2023 Volume 91 Issue 5 Pages 057005
2023 Volume 91 Issue 5 Pages 057005
The effect of electrolyte additive 1,2-dichloroethane (DCE) on the electrochemical stability of aluminum-graphite battery using acetamide-AlCl3 electrolyte is investigated comparatively. Original dendritic and dead aluminum are observed in Al-Al cells at a high operation rate. Original dendritic and dead aluminum are observed in the anode and separator of Al-Al cells at a high operation rate. DCE is a preferred electrolyte additive to reduce the polarization of the acetamide-AlCl3 electrolyte. Furthermore, DCE can effectively suppress the dendrites on the Al anode in Al|acetamide-AlCl3|Al cells. Besides, it has a positive effect on improving the discharge specific capacity and cycle stability of aluminum-graphite batteries with a high coulombic efficiency over 300 cycles. This result indicates that organic additive is suitable to improve the electrochemical performance of aluminum-graphite batteries.
AlCl3-based room temperature molten salts had been widely used in energy storage because of the reversible deposition/dissolution of aluminum at almost 100 % coulombic efficiency. Reynolds firstly reported a secondary aluminum battery with EMImCl-AlCl3 ionic liquid as electrolyte and aluminum as anode.1 Lin researched an ultrafast aluminum battery with EMImCl-AlCl3 as an electrolyte and graphitic foam as a cathode, which presented remarkable electrochemical performance.2 Tsuda’s group developed various graphite-based cathodes for aluminum ion batteries, such as graphene nanoplatelet, graphene-coated activated carbon fiber cloth, and graphene nanoplatelet-polysulfone, which exhibited a good cycle ability and satisfactory coulombic efficiency.3–5 Subsequently, researchers reported that aluminum-graphite batteries with urea-AlCl3 and acetamide-AlCl3 deep eutectic solvent as electrolytes had a high specific capacity and coulombic efficiency.6–10 In recent years, large numbers of research on aluminum batteries were reported, which mainly focused on the aspects of cathodes, including graphene/graphite foam, metal oxides, and metal sulfides.11–18
However, the existence of dendrite structures on the aluminum anode of the aluminum battery had been proved by Chen.19 An early study on aluminum electrodeposition in EMImCl-AlCl3 also confirmed that severe and even visible aluminum dendrite was formed on copper/aluminum cathodes.20,21 Besides, dendritic aluminum deposits were observed in AlCl3-amide.22–24
Those disadvantages of AlCl3-amide systems resulted in a negative impact on the electrochemical performance of AIBs, such as specific capacity, cycle stability, and safety. The aluminum dendrite would result in a “short circuit”, and the “dead aluminum” reduced the coulombic efficiency. Consequently, inhibiting dendrite growth is not only a key step to improve the safety of aluminum batteries, but also an important factor for the excellent electrochemical performances of aluminum batteries. Additives had been widely used to improve the quality of aluminum deposits obtained from AlCl3-based electrolytes. Endres reported the nanocrystalline Al with an average grain size of 14 nm was obtained from EMImCl-AlCl3 containing nicotinic acid as an organic additive.25 Zhang found that mirror-bright Al coatings could be electrodeposited with nicotinic acid and methyl nicotine as additives.26–28 They also showed that additives, including alkali metal chlorides, rare earth chlorides, small organic molecules, and surfactants influenced the morphology and nano-crystallinity of the aluminum deposit.29 Abbott showed that a mirror-finish Al coating was obtained from EMImCl-AlCl3 when toluene was used as an additive.30 Further, some studies have proposed that amide-AlCl3 systems were modified by additives to improve the quality of aluminum deposits and the electrochemical performance of aluminum-ion batteries.9,31,32 Therefore, it should be very interesting to study the inhibition of aluminum dendrite growth by electrolyte additives.
In this paper, the long-term charging and discharging cycles of Al|acetamide-1.3AlCl3|Al cells and Al|acetamide-1.3AlCl3|graphite batteries were carried out to evaluate the effect of additive 1,2-dichloroethane (DCE) on the cyclic stability of aluminum-graphite batteries at a high operation rate. Several analytical techniques, including cyclic voltammetry and linear voltammetry tests, charge/discharge test, and scanning electron microscopy, were used to determine the aluminum morphologies, the mechanism of aluminum dendrites growth, and the electrochemical performance of aluminum-graphite batteries.
