2023 Volume 91 Issue 10 Pages 102003
2023 Volume 91 Issue 10 Pages 102003
Lithium/transition-metal polysulfide batteries are a promissing candidate for next-generation batteries with high energy densities. Transition metal polysulfides and lithium- or sodium-containing transition metal sulfides exhibit large reversible capacities based on multi-electron processes, owing to the redox reactions of S in addition to the transition metal. This comprehensive paper aims to address the idea, research, and development of transition metal polysulfide electrode active materials and summarizes the author’s views on the concept of transition metal polysulfide electrodes. Furthermore, the diversity of coordination structures and unique structural changes during charging and discharging will be discussed.
Development of secondary batteries with energy densities of more than 300 Wh kg−1 that exceeds those of the current Li-ion batteries is required to meet the increased demand for high-energy secondary batteries. Electrode active materials with innovatively high capacities are some of the most important materials for the realization of such high-energy-density batteries.
Li metal and Si-based electrode active materials are promising active materials for negative electrodes and show approximately 10 times larger capacity than the conventional graphite negative electrode active material. S-based active materials have attracted considerable attention as positive electrodes. Theoretically, Li–S batteries have the potential to develop high-energy-density batteries with a gravimetric energy density of more than 500 Wh kg−1 and a volumetric energy density of more than 800 Wh L−1. However, achieving these high-energy-density batteries is challenging because of the drawbacks of the Li- and S-based electrode active materials for practical applications. Recently, transition metal polysulfides (MSx, M = Ti,1–5 V,6–8 Nb,9,10 or Mo,11–15 and x ≥ 3), which are metal sulfides with high S content, have been proposed as a new series of high-capacity positive electrode active materials. Several series including amorphous transition metal polysulfides, and lithium- and sodium-containing transition metal sulfides have been proposed. These novel high-capacity electrode active materials undergo a unique structural change with the redox of S during charging and discharging.
This comprehensive paper aims to show the general challenges of Li–S batteries and the series of transition metal polysulfide electrode active materials, such as amorphous transition metal polysulfides and lithium- and sodium-containing transition metal sulfides. Especially, the author’s views on the concept of transition metal polysulfide electrodes, and the challenges and perspectives in the development of transition metal polysulfides for practical battery applications is described.
S8 and Li2S are used as positive electrode active materials in Li–S secondary batteries. Since these materials are insulators, they must be used together with conductive additives. Most studies have used carbon as a conductive additive to carry electrons to S-based electrodes. However, it is generally necessary to mix in large amounts of carbon (20–50 wt%) when carbon is used as a conductor.16–20
Simply mixing S and carbon is unlikely to be sufficiently effective. Thus, in many cases, S is inserted into the mesopores or nanopores of the carbon.16–18 Because of both low specific gravity of carbon and its porous nature, a certain volume ratio of the composite electrode is occupied by carbon and pore. Furthermore, in both simple composite of S and nano carbon and S-inserted porous carbon, a large amount of electrolyte is required compared to the conventional oxide-based positive electrode active materials because its specific gravity is low and also large volume changes occur during charging and discharging.
The need for large amounts of carbon and electrolytes makes it difficult to achieve the originally anticipated high energy density, posing a challenge for the current Li–S batteries.
2.2 Dissolution of polysulfides into electrolyte solutionAnother issue is that Li polysulfides (Li2Sx; 2 ≤ x ≤ 8), intermediate products form during charging and discharging, and dissolve into the electrolyte solution, causing side reactions. When using a carbonate-based electrolyte solution, such as 1 M LiPF6 dissolved in a mixed solvent of ethylene carbonate (EC) and dimethyl carbonate (DMC), which is widely used as an electrolyte solution in Li-ion batteries, the S-based positive electrode faces difficulties in carrying out reversible charge-discharge, as shown in Fig. 1a. When S is used as the positive electrode active material, charging and discharging start from discharging direction (i.e., the direction in which sulfur becomes lithium sulfide). In this case, the initial discharge yielded a capacity of about 200 mAh g−1, but the subsequent charge had little capacity. This behavior is explained by an undesired chemical reaction (side reaction) between dissolved polysulfide ions and carbonate-based electrolytes. The chemical reactivity of polysulfide ions and carbonate-based electrolytes is expected to be high due to the strong nucleophilicity of polysulfide ions with large polarizability. Therefore, an ether-based electrolyte solution of 1 M lithium bis(trifluoromethanesulfonyl)amide (LiTFSA) dissolved in a mixed solvent of 1,3-dioxolane (DOL) and 1,2-dimethoxyethane (DME) is often used in Li–S batteries. When the ether-based electrolyte solution is used, the cell shows a large reversible capacity compared to the cells with the carbonate-based electrolyte solution, even though polysulfides are eluted into the electrolyte solution during charging and discharging. Dissolved polysulfide ions cause other challenges such as redox shuttles (polysulfide shuttles) and the other side reactions.21 Figure 1b shows a typical example of the charge-discharge curves of the Li–S cell using an ether-based electrolyte, showing a polysulfide shuttle. Owing to the shuttle reaction, the cell continued to charge at a low current density.
