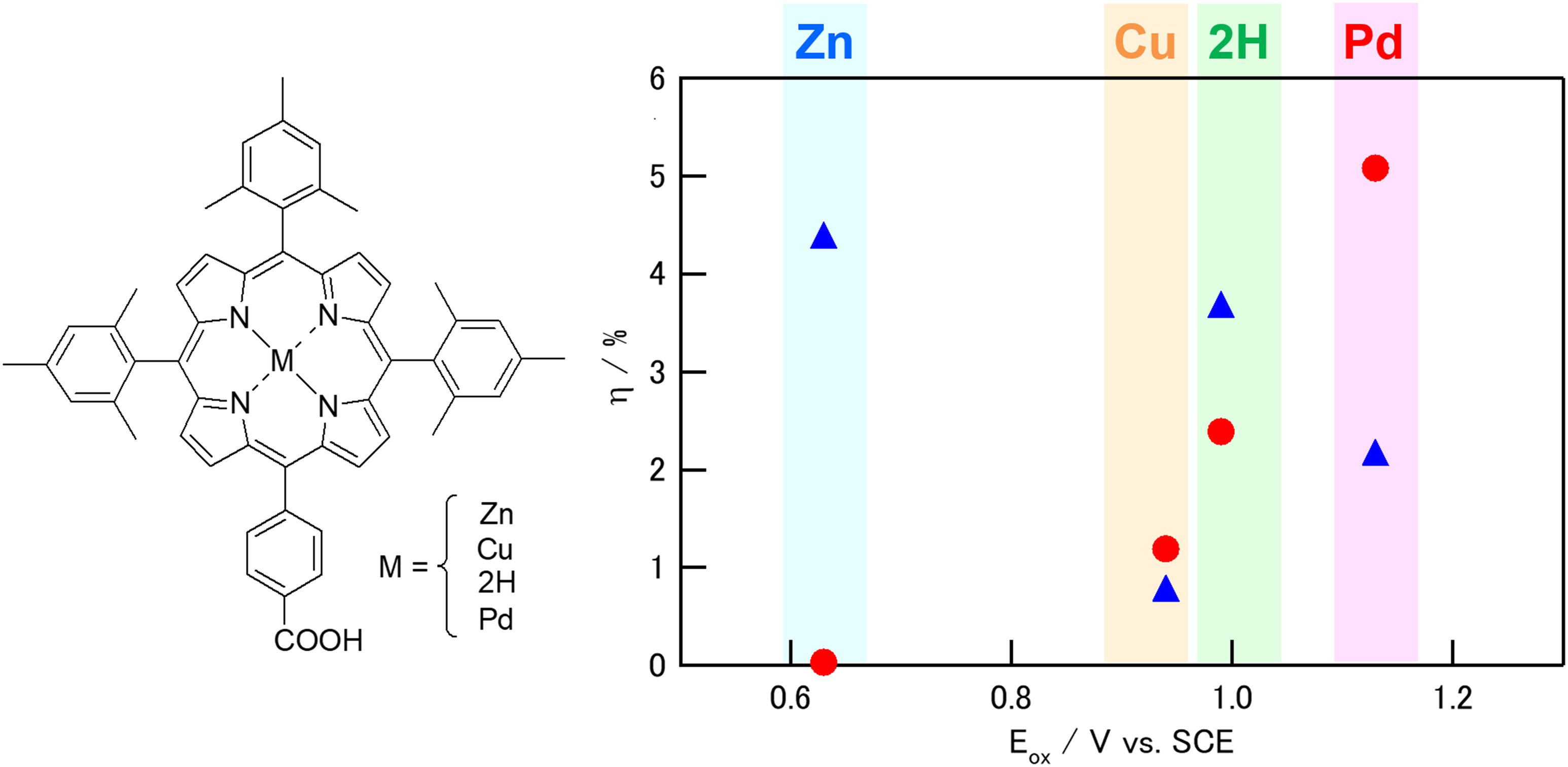
Abstract
Effects of central metal ions in porphyrin-sensitized solar cells were investigated by using 5-(4-carboxyphenyl)-10,15,20-tris(2,4,6-trimethylphenyl)porphyrins as sensitizers and I−/I3− or Br−/Br3− as redox mediators. Zn(II), Cu(II), Pd(II), and free-base porphyrins were synthesized, and the properties of them and the photovoltaic performances of the dye-sensitized solar cells (DSSCs) using them are compared. The electron injection processes from the dyes into TiO2 were investigated by changing Li+ concentration in the electrolytes. The regeneration processes of the dyes were examined by comparing the electrolytes with different redox potentials (I−/I3− or Br−/Br3−). With the Br−/Br3− redox mediator, palladium porphyrin yielded both higher short-circuit current density (Jsc) and open-circuit voltage (Voc) than that with the I−/I3− mediator and achieved the highest power conversion efficiency among all combinations in this study.