Dehydration of acetamide was carried out under vacuum at 60 °C for more than 72 h. Anhydrous aluminum chloride (AlCl3) and additive 1,2-dichloroethane (DCE) were used as received. All chemical reagents were stored in an argon atmosphere glove box (both water and oxygen contents were less than 0.1 ppm). AlCl3-acetamide electrolytes were prepared by slow addition of a certain weight of AlCl3 in a beaker under continuous stirring. Subsequently, the AlCl3-acetamide electrolyte was treated with Al foil at 60 °C for 12 h.14 The molar ratios (referred to as r) of AlCl3 to acetamide studied in this paper were 1.3, 1.4, and 1.5, which were widely selected in the studies of aluminum batteries.1–18 In some experiments, an additive (DCE) was added to the prepared AlCl3-acetamide electrolytes.
A three-electrode cell was employed to perform all the electrochemical measurements in an argon-filled glove box. A high-purity aluminum wire (99.99 %) was used as the reference electrode, and an aluminum sheet (99.99 %) was used as the counter electrode, high purity aluminum wire, and tungsten wire were used as working electrodes for cyclic voltammetry and linear voltammetry testing. Before use, all electrodes were polished with emery paper, cleaned with distilled water, and finally air-dried.
A pouch cell was assembled by using aluminum as both anode and cathode, glass fiber filter paper (GF/D, Whatman) as the separator, and one of AlCl3-acetamide room temperature molten salts as an electrolyte. The aluminum-aluminum cells were then charged and discharged at 4 C for 15 min in each half-cycle to evaluate the cycle stability of the aluminum anode (a high charging/discharge rate, 4 C derived from the rate performance of an aluminum-graphite battery using an AlCl3-urea electrolyte).3 Carbon paper (CP, 0.78 g/cm3, Shanghai Hesen Electric Co., Ltd.) was used as the cathode to study the effect of the electrolyte molar ratio on the electrochemical performance of an Al battery. To evaluate the effect of additive on the electrochemical stability of rechargeable aluminum-graphite batteries, a cell was constructed using a pyrolytic graphite foil (PG, 0.017 mm, Suzhou Dasen Electronics Materials) cathode and an Al foil anode. The long-term galvanostatic discharge/charge of the full cell was tested under a voltage range of 0.5–2.4 V. The cell was also measured by EIS in the frequency range of 105 Hz to 10−1 Hz without cycling. All electrochemical tests were performed at 25 ± 1 °C.
The electrochemical performances of the aluminum-graphite battery using AlCl3-acetamide with various molar ratios as electrolytes were studied. As shown in Fig. 1, the discharge specific capacity of Al|acetamide-1.3AlCl3|CP battery is maintained at 60 mAh g−1 during 400 cycles with a coulomb efficiency of near 90 %. With the molar ratio increasing, the discharge specific capacity of the aluminum-graphite battery slightly increases. As the molar ratio continues to increase to 1.5, the electrochemical stability of the Al-graphite cell becomes poor distinctly. The discharge specific capacity fluctuated in a wide range, and its coulomb efficiency was also unsatisfactory. After long-term electrochemical tests, the optical photographs of the aluminum anode and separator are shown in Fig. 1. It can be seen that as the molar ratio increases, the aluminum and separator are corroded severely in the electrochemical reaction region, which explains the unstable capacity and coulomb efficiency of the cells that the existing side reactions between the corrosive electrolyte with high molar ratio and aluminum anode and separator.

Cycle performance for Al-graphite batteries using AlCl3-acetamide with various molar ratios as electrolytes and the photos of anodes and separators of Al-graphite batteries after a long-term cycle.
Based on the above analysis, a room temperature molten salt with a molar ratio of 1.3 is more suitable as the electrolyte for aluminum batteries. Therefore, we will focus on the electrochemical behavior of aluminum cathode and graphite-type cathode in aluminum cells with AlCl3-based room temperature molten salts as electrolytes with a molar ratio of 1.3.