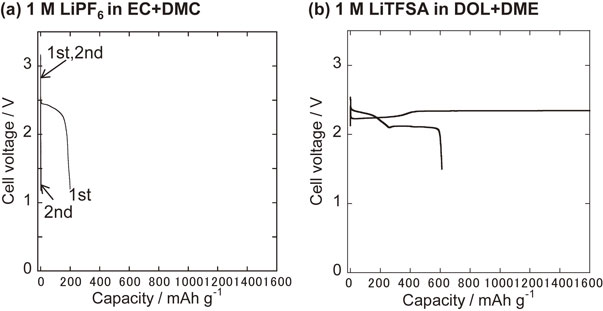
Typical charge-discharge curves of typical S-nano carbon composite electrodes in the cells using (a) a carbonate-based electrolyte (1 M LiPF6 in EC+DMC), and (b) an ether-based electrolyte (1 M LiTFSA in DOL+DME).
Figure 2a shows a schematic diagram of the polysulfide shuttle. Li polysulfides formed during charging and discharging are highly soluble in many solvents used in electrolyte solutions. As the Li polysulfide dissolves in the solvent, it becomes a polysulfide ion that diffuses throughout the electrolyte solution in the cell.17,21,22 During charging, short-chain polysulfide ions are electrochemically oxidized to long-chain polysulfide ions at the positive electrode. The long-chain polysulfide ions then migrate to the negative electrode and are chemically reduced to short-chain polysulfide ions on the Li metal surface. This reaction mechanism results in a phenomenon in which the charge capacity becomes extremely large, resulting in a decrease in the Coulomb efficiency.

(a) Schematic illustration of polysulfide shuttle and (b) examples of methods to control the polysulfide shuttle in Li–S batteries.
Each of these issues has been addressed in the recent studies of many researchers. For example, several solutions to the polysulfide shuttle problem have been proposed, as illustrated in Fig. 2b, and all of these have been proven to be useful.
The simplest solution is inhibition of dissolution of polysulfide ions into electrolyte. All-solid-state cells using solid electrolytes are the most attractive technology for Li-S batteries because the polysulfide does not dissolve into solid electrolytes and thus the side reaction due to the migration of polysulfides effectively suppressed. It is also effective to decrease the solubility of the sulfur electrode materials themselves. Sulfur impregnated in microporous carbon and metal (poly)sulfides are reprehensive materials with low solubility.
The use of electrolyte solution with low solubility of polysulfide is also effective. To avoid the redox shuttle, the suppression of migration of polysulfides between positive electrode and negative electrode is important. For example, polysulfide barrier coating on the positive electrode layer, the use of polysulfide adsorbent, Labyrinthine structure, and ion selective separator are also effective. Inhibition of reduction reaction on lithium surface is also important. For example, formation of electron-insulating solid electrolyte interphase (SEI) or coating on Li metal are effective. Several reviews on the study of Li–S batteries have been published.17,23–25 This comprehensive paper focuses on the development of insoluble S-based positive electrode materials, transition metal polysulfides.
The advantages of transition-metal polysulfides as high-capacity positive electrode materials are illustrated in Fig. 3a. Compared to S, transition metal polysulfides are expected to have the following characteristics:4,5,9 (1) suppression of the dissolution of polysulfides into the electrolyte solution by formation of chemical bonds between the transition metal and S, (2) large charge/discharge capacity based on the redox reactions of both transition metals and S, (3) increased electronic conductivity compared to S itself, and (4) increased material density compared to the S–C composite.

(a) List of expected advantages of transition metal sulfide electrode active materials, (b) UV-vis absorption spectra of ether-based electrolytes after charge-discharge measurements of the cell using a-TiS4 and the cell using the composite of TiS2 and S8,4 and (c) examples of positive electrode reactions in a typical conventional electrode of LiCoO2 and transition metal sulfide of a-TiS4. Reproduced with permission from Ref. 4, Elsevier.
Figure 3b shows the UV-vis absorption spectra of the ether-based electrolytes after charge-discharge measurements of the cells using amorphous TiS4 (a-TiS4) and a composite of TiS2 and S8.4 The spectra were measured to investigate the degree of polysulfide dissolution. It is reported that polysulfide ions (Sx2−, 2 ≤ x ≤ 8) show strong absorption in the UV region.26–28 The absorption intensity of the electrolyte formulated with a-TiS4 was much lower than that of the electrolyte based on the composite of TiS2 and S8. The dissolution of polysulfides in the electrolyte is significantly suppressed by amorphization. This improvement was achieved by the formation of chemical bonds between the Ti atoms and S for almost all S.
Figure 3c shows examples of positive electrode reactions in a typical conventional electrode of LiCoO2 and a transition metal sulfide, a-TiS4. In conventional positive electrode active materials, such as LiCoO2, the redox reaction of transition metals mainly occurs. By contrast, in a-TiSx, both Ti and S undergo redox reactions in the same voltage region of 2 V vs. Li+/Li. In other words, in addition to Ti, which is a cation, the redox reaction of S, which is an anion, also occurs. Furthermore, only a little more than half of the Co atoms contribute for the redox reactions in LiCoO2. Thus, the capacity is limited to approximately 160 mAh g−1. By contrast, in a-TiS4, all of the constituent atoms can be oxidized and reduced, enabling large-capacity charging and discharging of 700 mAh g−1 owing to multi-electron reactions. The redox reactions of Ti and S are influenced by each other because the Ti–S bonds in a-TiSx are considerably covalent.5,9 Many transition metal sulfides exhibit high electronic conductivities. Polysulfides often exhibit semiconducting properties and can provide higher electronic conductivities than S, enabling a decrease in the amount of conducting carbon. In addition, transition metal polysulfides have higher densities than S or S–C composites. Therefore, if transition metal polysulfides achieve the same capacity per weight as S–C composites, they will achieve a larger capacity per volume. Thus, metal polysulfide electrodes have the potential to achieve both large gravimetric and volumetric capacities.