1. Introduction
Dye-sensitized solar cells (DSSCs) have attracted wide attention because of their flexible design, ease of fabrication, and potential of the low-cost production.1,2 In DSSCs, photo-excited dyes inject electrons into the conduction band of mesoporous semiconductors such as titanium dioxide (TiO2). The oxidized dyes are then regenerated by the redox mediators in a surrounding electrolyte, where I−/I3− is widely used as a typical redox mediator.
In general, the I−/I3− limits open-circuit voltage (Voc) of DSSC, which is determined by energy difference between the redox potential and the quasi-Fermi level of semiconductors. Additionally, light absorption by I3− can be a disadvantage for short-circuit current density (Jsc) because of the optical loss. To overcome these problems, various alternative redox mediators have been explored. In particular, the conversion efficiency of 12.3 % with a Voc of 0.935 V have been achieved using a cobalt(II/III) tris(2,2′-bipyridine)-based redox mediator.3 However, highly efficient DSSCs with cobalt complexes usually require more elaborate control of surface coverage and porosity of TiO2 due to the faster recombination and the slower diffusion rates of cobalt complexes than the I−/I3− mediator. On the other hand, several application examples of the Br−/Br3− redox mediator, which has good transparency, deeper potential, and comparable diffusion rates to the I−/I3− mediator, were reported.4–7 Among them, a DSSC showing a record high potential over 1.4 V by the use of the Br−/Br3− mediator was reported.7
Porphyrin derivatives are one of the most promising dyes for the high-voltage DSSCs.3,8,9 To date, various metalloporphyrins6,10–19 and their dimers20–26 and trimers27–29 have been applied to DSSCs. Recently, porphyrin dyes have also been used in combination with organic dyes.30–32 There are many review papers on porphyrin dyes for DSSCs and hundreds of porphyrins are listed but almost all of them are zinc porphyrins except a few freebase ones.33–35 In porphyrin dimers as sensitizers, sometimes one of the two porphyrins was freebase porphyrins20–23 but in many cases the both porphyrins have zinc as central metal ions.24–26 Thus, the main topics of many researches on porphyrin dyes for DSSCs were not on central metal ions but on the modification of porphyrin ligands. On the other hand, some literatures reported that other metal ions have some advantages than Zn(II).6,11,12 Gervaldo et al. conducted a systematic study on the sensitizing properties of various metalloporphyrins with SnO2 semiconductor and concluded that a palladium porphyrin exhibits a higher photocurrent quantum yield than a zinc one.11 Alibabaei et al. reported that copper coproporphyrin yields higher efficiency than zinc or free-base ones, although much higher efficiencies of zinc porphyrins than copper counterparts were reported by different porphyrin derivatives.12 Moore et al. reported that palladium porphyrin yields higher efficiency in the combination of the Br−/Br3− redox mediator and SiO2 semiconductor than zinc and freebase counterparts.6 However, the reported efficiencies of metalloporphyrins except zinc one remains to be less than 3.8 %12 with I−/I3− redox. Understandings of the effects of central metal ions and halogen redox mediators on DSSC performances remain to be insufficient yet.
In this work, we focused on Zn(II), Cu(II), Pd(II), and free-base porphyrins (ZnP, CuP, PdP, and HP, Fig. 1) to investigate the effects of central metal ions on DSSC performances with I−/I3− or Br−/Br3− redox mediators. Compared to previously reported papers on central metal ions,6,11,12 four central metal ions with different electronegativities and two electrolytes with different redox potentials were systematically selected and each process in DSSC (i.e., electron injection, dye regeneration, and charge recombination) was investigated separately to obtain widely applicable findings. To scrutinize the energetics of the electron injection, photocurrents of DSSCs were measured and compared by varying Li+ concentration which lowers the conduction band (CB) edge of TiO2. The regeneration processes of the dyes were examined by comparing the electrolytes with different redox potentials (I−/I3− or Br−/Br3−). The charge recombination processes were evaluated by measuring the electron lifetime using the step-light induced measurements of photocurrent and photovoltage (SLIM-PCV) technique36 and the charge extraction method.37

2. Experimental Section
2.1 Materials and methods
Pyrrole was distilled from CaH2 under argon atmosphere. All other solvents and chemicals used in this work were analytical grade and used without further purification unless otherwise noted. 1H NMR spectra were recorded on a Bruker Avance 600 in CD2Cl2, where the chemical shifts were determined with residual CHDCl2 (δ 5.32). High-resolution mass spectra were obtained with a Shimadzu LCMS-IT-TOF through electrospray ionization (ESI).
2.2 Synthesis of dyes
HP and ZnP were synthesized as reported procedure.38 CuP and PdP were synthesized in a similar procedure as literatures.39,40 The general synthesis of dye molecules is presented as follows. To a stirred solution of 1 equiv of methyl 4-formyl-benzoate and 3 equiv of 2,4,6-trimethylbenzaldehyde and 4 equiv of pyrrole in CHCl3 was added BF3-OEt2 and refluxed under argon atmosphere for 2 h. P-chloranil was added and the solvent was removed in vacuo. Column chromatography on silica gel with CHCl3 afforded 5-(4-methoxy carbonylphenyl)-10,15,20-tris(2,4,6-trimethylphenyl)porphyrin (HP-est). For metalloporphyrins, excess of acetate salt of each metal ion was added to the solution of HP-est in CH2Cl2 and refluxed for 1 h. The reaction mixture was washed with saturated aqueous NaHCO3 and H2O, dried over anhydrous sodium sulfate, and purified by column chromatography on silica gel with CH2Cl2. Methyl ester of each porphyrin dye is hydrolyzed by adding NaOH and stirring at room temperature for 1 h. After adding 1 equiv of HCl, the solution was washed with H2O, dried over anhydrous sodium sulfate, and purified by column chromatography on silica gel (CH2Cl2/MeOH = 9 : 1).