The Raman spectra of the acetamide-AlCl3 electrolytes containing the additive DCE with various contents were shown in Fig. 2a. It can be seen that all Raman spectra contain two typical Raman characteristic peaks located at 347 cm−1 and 310 cm−1, corresponding to the [AlCl4]− and [Al2Cl7]− complexes, respectively. The Raman spectra of electrolytes were almost unchanged after one week and remained as orange-yellow transparent and clear liquids (Figs. 2b–2c). It indicates that the acetamide-AlCl3-DCE electrolytes have high chemical stability. For characterizing the effects of additive content on the complex change in the electrolyte, the ratio of the peak integral area of the corresponding [Al2Cl7]− to [AlCl4]− complexes (referred as I310/I347) was calculated. The ratio decreases slightly with the increase of additive content, but the content of [Al2Cl7]− to [AlCl4]− complexes in electrolytes still satisfies the electrochemical reaction process.

Raman spectra of (a) fresh and (b) after a week AlCl3-acetamide electrolyte containing DCE additive; (c) optical photograph of AlCl3-acetamide-DCE systems.
The highest current peak value is recorded in AlCl3-acetamide (Fig. 3a), while adding DCE, the current peak value reduces, which can be ascribed to the decrease of the relative content of active [Al2Cl7]− complex, thus agreeing with the results reported in Fig. 2. The aluminum plating-stripping efficiency was calculated for AlCl3-acetamide is 88.68 % and 93.80 % for AlCl3-acetamide-DCE system. The comparison suggests the AlCl3-acetamide-DCE as the most efficient media for the aluminum deposition/stripping process. The electrochemical stability of the investigated electrolytes upon oxidation on the tungsten current collectors, unchanged anode limit potential indicates the AlCl3-acetamide-DCE electrolyte can still provide a high charging cut-off voltage. To demonstrate aluminum dendrites on the anode surface in rechargeable aluminum batteries, the voltage profiles of the symmetric Al-Al cells with various AlCl3-based electrolytes are inspected. The voltage profiles of the symmetric Al-Al cells with acetamide-AlCl3 electrolytes containing DCE are inspected to evaluate the effect of the additive on the electrochemical process of the aluminum anode in rechargeable aluminum batteries. As seen from the voltage vs. time signature reported in stable operation (Fig. 3b), The Al-Al cell using acetamide-AlCl3-DCE as an electrolyte has a lower polarization than Al|AlCl3-acetamide|Al cell, attributing to the reduction of the viscosity of acetamide-AlCl3 electrolytes. The cross-sectional SEM images and EDX mapping were used to characterize the original state of the aluminum electrode surface of Al-Al batteries with various AlCl3-based electrolytes after a long-term cycle (Fig. 3d). Dendritic structures are observed on aluminum electrodes after a long-term cycle of Al-Al cells. Besides, large dead aluminum exists in separators for Al|acetamide-AlCl3|Al cell. The unstable voltage in the Al|AlCl3-acetamide|Al cell observed in Fig. 3c is related to the repeating formation and dissolution of aluminum dendrites. The voltage of the Al|acetamide-AlCl3-DCE|Al cell is larger in the initial stage and reduces to a stable value as the cell operates for 500 cycles without any short circuit. The cycling stability of the cell is dramatically improved because the dendritic structure is effectively inhibited by electrolyte additives.