3.2 Types of transition metal polysulfide electrode materialsIn this section, transition metal polysulfide electrode materials are classified into several types and examples of each are presented. Electrode materials based on the mixture of typical transition metals or transition metal sulfides and S or Li2S have been reported previously. Materials in which a large percentage of S forms chemical bonds with transition metals during charging and discharge are regarded as “transition metal polysulfides” electrode materials.
3.2.1 Amorphous transition metal polysulfidesNovel amorphous transition metal polysulfide materials such as amorphous Ti polysulfides (a-TiSx, x ≥ 3),1,2,4,5,29–32 amorphous V polysulfides (a-VSx, x ≥ 3),6,7 amorphous Nb polysulfides (a-NbSx, x ≥ 3),9,10 and amorphous Mo polysulfides (a-MoSx, x ≥ 3)12,13,33–37 have recently been developed. These materials are expected to exhibit high performance and the advantages of both transition metal sulfides and S electrodes.
The concept of amorphous transition-metal polysulfides originates from glass science. As shown in Fig. 3, we designed transition metal polysulfide electrodes that achieve high capacity by using S redox reactions and increasing the S content per transition metal; however, crystalline materials have a limited range of possible compositions. By contrast, the materials in the glass-forming region can be prepared at any stoichiometry. Because S is a typical glass-former, we believed that transition metal polysulfides with arbitrary compositions could be prepared. Thus, we first investigated whether glass materials with compositions of transition metal polysulfides (MSx, x ≥ 3) could be prepared by pulsed laser deposition (PLD)1 of the vapor phase and mechanochemical (MC)4,6,9,12,32 methods. Amorphous samples were obtained in several cases. Strictly speaking, we refer to these materials as a-MSx rather than MSx glass because the glass transition behavior, which is one of the definitions of a glass, was not clearly observed in many cases. An example of a high capacity achieved owing to an increased S content is presented below. Figure 4 shows the initial charge-discharge curves of the cells fabricated using a-NbS3, a-NbS4, a-NbS5, and carbonate-based electrolytes.9 The respective initial discharge capacities of 281, 446, and 596 mAh g−1, corresponds to 2.0, 3.7, and 5.6 electron processes per formula unit. As expected, the charge/discharge capacity increased with increasing S content. Multi-electron charging/discharging process in a-NbSx proved the redox of both transition metal and S. High reversible capacities were obtained in liquid electrolyte cells using carbonate-based electrolytes. This suggests that most of the S formed chemical bonds with Nb. The electronic conductivities of the compressed pellets of a-NbS3, a-NbS4, and a-NbS5, were >1000, 2.4, and 0.2 mS cm−1, respectively. The conductivity of a-NbS3 is too high to be measured using a two-electrode cell. The electronic conductivities of all of the a-NbSx samples were significantly higher than those of the insulating S.9

Figure 5 shows voltage versus capacity plots for various oxide and sulfide electrode materials.5 The dashed line in the figure shows the contour line of the energy density calculated from the total weight of the positive and Li negative electrodes. The oxide-based electrodes exhibited high voltages ranging from 3.5–5.0 V. By contrast, the voltages of the metal polysulfide electrodes were almost half of those of the oxide electrodes. However, the capacities of some transition-metal polysulfides are more than three times larger than those of the oxides. For example, a-TiS4 exhibits a high capacity of 688 mAh g−1, corresponding to the capacity provided by the insertion of approximately 4.5 Li+ into TiS4. For a fair comparison of the lithium-containing and non-lithium-containing electrode active materials, the weight of Li should be considered. Thus, the capacity was normalized to the total weight of the Li negative electrode and a-TiS4 as 4.5Li/TiS4. Thus, for a-TiS4 which showed a discharge capacity of 688 mAh g−1, a discharge capacity of 581 mAh g−1 (per weight of 4.5Li/TiS4) was used in the plot in Fig. 5.4,5 a-VSx, a-NbSx and a-MoSx are also high-capacity electrode active materials.6,7,11,12,14,15,36,38

Voltage versus capacity for oxide and sulfide electrode materials. The dashed line in the figure shows the contour line of energy density calculated by the total weights of the respective positive electrodes and Li negative electrode.5 Reproduced with permission from Ref. 5. Copyright 2017, American Chemical Society.
TiS2,39 TiS3,3 and FeS2,40 are well-known conventional sulfide positive electrode active materials. TiS2 has a layered structure in which S exists as S2−, FeS2 has a pyrite structure in which S exists as disulfide ions (S–S2−), and TiS3 has both sulfide and disulfide ions in the crystal structure. A typical crystalline transition metal polysulfide with high S content is patronite VS4. In VS4, there are two disulfides (four S atoms) per V atom, and they form a 1D quasi-chain structure; although denoted above as disulfide ions, the disulfides are bonded rather covalently to a transition metal. Grayfer et al. discussed the role of disulfide anions in the electrochemical transformations of transition-metal polysulfide electrodes.41 The nano-composite of VS4 and graphene-oxide charged and discharged with a large reversible capacity of 900 mAh g−1.42 Crystalline MSx is attractive because of its high mass production efficiency.