Copper 5-(4-carboxyphenyl)-10,15,20-tris(2,4,6-trimethyl-phenyl)porphyrin (CuP). HRMS (ESI-MS): m/z = 844.2841 [M−H]−, (calcd. for C54H45N4O2Cu: 844.2844).
Palladium 5-(4-carboxyphenyl)-10,15,20-tris(2,4,6-trimethyl-phenyl)porphyrin (PdP). 1H NMR (600 MHz, CD2Cl2) δ (ppm): 8.72 (d, J = 5.2 Hz, 2H), 8.65 (d, J = 5.1 Hz, 2H), 8.61 (m, 4H), 8.47 (d, J = 8.4 Hz, 2H), 8.32 (d, J = 8.3 Hz, 2H), 7.28 (s, 6H), 2.60 (s, 9H), 1.85 (s, 6H), 1.82 (s, 12H). HRMS (ESI-MS): m/z = 887.2598 [M−H]−, (calcd. for C54H45N4O2Pd: 887.2601).
2.3 Device fabrication
Mesoporous TiO2 films were prepared using commercial pastes of pure anatase TiO2 (Ti-Nanooxide, Solaronix) with mean particle sizes of 13 nm and 400 nm for the transparent power-generating and opaque scattering layers, respectively. The pastes were screen-printed on a fluorine-doped SnO2 glass plate (FTO, Nippon Sheet Glass Co. Ltd.). The printed TiO2 films on FTO glass were sintered at 500 °C for 30 min, treated with TiCl4 aqueous solution at 70 °C for 30 min, and sintered once again at 500 °C for 30 min. For the photoanode preparation, the TiO2 films were immersed into a methanol solution of 0.2 mM porphyrin at 30 °C for 1 h. The films were rinsed with the immersing solvent before use.
DSSCs were fabricated by injecting an electrolyte between a photoanode and a Pt-coated glass counter electrode (Geomatec Co. Ltd.). Two electrodes were separated by a 20 µm spacer (Himilan, DuPont) and tightly held by clips. Iodine-based electrolytes were composed of 25 mM (M = mol dm−3) I2 and arbitrary concentrations of LiI and 1,2-dimethyl-3-propylimidazolium iodide (DMPII) in acetonitrile. Bromine-based electrolytes were composed of 25 mM Br2 and arbitrary concentrations of LiBr and 1,2-dimethyl-3-propylimidazolium bromide (DMPIBr) in acetonitrile. The active area of the cells was 0.16 cm2.
2.4 Photovoltaic characterization
Photovoltaic properties of the cells were measured under illumination of standard air mass (AM) 1.5G simulated sunlight (YSS-80, Yamashita Denso) with a potentiostat (HSV-100, Hokuto Denko). The light intensity was calibrated to 1 Sun (100 mW cm−2) by a standard silicon cell (BS-520 S//N 235, Bunkoukeiki Co. Ltd.). Collected data were used to calculate the power conversion efficiency (η) according to the following equation: η = Jsc × Voc × FF/Pin, where Jsc is the short-circuit current density, Voc is the open circuit voltage, FF is the fill factor, and Pin is the incident radiation power.
Incident photon-to-current conversion efficiency (IPCE) spectra were measured by an IPCE measurement system (SM-250E, Bunkoukeiki Co. Ltd.). A standard silicon solar cell (SiPD S1337-1010BQ, Bunkoukeiki Co. Ltd.) was used as the reference. IPCE values were calculated from the current ratio and the IPCE value of the reference cell at each wavelength.
2.5 Absorption measurements
The UV-visible absorption spectra of the materials in solution or on TiO2 films were recorded with a JASCO V-570 UV/vis/NIR spectrophotometer. For the absorption spectra of dyes on TiO2, 6 µm-thick transparent TiO2 films were used.
2.6 Electrochemical measurements
Electrochemical measurements were performed by the differential pulse voltammetry (DPV) method on a BAS100 electrochemical analyzer. Voltammograms were recorded under argon flow using dehydrated dichloromethane and 0.1 M nBu4NPF6 as the supporting electrolyte. A two-compartment cell connected by a salt bridge was employed, containing a porphyrin solution, a platinum disk (BAS) working electrode and a Pt wire counter electrode in one cell, and a glass Ag/AgCl reference electrode and a saturated KCl aqueous solution in the other cell. Ferrocene/ferrocenium (0.53 V vs. Ag/AgCl) was used as the external potentiometric standard. All of measured values were converted to potentials vs. standard calomel electrode (SCE).