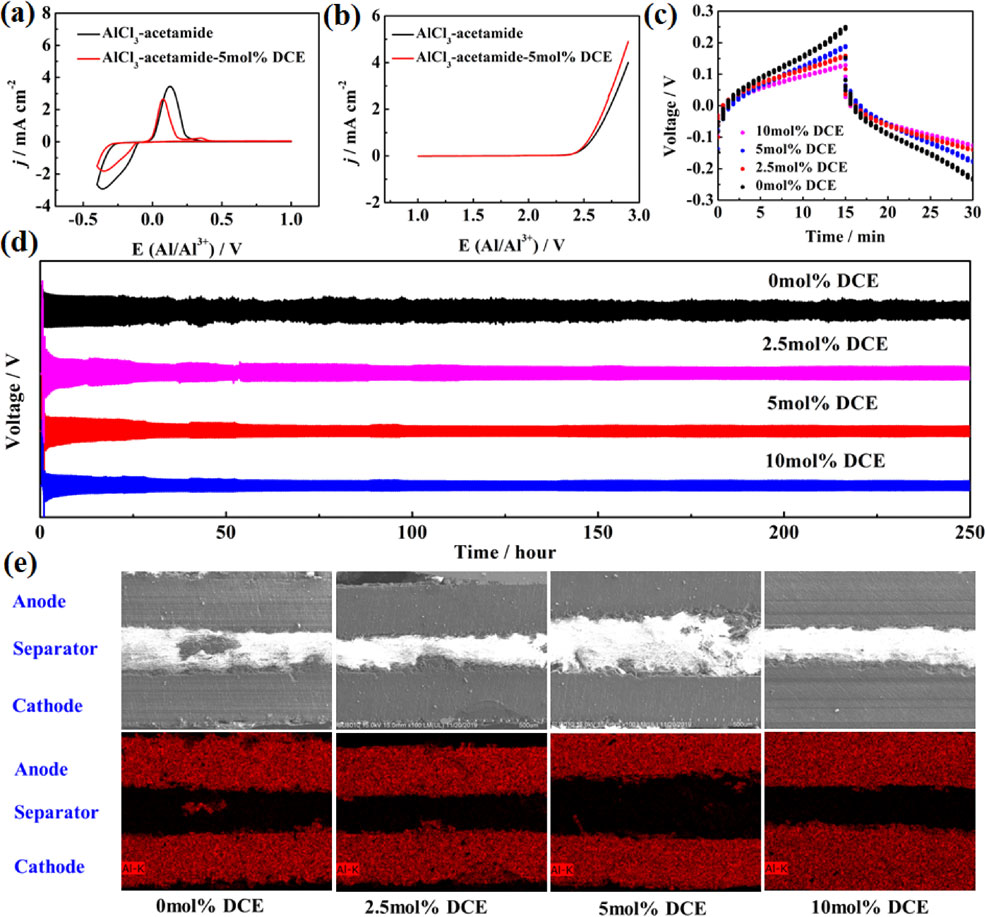
(a) CVs recorded on Al electrode and (b) LSVs recorded on tungsten of acetamide-AlCl3 electrolyte containing DCE (5 mol%); Voltage vs. time signature of the galvanostatic cycling test performed on Al-Al cell using AlCl3-acetamide-based electrolyte electrolytes reported in (c) stable operation and (d) the full test performed; (e) Cross-sectional SEM images and EDX mapping of symmetric Al-Al cells.
The galvanostatic charge/discharge test was proceeded using pouch cells with aluminum as the anode and pyrolytic graphite (PG) as the cathode to evaluate the effect of additive DCE on the electrochemical performance of rechargeable aluminum-graphite batteries. The electrochemical performances of Al-PG batteries using acetamide-AlCl3 with/without DCE as electrolytes were illustrated in Fig. 4. During charging and discharging, all systems have similar voltage characteristic curves (Fig. 4a). For acetamide-AlCl3 electrolyte, Al-PG battery exhibits an unstable discharge specific capacity of 50–60 mAh g−1 with a low coulombic efficiency of 90 % even over 300 cycles. The discharging process consists of two stages. Acetamide-AlCl3-DCE electrolyte provides a specific capacity of 40 mAh g−1, which is double compared with that (20 mAh g−1) of the acetamide-AlCl3 system. It is consistent with the result that a significantly higher intensity of the peak (located at 2.25–1.75 V) in the differential capacity curves than that in the system without the additive. The intensity of the peak increases when DCE is added to the acetamide-AlCl3 electrolyte, indicating an increased intercalation/de-intercalation ability of the [AlCl4]− complex ions between the graphite layers (Fig. 4b). When DCE was added into the electrolyte, the coulombic efficiency of the Al-PG battery increases to nearly 100 %, and the discharge specific capacity increases to 68 mAh g−1 for acetamide-AlCl3-DCE electrolytes (Fig. 4d).