3.2.3 Lithium containing transition metal sulfidesThe development of lithium containing positive electrode active materials eliminates the need for Li in the negative electrode and allows the use of typical negative electrode materials, such as graphite and Si. The ability to fabricate batteries in a discharged state, in which energy is not charged inside the cell, is advantageous with regard to safety during battery production. In addition, by synthesizing transition metal sulfide electrodes with a structure containing Li in advance, reversibility during charging and discharging is expected to be enhanced. Therefore, the development of transition metal sulfides with high Li content is attractive. However, there are far fewer reports on lithium-containing transition metal sulfide electrode materials than on oxide materials. One reason for this is that these materials often exhibit low chemical stability in humid environments.
In this comprehensive paper, lithium containing transition metal sulfides which involve multi-electron process charging and discharging are considered as one of the series of transition metal polysulfides. In most lithium-containing transition metal sulfides, sulfur is present as a sulfide ion rather than a disulfide or polysulfide ion. However, lithium-containing transition metal sulfides with high lithium and sulfur content produce S–S bonds during high-capacity charging.
Some lithium-containing metal sulfide positive electrode active materials, such as Li2FeS2,43 Li2TiS3,44 Li3NbS4,44,45 Li1.13Ti0.57Fe0.3S2,46 Li8FeS5 (Li2S–FeS),47 and Li3CuS2 (Li2S–Cu2S),48 have been reported.
3.2.3.1 Antifluorite Li2S-based lithium transition metal sulfidesLi2S has a theoretical capacity of 1167 mAh g−1 but is ionically and electronically insulating.49–51 The addition of a transition metal to Li2S enhances both the ionic and electronic conductivities, thus improving the electrode performance. For example, all-solid-state batteries with Li2S–Cu operate at a high capacity.49 Formation of a solid solution is an effective approach for improving the electrode activity of Li2S. In pioneering research, Takeuchi et al. developed Fe-substituted Li2S-based materials Li2S–FeS with high capacity.47,52
Li2S–Cu2S solid solutions are a typical example of a solid solution system.48,53 Li3CuS2 has an antifluorite-type structure ($Fm\bar{3}m$) with a disordered cation arrangement, in which the Li+ of Li2S is partially replaced by Cu+. Activation of Li2S by formation of a solid solution with Cu2S led to a significant improvement in the electronic and ionic conductivities of the antifluorite-type structure. The galvanostatic charge-discharge curves of the Li-In/Li3PS4 glass/Li3CuS2–Li3PS4 cell in the voltage range of 1.5–2.5 V vs. Li+/Li (0.9–1.9 V vs. Li-In) are shown in Fig. 6. The Li3CuS2 active material showed an initial charge capacity of 389 mAh g−1 and an initial discharge capacity of 376 mAh g−1. The charge-discharge capacities corresponded to the two-electron reaction per Li3CuS2 formula unit. The average discharge voltage was 2.09 V vs. Li+/Li and did not change in the subsequent cycles.

A notable feature of Li3CuS2 is its relatively small potential hysteresis during the charging and discharging processes. This indicates that the symmetry of the charge and discharge reactions was relatively high. The typical charge-discharge reaction between Li2S and S is a conversion-type reaction. The charge-discharge between Li2S, which is an ionic crystal, and S8, which is a molecule with high covalency, involves a large structural change with a change in the bonding character, and it is expected that the reaction routes for the charge and discharge reactions will be different from each other. This is believed to be the main cause of the potential hysteresis in the charge-discharge curves of the S electrode. The substitution of Cu+ for Li+ in antifluorite-type Li2S changes not only the Li-ion and electronic conductivities, but also the reaction mechanism. Cu introduced by substitution may interfere with the Li2S–to–S8 conversion reaction and effectively preserve the skeletal structure based on the Li2S crystal structure.
In addition to transition metals, multivalent cations, such as Mg2+ and Al3+, and halogens, such as I−, also enhance the ionic conductivity, reversible capacity, and cycle performance, of Li2S.54–59 This concept is more effective for all-solid-state batteries than for the conventional battery systems that use organic liquid electrolytes because ionic conductivity of electrodes is more important in all-solid-state batteries.
3.2.3.2 Rocksalt-type lithium transition metal sulfidesWe have developed rocksalt-type lithium transition metal sulfides as a new series of large-capacity electrode active materials.10,44,45,60,61
Mechanochemically-prepared Li2TiS3 and Li3NbS4 have a cation disordered cubic rocksalt structure and can charge/discharge with a capacity greater than 400 mAh g−1.44 Figure 7 shows (a) a crystal model of cubic (rock salt) Li2TiS3 and (b) the charge-discharge curves of the cell using cubic Li2TiS3 and 1 M LiPF6 in EC+DMC electrolytes. Upon 3.0 V (vs. Li+/Li) charging and 1.5 V discharging, approximately 2.5 and 3.5 Li ions can reversibly insert into and extract from the structure with a composition range of 0.4 < x < 2.9 for LixTiS3 and 0.4 < y < 4.0 for LiyNbS4, respectively. These materials exhibit excellent cycle performance in all-solid-state Li cells.44,45

Material design that considers ionic conductivity after charging and discharging is important for the development of high-capacity electrode materials. Recently, we developed electrode–electrolyte bifunctional materials in the Li2S–V2S3–LiI system with high ionic and electronic conductivities.8 Figures 8a–8d show (a) schematic images of conventional composite, nano-composite, and homogeneous materials; (b) all-solid-state cells with the conventional composite electrode and (c) electrode-electrolyte bifunctional material; and (d) charge-discharge curves of all-solid-state cell Li–In alloy/Li3PS4 glass/90(0.75Li2S·0.25V2S3)·10LiI. It is usually necessary to add solid electrolytes and conductive carbon to the composite electrode; however, this reduces the ratio of the active material in the electrode layer and reduces the capacity per electrode layer. To increase the energy density of all-solid-state batteries, we proposed electrode–electrolyte bifunctional materials that can function as active materials and solid electrolytes. The usual composite electrode is a submicron-to-micrometer composite; however, our bifunctional material concept aims to achieve a homogeneous material or an almost homogeneous nano-composite with the size of constituent material particles in the order of several nanometers.