2.7 Electron lifetime and electron density measurements
Electron lifetimes in DSSCs were measured by the step-light induced measurements of photocurrent and photovoltage (SLIM-PCV) technique23 using a violet LED (λ = 405 nm) as the light source. Transient photovoltage decay was induced by a small stepwise change in light intensity, and electron lifetimes were estimated by a single exponential fitting. Electron densities in the TiO2 film of DSSCs were measured by a charge extraction method,37 where DSSCs were changed from open circuit to short circuit and light was simultaneously switched off. The amount of extracted charge was estimated by integrating the transient current to calculate the electron density.
2.8 Fluorescence lifetime measurements
The fluorescence lifetimes were determined by a two-dimensional photon-counting technique using a streak camera (C4334 streakscope, Hamamatsu Photonics) and the excitation pulse source of a model-locked Ti-sapphire laser (Tsunami, Spectra Physics; frequency-doubled excitation pulse: 400 nm). A glass cutoff filter was used to isolate the fluorescence from the scattered laser light.
3. Results and Discussion
3.1 Optical and electrochemical properties of porphyrins
Figure 2 shows the absorption spectra of ZnP, HP, CuP, and PdP in MeOH and those on transparent TiO2 films (∼6 µm thick) in acetonitrile. Characteristic data of the spectra are summarized in Table 1. The solution spectra show the typical spectral pattern of porphyrins with Soret band (400–440 nm) and Q bands (500–650 nm). While the Q bands of HP exhibit four peaks, the metalloporphyrins have fewer Q band peaks because of degenerate excited states with square symmetry. With the increase in the electronegativity of central metal ions, the absorption spectral edge is blue-shifted, which is attributed to the lowering of the HOMO levels. The absorption spectra around Q bands were enlarged and normalized at the peak of that on TiO2 to compare with in Fig. 2. On TiO2, the Q bands of all the porphyrins showed very similar spectral features to those in solution, indicating that the adsorption on TiO2 has negligible influence on the electronic properties of the porphyrins, while the Soret band absorptions are saturated in this condition.

Table 1. Optical and electrochemical properties of the porphyrins.
| compound |
absorption λmaxa/nm
(ε/104 M−1cm−1) |
Eoxb
/V vs.
SCE |
E0–0c
/V |
Eox–E0–0
/V vs.
SCE |
τ
/ns |
| ZnP |
402 (3.7), 423 (50.0), 558 (1.6), 596 (0.5) |
0.63 |
2.00 |
−1.37 |
2.37 |
| CuP |
412 (51.1), 537 (1.9) |
0.94 |
2.07 |
−1.13 |
— |
| HP |
414 (42.4), 512 (1.7), 546 (0.6), 589 (0.5), 646 (0.3) |
0.99 |
1.88 |
−0.89 |
9.49 |
| PdP |
413 (26.2), 522 (2.3), 553 (0.2) |
1.13 |
2.17 |
−1.04 |
<0.1 |
aWavelengths of absorption peaks in MeOH. bGround state redox potentials measured by DPV in CH2Cl2 containing 0.1 M n-Bu4NPF6 as the supporting electrolyte. cE0–0 was determined by the onset wavelengths of corresponding absorption spectra on TiO2 in acetonitrile.
The oxidation potentials of the porphyrins (Eox) were obtained by DPV and summarized in Table 1. The Eox value positively shifts with the increase in the electronegativity of central metal ions, while that of the freebase is between those of CuP and PdP. The singlet excited-state oxidation potentials (Eox*) were calculated by Eox–E0–0, in which E0–0 values were estimated from the onset wavelength of the corresponding absorption spectra of porphyrins on TiO2 film. From these values, energy diagram of the dyes, conduction band (CB) of TiO2, Br−/Br3−, and I−/I3− was illustrated as Fig. 3. The Eox* values show that electron injection from their singlet excited states to TiO2 CB edge level is energetically feasible in each dye.