(a) Charge and discharge curves and (b) differential capacity curves of Al-GP batteries with acetamide-AlCl3 based electrolytes operated at a high rate of 4 C; (c) electrochemical impedance spectroscopy (EIS) of the Al-GP batteries; (d) cycling stability of Al-GP batteries with acetamide-AlCl3 based electrolytes operated at a high rate of 4 C.
The electrochemical impedance spectroscopy (EIS) of both batteries with acetamide-AlCl3 and acetamide-AlCl3-DCE (5 mol%) electrolytes were shown in Fig. 4c. The charge-transfer resistance (Rct) in the high-medium frequency region represents the charge transfer ability of ions at the electrolyte and cathode interface. The Rct value (779.5 Ω) of acetamide-AlCl3-5 mol% DCE electrolyte is smaller than that (1303.1 Ω) of acetamide-AlCl3 electrolyte, reflecting the charge transfer ability at the electrolyte and cathode interface become higher after adding additive DCE into acetamide-AlCl3. The Warburg impedance (W) in the low-frequency region reflects the diffusion ability of anions into the graphite layer. After adding the additive DCE, the W value decreases to 116.6 Ω cm−2 from 214.5 Ω cm−2 (acetamide-AlCl3), which means the diffusion ability of anions into the graphite layer becomes stronger with the existing of additive DCE in the electrolyte. The viscosity (η) and electrical conductivity (σ) of acetamide-AlCl3-5 mol% DCE are measured as 67.87 mP s and 1.88 mS cm−1 (25 °C), respectively. Compared with the acetamide-AlCl3 system (η = 78.76 mP s, σ = 1.55 mS cm−1), the improved physicochemical properties by additive DCE increased the kinetics of ion transport. Based on the above analysis, the presence of DCE in electrolyte prompt the [AlCl4]− complex ions to intercalate into graphite.
The organics additive significantly improved the capacity and coulomb efficiency of aluminum-graphite type cells with the AlCl3-based system as electrolytes, especially in aluminum cells with the AlCl3-acetamide system as an electrolyte. Therefore, the performance of aluminum-graphite cells can be further improved by adjusting the additive composition, such as optimization of additive content, binary/tertiary additives, or organic substances containing heterocyclic structures.
Electrolyte additive, 1,2-dichloroethane (DCE), is considered to investigate its effect on the electrochemical performance of aluminum-graphite batteries using the acetamide-AlCl3 electrolyte. Original dendritic and dead aluminum are observed in the anode and separator of Al-Al cells at a high operation rate. DCE is a preferred electrolyte additive to reduce the polarization of the acetamide-AlCl3 electrolytes. Furthermore, DCE can effectively suppress the dendrites on Al anode in Al|acetamide-AlCl3|Al cells. Besides, it has a positive effect on improving the discharge specific capacity and cycle stability of aluminum-graphite batteries with a high coulombic efficiency over 300 cycles. This result indicates that organic additive is suitable to improve the electrochemical performance of aluminum-graphite batteries.
This work was financially supported by the Key Scientific Research Project of Colleges and Universities in Henan Province (Nos. 23B480002, 22B480001), Henan Provincial Science and Technology Research Project (No. 232102240083).
The data that support the findings of this study are openly available under the terms of the designated Creative Commons License in J-STAGE Data at https://doi.org/10.50892/data.electrochemistry.22729091.
Fengcui Li: Conceptualization (Equal), Data curation (Lead), Formal analysis (Lead), Funding acquisition (Supporting), Investigation (Lead), Methodology (Lead), Visualization (Lead), Writing – original draft (Lead)
Chengyuan Liu: Conceptualization (Equal), Funding acquisition (Lead), Supervision (Equal), Writing – review & editing (Supporting)
Rujia Liu: Data curation (Equal), Formal analysis (Equal), Writing – review & editing (Equal)
Jiangyu Yu: Data curation (Equal), Formal analysis (Equal), Writing – review & editing (Equal)
Zhiwei Liu: Data curation (Equal), Formal analysis (Equal), Writing – review & editing (Equal)
The authors declare no competing financial interest.
Key Scientific Research Project of Colleges and Universities in Henan Province: 23B480002, 22B480001
Henan Provincial Science and Technology Research Project: 232102240083