Schematic images of (a) conventional composite, nano-composite, and homogeneous materials, (b, c) all-solid-state cells with (d) conventional composite electrode and (c) electrode-electrolyte bifunctional material, and (d) charge-discharge curves of all-solid-state cell Li-In alloy/Li3PS4 glass/90(0.75Li2S·0.25V2S3)·10LiI.8 Reproduced with permission from Ref. 8. Copyright 2022, American Chemical Society.
It is essential for an electrode-electrolyte bifunctional material to satisfy the following two requirements: (i) high ionic and electronic conductivities and (ii) sufficiently high ionic and electronic conductivities after structural changes during Li extraction and insertion. In other words, only high mixed conductivity is not sufficient for bifunctional materials.
All-solid-state batteries with Li2S–V2S3–LiI in the positive electrode layer showed a relatively large reversible capacity of 400 mAh g−1 at 25 °C without mixing solid electrolytes and conductive carbons in the electrode (Fig. 8d). The Li2S–V2S3–LiI system was a nano-composite material consisting of amorphous Li2S–V2S3–LiI and Li1−xVS2 nanocrystal with sizes on the order of several nanometers.
The basic concept of a metal polysulfide electrode active material is to obtain high capacity by exploiting the presence of a large amount of S relative to the transition metal. However, in many cases, a high capacity is not obtained solely by compositing metal sulfides and S, but also by a novel charge-discharge mechanism. In a typical S positive electrode, a large structural change occurs between the Li2S ionic crystal and molecular S8. Because both materials are insulating, they were used as nano-composites with conductive carbon. In transition metal polysulfides, S and transition metals form relatively strong covalent bonds that have a significant effect on the charge-discharge mechanism. For example, bond formation with a transition metal prevents the isolation of polysulfide ions during charging and discharging, thereby significantly reducing the elution of S components into the electrolyte. High-capacity charging and discharging via the redox reaction of S (anions) is also possible. Redox reactions involve the dissociation and formation of S–S disulfide bonds, with a change in the oxidation state of S. Figure 9a shows an example of the peculiar amorphous-mediated structural changes observed during the charging and discharging of transition-metal sulfide positive electrodes. With the insertion and extraction of Li, the cation-to-anion ratio changed dramatically, and dissociation and formation of S–S bonds occurred. In this case, the number of Ti–S bonds and the coordination number of S changed significantly. In a-TiSx electrodes, this large change in the coordination number occurred gradually. This leads to a long plateau region at 2 V, with a gradual change in the charge-discharge voltage in the charge-discharge curve. This conformational change is both a conversion type because of the formation and dissociation of covalent S–S bonds, and an insertion/extraction type because of the Li insertion/extraction within the structure. a-TiS4 forms a network framework with Ti and S–S, and Li-inserted a-Li4TiS4 forms a chain-like framework with Ti and S. Ionic bonding between Li and S of TiSx frameworks is also formed. When further discharged to a lower voltage region below 1 V vs. Li+/Li, a conversion-type reaction occurs that produces Li2S and Ti. This was elucidated by combining an experimental structural analysis using synchrotron radiation measurements with computational chemistry studies performed on a supercomputer.5

The following summarizes the findings regarding the charge-discharge mechanism of a-TiSx. (1) High capacity charging and discharging due to multi-electron process occur at 2 V Li+/Li region. (2) Charging and discharging tend to proceed while maintaining the amorphous state (e.g. a-TiS4). (3) Formation and dissociation of S–S bonds with anion redox are often involved and the local structure changes continually. The formation and dissociation of S–S bonds increase or decrease in the coordination number of S to Ti. (4) Many Ti–S bonds are retained during charging and discharging to form chain-like and reticular-like frameworks. The decrease in the coordination number by Li insertion results in a structural change toward a chain framework, and the increase in the coordination number by Li extraction results in a structural change toward a reticular framework. (5) Redox of both S and Ti occurs. That is, the electronic states of both S and Ti change together with the structural changes associated with Li insertion and extraction.
Figure 9b shows examples of the peculiar structural changes involving amorphization observed during charging and discharging of the transition-metal sulfide positive electrodes.60
In cubic rocksalt lithium transition metal sulfides, a highly reversible structural phase transition between rocksalt and amorphous structures occurs because a part of the rocksalt-type six-coordination framework consisting of –Ti–S–Ti– chains is retained during amorphization by Li extraction.60 This phase transition is similar to that of the optical phase-change recording material used in DVD-RWs, such as Ge2Sb2Te with Ge–Te–Ge bonding.62 It is expected that the structural phase transition between the rocksalt and amorphous phases because of the Li insertion/extraction reactions is fast and highly reversible.60 As expected, S atoms that lost more than one neighbor Li preferentially changed their positions to form homopolar S–S bonds, implying that S is oxidized during charging. Furthermore, most disulfides (S) directly interacted with Ti. This result shows the charge-discharge mechanism involves anion redox between S2− and S–S2−, leading to high capacity. However, ab initio molecular dynamics simulations suggest that even after full charging, the ratio of S atoms forming S–S homo bonds is not high, indicating that redox reactions occur not only at S but also in the entire structure formed by –Ti–S–Ti– chains.60
Although the formation and dissociation of covalent bonds tend to change the order of both the reaction and structural changes during charging and discharging, high symmetry of the charge and discharge reactions is necessary to reduce the hysteresis of the charge-discharge potentials.