In addition to the energy level, lifetime of the excited states is also important factor for the efficient electron injection into the TiO2. To estimate the excited-states lifetimes, fluorescence decays of the porphyrins were measured. Fluorescence spectra and decays are shown in Fig. 4 and estimated lifetimes are listed in Table 1. For the CuP, no fluorescence was detected. It could be explained by the fast intersystem crossing of sing-doublet excited state due to having the unpaired electron of Cu, which produces trip-doublet and trip-quartet states.41,42 Obtained transients of ZnP and HP are fitted by a single exponential decay. The excited-state lifetimes of ZnP and HP are estimated from the exponents to be 2.37 and 9.49 ns, respectively. For the PdP, observed emission was very weak and the decay was too fast to analyze in the system. The excited-state lifetime of PdP should be less than 0.1 ns which is the total apparatus response function of the measurement system. The short lifetime of PdP can be explained by the heavy atom effect of Pd. It is well known that heavy atom induces fast intersystem crossing in porphyrins.43 In the case of Pd-porphyrins with similar structure, excited singlet state lifetime is reported to be around 20 ps.43,44

Using these porphyrins as sensitizers, DSSCs were fabricated and IPCEs were investigated varying the Li+ concentration in the I−/I3− based electrolytes. Li+ concentrations were controlled by changing the LiI amount in the electrolyte, and at the same time, the amount of DMPII is also adjusted to keep the concentrations of I− and I3− at the same value. It is well known that the IPCE of DSSCs is greatly affected by the Li+ concentration in the electrolytes. Li+ ions can penetrate into the mesoporous TiO2 and increase the driving force for the electron injection from the excited dyes to the TiO2 by lowering the CB edge level.45 Figure 5 is the plots of IPCE maxima as a function of Li+ concentration of DSSCs using each porphyrin dye and I−/I3− redox. IPCE spectra with I−/I3− or Br−/Br3− redox mediator are also summarized in Fig. 6, where the Li+ concentration is 100 mM, which gave near IPCE maxima for all the DSSCs. It is noteworthy that the IPCE maxima were observed around the peaks of Soret band absorption. As shown in Fig. 2, ZnP, HP, CuP, and PdP on 6 µm-thick transparent TiO2 films showed saturated absorption around this region. Since the photoelectrodes of the DSSCs used here are 10 µm-thick and with a scattering layer, the light harvesting efficiency (LHE) can be considered as sufficiently high in this region. The LHE spectra of the thick TiO2 films including scattering layers for DSSCs sensitized with ZnP, HP, CuP, and PdP were also shown in Fig. 6. The LHE spectra were calculated by subtracting transmittance and reflectance from 100 %. Because of the scattering layer in the TiO2 film, there were some leaked lights through the open window of the integrated sphere or the clearance gap around the sample. Thus, LHEs didn’t reach zero even in the long wavelength region where there was no absorption of dyes. It is also notable that there was new absorption around 700 nm in the case of ZnP. This can be the absorption of the aggregated porphyrins but they didn’t contribute to the photon-to-electron conversion.
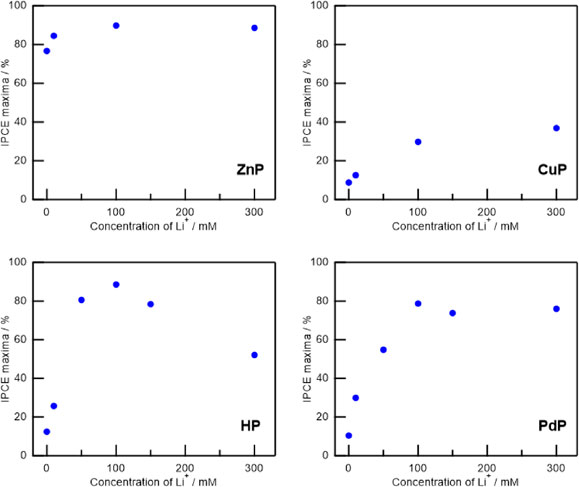
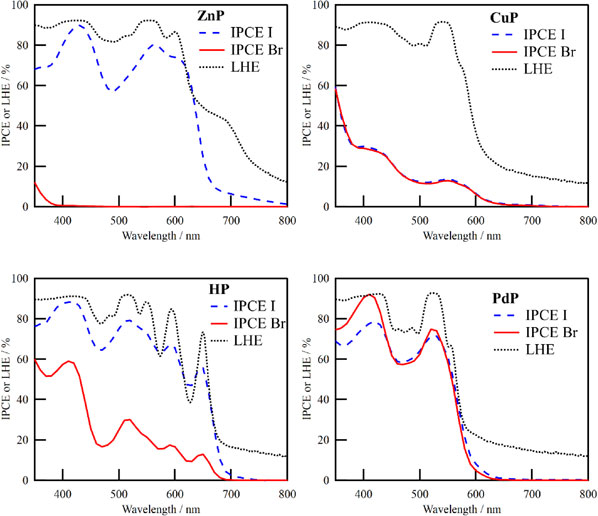
Figure 5 shows that ZnP, HP, and PdP yield high IPCE values of more than 79 %, while the IPCE maximum of CuP is less than 40 %. The considerably low IPCE value for CuP (<40 %) could be explained by the fast intersystem crossing of sing-doublet excited state within 8 ps.46 In the case of HP, the highest IPCE reached 89 % at 100 mM of Li+ but large decrease in IPCE maxima at higher Li+ concentrations were observed, in contrast to the other metalloporphyrins. This trend can be explained from the complexation of HP with Li+.47 It is notable that PdP exhibits a substantial IPCE increase from 5 to 79 % by increasing Li+ concentration. It is reported that the electron injection process in DSSC occurs in <5 ps48,49 and the singlet excited-state lifetime of PdP is around 20 ps.43,44 Thus, these two processes can compete with each other in DSSCs using PdP. With the increase of the Li+ concentration, the electron injection was accelerated and happened more compared to the relaxation of the singlet excited state of PdP. The difference in IPCEs between PdP cell and CuP cell can be explained from the longer lifetime of the singlet state of PdP compared to the sing-doublet state of CuP. In addition, if the CB of TiO2 was sufficiently lowered by Li+ ions, the electron injection from the triplet state of PdP or the trip-doublet and trip-quartet states of CuP might be also possible. In that case, the longer lifetime of the triplet state of PdP (∼200 µs44) compared to the trip-doublet and trip-quartet states of CuP (∼0.1 µs50) can be another reason to explain the difference in IPCE values between PdP and CuP under the high Li+ concentration condition.
