npas2 and Per2 modifications in zinc-induced acute diurnal toxicity in mice. J Toxicol Sci. 2022;47:547–53. https://doi.org/10.2131/jts.47.547.
- 22. Yoshioka H, Wu S, Moriishi T, Tsukiboshi Y, Yokota S, Miura N, et al. Sasa veitchii extract alleviates nonalcoholic steatohepatitis in methionine–choline deficient diet-induced mice by regulating peroxisome proliferator-activated receptor alpha. Traditional & Kampo Medicine. 2023;10:259–68. https://doi.org/10.1002/tkm2.1385.
- 23. Yoshioka H, Fukaya S, Onosaka S, Nonogaki T, Nagatsu A. Kampo formula “Hochu-ekki-to” suppressed carbon tetrachloride-induced hepatotoxicity in mice. Environ Health Prev Med. 2016;21:579–84. https://doi.org/10.1007/s12199-016-0571-x.
- 24. Yoshioka H, Nonogaki T, Fukaya S, Ichimaru Y, Nagatsu A, Yoshikawa M, et al. Sasa veitchii extract protects against carbon tetrachloride-induced hepatic fibrosis in mice. Environ Health Prev Med. 2018;23:49. https://doi.org/10.1186/s12199-018-0739-7.
- 25. Yoshioka H, Tanaka M, Fujii H, Nonogaki T. Sasa veitchii extract suppresses carbon tetrachloride-induced hepato- and nephrotoxicity in mice. Environ Health Prev Med. 2016;21:554–62. https://doi.org/10.1007/s12199-016-0581-8.
- 26. Yoshioka H, Usuda H, Fujii H, Nonogaki T. Sasa veitchii extracts suppress acetaminophen-induced hepatotoxicity in mice. Environ Health Prev Med. 2017;22:54. https://doi.org/10.1186/s12199-017-0662-3.
- 27. Greco CM, Koronowski KB, Smith JG, Shi J, Kunderfranco P, Carriero R, et al. Integration of feeding behavior by the liver circadian clock reveals network dependency of metabolic rhythms. Sci Adv. 2021;7:eabi7828. https://doi.org/10.1126/sciadv.abi7828.
- 28. Hosseini MJ, Shaki F, Ghazi-Khansari M, Pourahmad J. Toxicity of copper on isolated liver mitochondria: impairment at complexes I, II, and IV leads to increased ROS production. Cell Biochem Biophys. 2014;70:367–81. https://doi.org/10.1007/s12013-014-9922-7.
- 29. Kakan X, Chen P, Zhang J. Clock gene mPer2 functions in diurnal variation of acetaminophen induced hepatotoxicity in mice. Exp Toxicol Pathol. 2011;63:581–5. https://doi.org/10.1016/j.etp.2010.04.011.
- 30. Ge W, Wang T, Zhao Y, Yang Y, Sun Q, Yang X, et al. Period1 mediates rhythmic metabolism of toxins by interacting with CYP2E1. Cell Death Dis. 2021;12:76. https://doi.org/10.1038/s41419-020-03343-7.
- 31. Foo JB, Ng LS, Lim JH, Tan PX, Lor YZ, Loo JSE, et al. Induction of cell cycle arrest and apoptosis by copper complex Cu(SBCM)(2) towards oestrogen-receptor positive MCF-7 breast cancer cells. RSC Adv. 2019;9:18359–70. https://doi.org/10.1039/c9ra03130h.
- 32. Ackerman CM, Chang CJ. Copper signaling in the brain and beyond. J Biol Chem. 2018;293:4628–35. https://doi.org/10.1074/jbc.R117.000176.
- 33. Wang L, Yang L, Yang F, Li X, Song Y, Wang X, et al. Involvements of H2O2 and metallothionein in NO-mediated tomato tolerance to copper toxicity. J Plant Physiol. 2010;167:1298–306. https://doi.org/10.1016/j.jplph.2010.04.007.