Na is a well-known cost-effective carrier cation because of its abundance in the Earth’s crust and its widespread distribution. Therefore, the development of electrode active materials for Na batteries is desirable. Transition metal polysulfide electrodes are expected to be useful in Na batteries.30,34–36,63–66 Amorphous transition polysulfides such as a-TiSx30 and a-MoSx14,34–36 are used as high-capacity electrode active materials in Na batteries. Sodium-containing transition metal sulfides such as Na2FeS2,66 Na2TiS3,64,67 and Na3NbS4,68 also serve as high-capacity electrode active materials for Na batteries. Although the crystal phase, diffusion mechanism, and ratio of volume change are different for Na because of its larger size than that of Li, the concept of high-capacity reversible charging and discharging with multi-electron reactions using the redox reactions of S in addition to transition metals is applicable. To obtain high-performance materials, it is important to design a structure that allows effective insertion and extraction of Na. Here, amorphization is a design guideline. As in the case of Li, in high-capacity transition metal polysulfides, the formation and dissociation of S–S and M–S bonds during charging and discharging and the accompanying structural changes should be reversible.
Herein, Na2TiS3 is introduced as a representative example of an electrode-active material for Na batteries. Figure 10 shows the X-ray diffraction (XRD) patterns of the Na2TiS3 prepared with various compositions and the electrode performance of the all-solid-state Na cells.64,65,69 The samples were prepared using a conventional solid-state reaction and different mechanochemical treatment conditions at 360 and 510 rpm to develop a novel crystalline phase. Synthesis by conventional heat treatment yielded monoclinic Na2TiS3 with an ordered arrangement of the Na and Ti cations, and mechanochemical syntheses using ball milling at 510 and 360 rpm yielded Na2TiS3 with a cubic rocksalt-type structure and a disordered arrangement of the Na and Ti cations, and a-Na2TiS3, respectively. In Na2TiS3, the monoclinic phase is stable at room temperature.

(a) XRD patterns of Na2TiS3 prepared with various compositions, (b) structure models of (b) cubic rocksalt structure and (c) layered rocksalt structure, (d) charge-discharge curves of all-solid-state Na cells Na-Sn/Na3PS4 solid electrolyte/Na2TiS3, and (e) cycle performance of all-solid-state cells using cubic Na2TiS3.64,65,69 Reproduced with permission from Ref. 64. Copyright 2019, The Ceramic Society of Japan. Reproduced with permission from Ref. 65. Copyright 2019, The Chemical Society of Japan. Reproduced with permission from Ref. 69. Cooyright 2019, The Electrochemical Society.
Figure 10d shows the charge-discharge curves of all-solid-state cells using amorphous, cubic, and monoclinic Na2TiS3. The reversible capacities of amorphous Na2TiS3 and cubic Na2TiS3 were twice as large as that of monoclinic Na2TiS3. Materials with two types of cations that favor octahedral coordination tend to have a layered (or ordered) rock salt-analog structure as a stable phase. By contrast, mechanochemical synthesis tends to yield materials with cation-disordered cubic rocksalt structures. This cation-disordered cubic rocksalt structure is typically a metastable phase. This is a general tendency found in Li systems, such as Li2TiS360 and Li2SnS361 and in oxide-based materials. Compared to the cation-ordered layered structure, the disordered structure is relatively favorable for cation migration, particularly in alkali-metal-rich compositions.70
5.2 All-solid-state Li and Na/polysulfide batteriesTransition-metal polysulfide electrode active materials are useful in both liquid- and solid-electrolyte-type batteries. As described above, the dissolution of polysulfides in an electrolyte solution is suppressed by the formation of chemical bonds between the transition metal and the polysulfides, which is a major feature of batteries using electrolyte solutions. This has enabled the use of carbonate-based electrolytes. Transition-metal polysulfides usually exhibit longer cycle performances in all-solid-state cells than in conventional liquid-type electrolytes. A long cycling performance of several hundred cycles was achieved in most cases. This proves that the transition metal polysulfide electrode active materials show inherently greater ability to resist degradation, even after several hundred charge-discharge cycles. The stable operation of all-solid-state batteries suggests that the degradation in the cells with an electrolyte solution is because of the chemical reactions at the surface of the electrode particles and/or the loss of conduction pathways towing to mechanical degradation, rather than owing to the degradation arising from the structural change of the bulk materials.
This series of materials also has unique mechanical properties such as ductility.45 A favorable solid-solid contact between the electrode and electrolyte particles is a key factor in the fabrication of high-performance all-solid-state batteries. Conventional oxide-based positive electrode materials tend to produce cracks during fabrication and/or charge–discharge processes. Transition-metal polysulfide electrode active materials show a much larger capacity than conventional oxide-electrode active materials, giving rise to a large volume change. Thus, heavy mechanical degradation of the electrode structure is a concern. However, all-solid-state cells using these materials exhibit a relatively long cycle performance. Herein, we explain one of the reasons for the long cycle performance observed in all-solid-state cells containing metal-polysulfide-based electrode active materials with unique mechanical properties, using Li3NbS4 as an example.