In the IPCE spectra of PdP (Fig. 6), the effect of light absorption by I3− can be also confirmed. The smaller IPCE maximum with I−/I3− for PdP (79 %) than that for ZnP or HP (90 and 89 %, respectively) can be explained from the competitive light absorption of I3−. In Table 1, the molar absorbance coefficient (ε) at the Soret band of PdP is 26.2 × 104 M−1cm−1, which is smaller than that of ZnP (50.0 × 104 M−1cm−1) or HP (42.4 × 104 M−1cm−1). Although the LHEs of this region are saturated, the electrolytes can penetrate into the mesoporous TiO2 layer and its absorption can compete with that of the dyes on TiO2. The less ε of the dye, the more I3− can absorb the light and IPCE will be reduced. This explanation is also consistent with the comparison of the IPCEs of PdP sensitized cells between two electrolytes. In the shorter wavelength region, PdP cell showed higher IPCE value with the Br−/Br3− (92 %) than that with the I−/I3− (79 %), in contrast to the similar IPCEs in the longer wavelength region. This can be attributed to the less light absorption of the Br−/Br3− redox mediator around this region than the I−/I3− one. With the I3− absorption and the reflection of light at the FTO-glass surface in mind, it can be said that the absorbed photons by ZnP, HP, or PdP are fully extracted as currents. In other words, IPCE with I−/I3− were not inhibited by other processes such as the dye regeneration process.
On the other hand, IPCE with Br−/Br3− showed large difference depending on the central metal ions of porphyrins. First of all, the IPCE of CuP showed no difference between I−/I3− and Br−/Br3−. As discussed above, the IPCE of DSSC with I−/I3− using CuP was limited by the electron injection process because of the short excited-state lifetime of CuP. The IPCE of DSSC using CuP was considered to be still limited by that injection process even with Br−/Br3− mediator. Thus, there was no difference in IPCE between I−/I3− and Br−/Br3− in the case of CuP. In other words, the regeneration process of oxidized CuP by Br−/Br3− mediator is more efficient than the electron injection process from excited CuP into TiO2. Except for CuP, the IPCE with Br−/Br3− showed different trend compared to that with I−/I3−. Considering the sufficient electron injection efficiencies of DSSCs with ZnP, HP, and PdP at optimized Li+ concentration, the observed difference in their IPCE values with the Br−/Br3− mediator can be explained from the regeneration process of the oxidized dyes. To examine the driving forces for regeneration, the energy differences between Eoxs of the porphyrins and the redox mediator potentials (ΔG) were summarized in Table 2 with the IPCE maxima of the DSSCs. Table 2 shows that the smallest ΔG value for the I−/I3− mediator is 0.52 eV of ZnP, which meets the offset potential usually required for efficient regeneration (>0.5 eV).51 On the other hand, the ΔG values of ZnP and HP for the Br−/Br3− mediator are lower than 0.5 eV. Since the I−/I3− and Br−/Br3− mediators show similar redox reactions with similar potential differences,51 the Br−/Br3− mediator is presumed to require a similar ΔG value with the I−/I3− one for efficient regeneration. Thus, the significantly lower IPCE values of ZnP and HP with the Br−/Br3− than that with the I−/I3− were attributed to the insufficient ΔG values.
Table 2. Driving forces for electron injection from the I
−/I
3− or Br
−/Br
3− redox mediator to porphyrins and IPCE maxima of the DSSCs.
| dye |
I |
Br |
| ΔG/eV |
IPCE/% |
ΔG/eV |
IPCE/% |
| ZnP |
0.52 |
90 |
0.08 |
1 |
| CuP |
0.83 |
30 |
0.39 |
29 |
| HP |
0.88 |
89 |
0.44 |
59 |
| PdP |
1.02 |
79 |
0.58 |
92 |
3.3 Photocurrent-photovoltage characteristics
The J-V curves of the porphyrin-sensitized solar cells with the I−/I3− or Br−/Br3− redox mediator are shown in Fig. 7 and obtained photovoltaic parameters are listed in Table 3. The results are also summarized as a function of Eox of dyes in Fig. 8. All the cells show higher open-circuit voltage (Voc) with the Br−/Br3− mediators than with the I−/I3− ones. With the I−/I3− mediator, ZnP exhibits the largest Jsc, Voc, and η, and it is consistent with the fact that Zn ion has been used as a popular central ion for the porphyrin dyes for long time. However, Fig. 8 shows that Jsc of the DSSCs with Br−/Br3− mediator can be significantly increased by using dye with deep Eox. As a result, PdP with Br−/Br3− mediator yielded the highest η value, which exceled that of ZnP with the I−/I3− mediator. It is noteworthy that not only the Voc but also the Jsc of the PdP cell with the Br−/Br3− mediator was larger than that with the I−/I3− one, which can be attributed to smaller electrolyte absorption of the Br−/Br3− mediator as mentioned above in the IPCE measurement section. These results indicate that the use of the Br−/Br3− mediator for porphyrin-sensitized solar cells with sufficiently deep Eox is a promising approach.