- 34. Chen Z, Meng H, Xing G, Chen C, Zhao Y, Jia G, et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol Lett. 2006;163:109–20. https://doi.org/10.1016/j.toxlet.2005.10.003.
- 35. Mitra S, Keswani T, Dey M, Bhattacharya S, Sarkar S, Goswami S, et al. Copper-induced immunotoxicity involves cell cycle arrest and cell death in the spleen and thymus. Toxicology. 2012;293:78–88. https://doi.org/10.1016/j.tox.2011.12.013.
- 36. Musacco-Sebio R, Ferrarotti N, Saporito-Magrina C, Semprine J, Fuda J, Torti H, et al. Oxidative damage to rat brain in iron and copper overloads. Metallomics. 2014;6:1410–6. https://doi.org/10.1039/c3mt00378g.
- 37. Hastings M. The brain, circadian rhythms, and clock genes. BMJ. 1998;317:1704–7. https://doi.org/10.1136/bmj.317.7174.1704.
- 38. Alifu Y, Kofuji S, Sunaga S, Kusaba M, Hirayama J, Nishina H. The Light-Inducible Genes Per2, Cry1a, and Cry2a Regulate Oxidative Status in Zebrafish. Biol Pharm Bull. 2021;44:1160–5. https://doi.org/10.1248/bpb.b21-00432.
- 39. Zhanfeng N, Yanhui L, Zhou F, Shaocai H, Guangxing L, Hechun X. Circadian genes Per1 and Per2 increase radiosensitivity of glioma in vivo. Oncotarget. 2015;6:9951–8. https://doi.org/10.18632/oncotarget.3179.
- 40. Johnson BP, Walisser JA, Liu Y, Shen AL, McDearmon EL, Moran SM, et al. Hepatocyte circadian clock controls acetaminophen bioactivation through NADPH-cytochrome P450 oxidoreductase. Proc Natl Acad Sci U S A. 2014;111:18757–62. https://doi.org/10.1073/pnas.1421708111.
- 41. Malhi H, Gores GJ, Lemasters JJ. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43:S31–44. https://doi.org/10.1002/hep.21062.
- 42. Keswani T, Mitra S, Bhattacharyya A. Copper-induced immunotoxicity involves cell cycle arrest and cell death in the liver. Environ Toxicol. 2015;30:411–21. https://doi.org/10.1002/tox.21916.
- 43. Loudianos G, Gitlin JD. Wilson’s disease. Semin Liver Dis. 2000;20:353–64. https://doi.org/10.1055/s-2000-9389.
- 44. Ferenci P. Regional distribution of mutations of the ATP7B gene in patients with Wilson disease: impact on genetic testing. Hum Genet. 2006;120:151–9. https://doi.org/10.1007/s00439-006-0202-5.
- 45. Dancis A, Haile D, Yuan DS, Klausner RD. The Saccharomyces cerevisiae copper transport protein (Ctr1p). Biochemical characterization, regulation by copper, and physiologic role in copper uptake. J Biol Chem. 1994;269:25660–7.
- 46. Petris MJ, Smith K, Lee J, Thiele DJ. Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J Biol Chem. 2003;278:9639–46. https://doi.org/10.1074/jbc.M209455200.
- 47. Landon CD, Benjamin SE, Ashcraft KA, Dewhirst MW. A role for the copper transporter Ctr1 in the synergistic interaction between hyperthermia and cisplatin treatment. Int J Hyperthermia. 2013;29:528–38. https://doi.org/10.3109/02656736.2013.790563.
- 48. Huster D, Finegold MJ, Morgan CT, Burkhead JL, Nixon R, Vanderwerf SM, et al. Consequences of copper accumulation in the livers of the Atp7b-/- (Wilson disease gene) knockout mice. Am J Pathol. 2006;168:423–34. https://doi.org/10.2353/ajpath.2006.050312.
 https://orcid.org/0000-0002-0977-434X
https://orcid.org/0000-0002-0977-434X