The initial charge and discharge capacities of all-solid-state cell using Li3NbS4 were 263 and 370 mAh g−1, respectively. After the first cycle, a capacity of approximately 370 mAh g−1 was maintained for the charge and discharge processes. This capacity corresponds to the structures ranging from Li0.6NbS4 to Li4.0NbS4. Figure 11a shows the self-healing of the interfacial contacts in the composite electrode of the all-solid-state cells using Li3NbS4. The cross-sectional scanning electron microscopy (SEM) images of the charged and discharged composite electrode layers showed that the composite electrode layers were free of large cracks despite the presence of voids. During charging, the Li3NbS4 electrode shrank by 25 % compared to charged Li4NbS4. The void volume increases after charging and decreases after discharging. The Li3NbS4 particles were well-connected to the composite electrode after discharging. The fragmentation of Li3NbS4 was hardly observed during either the charging or discharging processes. Although large volume changes usually cause fragmentation of electrode particles, loss of interfacial contact, and decreased packing density, a dense electrode was observed. This result suggests that room-temperature pressure sintering of the electrode particles occurs during the discharging (expanding) process, increasing the contact area of both the electrode-electrolyte and electrode-electrode interfaces. That is, room-temperature sintering-assisted self-healing of the interfacial contacts occurs. Room-temperature pressure-sintering is typically observed in solid sulfide electrolytes.71 Room-temperature pressure sintering also occurred in Li3NbS4 and other polysulfide electrodes. Figure 11b shows the cycle performance of the all-solid-state cell using Li3NbS4 at 50 °C. The all-solid-state cell exhibited high cyclability, retaining 92 % of its capacity from the 7th–200th cycles. The high cyclability is partially attributed to the unique mechanical properties of Li3NbS4.

(a) Cross-sectional SEM images of the composite electrode of all-solid-state cells using the Li3NbS4 (upper) charged state and (bottom) discharged state and schematics of self-healing of interfacial contacts, and (b) cycle performance of all-solid-state cell using Li3NbS4 at 50 °C.45 Reproduced with permission from Ref. 45. Copyright Authors 2018. CC BY 4.0.
Compared to oxide-based high potential positive electrode materials, the electrode potential of transition metal polysulfides is approximately half. Thus, it is necessary to develop an electrode with at least twice the capacity, and ideally with four times the capacity, of the oxide-based positive electrode materials. When using a 2 V class high-capacity positive electrode, the capacity of the negative electrode must also be large, or a large amount of negative electrode requires. The low working voltage makes it difficult to design a battery with a high energy density. In addition, large expansion and shrinkage are inevitable in high-capacity materials because large-capacity electrode active materials require the insertion and extraction of a large amount of Li. High-capacity S-rich metal sulfides, such as a-TiS4, a-NbS5, a-MoSx and VS4, can be reversibly charged and discharged with high capacity. However, the cyclability of the cells with high-S-content transition metal polysulfides tends to decease with an increase in the S content. The volume change rate, conductivity, and side reactions are related to the charge/discharge cycle degradation.
When considering the degradation mechanisms, it is preferable to first classify them into the categories of chemical and mechanical degradation. Chemical degradation includes the degradation of the bulk structure during charging and discharging (change in the crystallinity of the electrode or irreversible structural change at the atomic level) and the side reactions between the electrode active material surface and the electrolyte. However, these factors are often interrelated.
The fact that high reversibility has often been confirmed in all-solid-state batteries indicates that transition-metal polysulfides are inherently highly conformationally reversible.
Some transition metal polysulfides and the charged and discharged are not thermodynamically most stable although most electrode active materials of charged and discharged state are metastable phase or thermodynamically non-equilibrium. To achieve long-life charging and discharging, these metastable or thermodynamically nonequilibrium materials require a certain degree of stability in the structure of the charged and discharged states to suppress irreversible phase transitions to another material chemically.
As typified by the polysulfide electrode active materials of Ti, V, Nb, and Mo, multi-electron reactions beyond four-electron reactions occur. In these cases, the cation-to-anion ratios change significantly. Compositions with high alkali metal ion contents, such as Li and Na, have a high degree of ionic bonding, whereas compositions with low alkali metal ion contents have a relatively high covalent bonding contribution in the overall structure. The polarity changed significantly; therefore, the compatibility with the electrolyte also changed significantly. Therefore, it is important to investigate favorable electrolytes.72–74 Phase separation in the structure during charging and discharging is also important.
Mechanical degradation includes particle miniaturization and the loss of conduction pathways owing to expansion and contraction. For example, expansion and contraction can cause cracks inside the particles, cracks in the electrode layer, and detachment of the electrode from the current collector.
Because the degradation factors depend on the type of metal polysulfide electrode, and the environment and operating conditions under which each cell is fabricated, it is necessary to identify the main degradation factors in each case and address them individually. To date, few studies investigating metal polysulfide electrode active materials have been reported, and it is still necessary to elucidate the possible degradation factors individually.