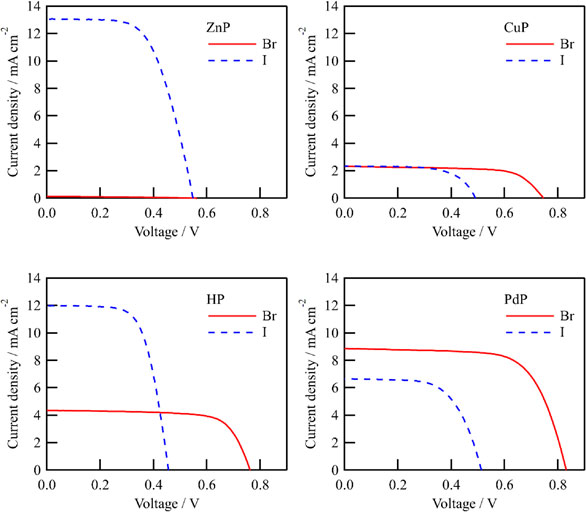
Table 3. Photovoltaic Performances of DSSCs with the porphyrins in combination with the I
−/I
3− or Br
−/Br
3− redox mediator.
| dye |
electrolyte |
Jsc/mA cm−2 |
Voc/V |
FF/− |
η/% |
| ZnP |
Ia |
13.10 |
0.55 |
0.61 |
4.35 |
| Brb |
0.13 |
0.56 |
0.37 |
0.03 |
| CuP |
Ia |
2.34 |
0.49 |
0.64 |
0.74 |
| Brb |
2.33 |
0.75 |
0.69 |
1.19 |
| HP |
Ia |
12.00 |
0.45 |
0.67 |
3.64 |
| Brb |
4.36 |
0.76 |
0.72 |
2.39 |
| PdP |
Ia |
6.66 |
0.51 |
0.63 |
2.13 |
| Brb |
8.86 |
0.83 |
0.69 |
5.08 |
a0.1 M LiI, 0.6 M DMPII, and 25 mM I2. b0.1 LiBr, 0.6 M DMPIBr, and 25 mM Br2.
3.4 Electron lifetime in DSSCs
In addition to the electron injection and the dye regeneration, there is another important factor in DSSCs: the recombination. The injected electrons in TiO2 can recombine with the redox mediators or the oxidized dyes and that process also affects the photovoltaic performances of DSSCs. To evaluate the recombination process depending on the central metal ions of porphyrins, electron lifetime vs. electron density of the DSSCs were measured by the SLIM-PCV technique and the charge extraction method. Before the measurements, the adsorption amounts of porphyrins on the TiO2 film were estimated because the adsorption amount also affects the recombination process. The adsorbed dyes on TiO2 were dissolved again in 4 : 1 MeOH/water solution with NaOH and the amounts were calculated from the absorbance of the solution. The estimated adsorption amounts are summarized in Table 4. As a result, the adsorption amounts of all the porphyrins were found to be almost equal, assuring that difference in the adsorption amount has negligible influence on measured lifetimes. Figure 9 shows the plots of electron lifetime of DSSCs as a function of electron density. For both the redox mediators, the metalloporphyrins showed longer electron life time than the free-base ones. Among the four porphyrins, ZnP showed the longest lifetime with I−/I3− redox mediator, although the measurement of ZnP with Br−/Br3− was difficult because of the low current. It interesting that the electron lifetimes in TiO2 were such different depending on the central metal ions even with the almost same adsorption mount. The details mechanisms should be researched in the future but for the improvement of DSSC performances, the differences of the electron lifetimes between I−/I3− and Br−/Br3− can be more important information. The electron lifetime in DSSCs with the Br−/Br3− mediator showed larger decrease as a function of electron density than that with the I−/I3− mediator, which is independent of the central metal ions. The Br−/Br3− showed the faster recombination in DSSCs than I−/I3−. In other words, there is still a room for efficiency improvement in porphyrin sensitized solar cells with Br−/Br3− redox mediators by hindering the fast recombination.