6.2 ProductionSynthesis of transition metal polysulfides must overcome challenges. Because many transition metals do not have stable polysulfide crystal phases and because of the high vapor pressure of S, it is difficult to synthesize transition metal polysulfides using the usual solid-phase reaction method. Therefore, we chose to synthesize amorphous transition metal sulfide particles using mechanochemical methods during the initial material exploration stage. This mechanochemical process is useful for the preparation of glasses and metastable crystalline phases at room temperature. Mechanochemical synthesis, such as ball milling, is a chemical reaction involving mechanical energy. Mechanochemical treatments are often used for miniaturization or for chemical reactions on the surfaces during powder processing. However, for high-energy mechanochemical treatments, bulk chemical reactions such as compounding, amorphization, and crystal phase transitions occur. This process is useful for the synthesis of amorphous transition metal polysulfides and lithium- or sodium-containing transition metal sulfides with metastable crystalline phases.4,5,9,69
Thermal decomposition of ammonium transition metal polysulfides is a more practical process for the synthesis of amorphous transition metal polysulfides, even though synthesis is only possible for a limited number of transition metals. For example, a-MoSx of various compositions can be synthesized using various ammonium Mo polysulfides as the starting materials.
This comprehensive paper summarizes the ideas, research, and development of transition-metal polysulfide electrode active materials. Transition-metal polysulfide electrode active materials containing a large amount of S relative to the transition metal allow for high-capacity charging and discharging using S redox reactions. Chemical bonding between the polysulfide and transition metal can inhibit the dissolution of polysulfide into the electrolyte solution. The inclusion of transition metals often results in semiconducting electronic conductivity. A low carbon content leads to a high volumetric energy density. Alkali-metal-containing transition-metal sulfides are also important. In the discharged state, S exists as a sulfide ion and is a normal sulfide material. However, during charging, multiple Li or Na ions are withdrawn from the structure, causing a structural change that significantly changes the cation-to-anion ratio. The redox reaction of S involves the formation and dissociation of S–S bonds. The coordination number of S around transition metals can change significantly during charging and discharging. In all-solid-state batteries, metallic polysulfide electrode active materials exhibit high cyclability. This is believed to be related to the electrode and the electrolyte being sulfide materials that are less likely to undergo side reactions. In addition, many transition metal polysulfides exhibit ductility. In particular, room-temperature pressure sintering, which is a property of stress densification, occurs in post-discharge materials with high Li content. In response to the large volume change of the electrode active material during high-capacity charging and discharging, the electrode active material and solid electrolyte exhibit ductile behavior, and self-healing of the surface contact interface occurs during charging and discharging, leading to a long cycle life. Further improvements in the cyclability and mass production are challenges that must be overcome for the practical application of transition-metal polysulfide electrode active materials. In recent years, the number of studies on transition-metal polysulfide electrode active materials has increased rapidly. The author hopes that metal polysulfide batteries will be achieve practical use as next-generation rechargeable batteries by achieving high performance through multiple breakthroughs.
The author sincerely thanks Prof. Masahiro Tatsumisago, Prof. Akitoshi Hayashi, Prof. Shigeo Mori, Prof. Kiyoharu Tadanaga, Dr. Takuya Matsuyama, Dr. Akira Nasu, Prof. Hikari Sakaebe, Dr. Hironori Kobayashi, Dr. Tomonari Takeuchi, Dr. Masahiro Shikano, Dr. Kentaro Kuratani, Mr. Kazuto Koganei, Prof. Zempachi Ogumi, Prof. Yoshiharu Uchimoto, Prof. Hajime Arai, Prof. Katsutoshi Fukuda, Prof. Koji Ohara, Prof. Tomoya Kawaguchi, Prof. Koji Nakanishi, and many students and collaborators. The research summarized in this paper was supported by several projects including the JST-ALCA SPRING Project and the RISING and RISING2 projects of NEDO and JSPS KAKENHI.
Atsushi Sakuda: Conceptualization (Lead), Writing – original draft (Lead), Writing – review & editing (Lead)
The authors declare no conflict of interest in the manuscript.
Japan Society for the Promotion of Science: JP18H05255
Japan Society for the Promotion of Science: JP21H04625
Japan Society for the Promotion of Science: JP20K05688
New Energy and Industrial Technology Development Organization: RISING1+2
Japan Science and Technology Corporation: JPMJAL1301
This paper summarizes the research results of “Progress of Next-Generation Battery Research by Development of Sulfide Electrode Materials and Solid Electrolytes and Construction of Solid-State Interface”, which was the subjects of 2019 Young Researcher Award of ECSJ (Sano Award).
A. Sakuda: ECSJ Active Member

Atsushi Sakuda (Associate Professor, Osaka Metropolitan University (OMU))
Atsushi SAKUDA is currently an associate professor of Osaka Metropolitan University (OMU). He received his Ph.D. from Osaka Prefecture University (OPU) in 2011. He worked as a JSPS postdoctoral fellow at OPU, and then worked as a researcher and a senior researcher at the National Institute of Advanced Industrial Science and Technology (AIST). He moved to OPU as an assistant professor in 2017 and promoted an associate professor in 2020. In 2022, OPU and Osaka City University united to form the OMU. He was awarded Young Ceramist Awards of the Ceramic Society of Japan (2018), Young Researcher Award of The Electrochemical Society of Japan (Sano Award) (2019), and The Young Scientists’ Prize in the 2020 Commendation for Science and Technology, from the Japanese Minister of Education, Culture, Sports, Science and Technology (2020). He has made contributions to the field of Electrochemistry. In particular, he has played an important role in the research on inorganic materials chemistry for all-solid-state secondary batteries. He has published 175 research papers which are divided into the following categories: lithium-ion and sodium-ion conducting glasses and glass-ceramics, amorphous transition metal polysulfide electrode materials, mechanical properties of battery materials, core technology for all-solid-state batteries.