Table 4. Porphyrin adsorption amounts (mol) on TiO
2 film.
| ZnP |
CuP |
HP |
PdP |
| 2.8 × 10−8 |
2.8 × 10−8 |
2.6 × 10−8 |
3.0 × 10−8 |
4. Conclusions
Investigations of DSSCs using a tetra-aryl porphyrin ligand with various central metal ions revealed that zinc, palladium, and free-base porphyrins can attain the efficient electron injection into TiO2 in the presence of Li+, while the cupper one remains to show low IPCE of less than 40 %. Also, the free-base porphyrin showed large decrease in IPCE at higher Li+ concentrations compared with the other metalloporphyrins. When sufficiently deep Eox of porphyrin is satisfied, the Br−/Br3− mediator can give higher Jsc than the I−/I3− one in addition to Voc increase. As a result, the palladium porphyrin yielded the highest power conversion efficiency among all combinations in this study. The electron lifetime in DSSCs with the Br−/Br3− mediator showed larger decrease as a function of electron density than that with the I−/I3− mediator irrespective to metal ion types. It is suggested that hindering recombination can make the porphyrin-sensitized solar cells with the Br−/Br3− mediator more efficient. The replacement of the central metal ion is one of the easiest ways to control the properties of porphyrin dyes and can be widely adopted to various porphyrin ligands without any difficulty. Thus, these findings in this research will be the important solution for DSSCs not only with the Br−/Br3− but also with various redox mediators which have different redox potentials compared to I−/I3−. For those future applications, the importance of hindering recombination was also shown in this research by the electron lifetime measurement.
Acknowledgment
This work was supported by the New Energy and Industrial Technology Development Organization (NEDO) under the Ministry of Economy Trade and Industry (METI) of Japan.
CRediT Authorship Contribution Statement
Fumiyasu Awai: Conceptualization (Lead), Data curation (Lead), Formal analysis (Lead), Investigation (Lead), Writing – original draft (Lead)
Yonbon Arai: Formal analysis (Equal), Investigation (Equal), Methodology (Lead), Project administration (Equal)
Jotaro Nakazaki: Writing – review & editing (Equal)
Satoshi Uchida: Methodology (Equal), Resources (Equal), Supervision (Supporting)
Hiroshi Segawa: Project administration (Lead), Supervision (Equal), Writing – review & editing (Supporting)
Conflict of Interest
The authors declare no conflict of interest in the manuscript.
Funding
New Energy and Industrial Technology Development Organization: P20005
New Energy and Industrial Technology Development Organization: P20015
Footnotes
J. Nakazaki, S. Uchida, and H. Segawa: ECSJ Active Members
References
- 1) B. O’Regan and M. Grätzel, Nature, 353, 737 (1991).
- 2) A. Hagfeldt and M. Grätzel, Acc. Chem. Res., 33, 269 (2000).
- 3) A. Yella, H. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W. Diau, C. Yeh, S. M. Zakeeruddin, and M. Graetzel, Science, 334, 629 (2011).
- 4) Z. S. Wang, K. Sayama, and H. Sugihara, J. Phys. Chem. B, 109, 22449 (2005).
- 5) C. Teng, X. Yang, S. Li, M. Cheng, A. Hagfeldt, L. Wu, and L. Sun, Chem. Eur. J., 16, 13127 (2010).
- 6) G. F. Moore, S. J. Konezny, H. Song, R. L. Milot, J. D. Blakemore, M. L. Lee, V. S. Batista, C. A. Schmuttenmaer, R. H. Crabtree, and G. W. Brudvig, J. Phys. Chem. C, 116, 4892 (2012).
- 7) K. Kakiage, H. Osada, Y. Aoyama, T. Yano, K. Oya, S. Iwamoto, J. Fujisawa, and M. Hanaya, Sci. Rep., 6, 35888 (2016).
- 8) M. J. Griffith, K. Sunahara, P. Wagner, K. Wagner, G. G. Wallace, D. L. Officer, A. Furube, R. Katoh, S. Mori, and A. J. Mozer, Chem. Commun., 48, 4145 (2012).
- 9) L. Li and E. W. Diau, Chem. Soc. Rev., 42, 291 (2013).
- 10) M. K. Nazeeruddin, R. Humphry-Baker, D. L. Officer, W. M. Campbell, A. K. Burrell, and M. Grätzel, Langmuir, 20, 6514 (2004).
- 11) M. Gervaldo, F. Fungo, E. N. Durantini, J. J. Silber, L. Sereno, and L. Otero, J. Phys. Chem. B, 109, 20953 (2005).
- 12) L. Alibabaei, M. Wang, R. Giovannetti, J. Teuscher, D. Di Censo, J. Moser, P. Comte, F. Pucciarelli, S. M. Zakeeruddin, and M. Graetzel, Energy Environ. Sci., 3, 956 (2010).
- 13) N. Xiang, W. Zhou, S. Jiang, L. Deng, Y. Liu, Z. Tan, B. Zhao, P. Shen, and S. Tan, Sol. Energy Mater. Sol. Cells, 95, 1174 (2011).
- 14) H. He, M. Dubey, Y. Zhong, M. Shrestha, and A. G. Sykes, Eur. J. Inorg. Chem., 2011, 3731 (2011).
- 15) S. Ardo, D. Achey, A. J. Morris, M. Abrahamsson, and G. J. Meyer, J. Am. Chem. Soc., 133, 16572 (2011).
- 16) I. Radivojevic, G. Bazzan, B. P. Burton-Pye, K. Ithisuphalap, R. Saleh, M. F. Durstock, L. C. Francesconi, and C. M. Drain, J. Phys. Chem. C, 116, 15867 (2012).
- 17) S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. F. E. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, M. K. Nazeeruddin, and M. Grätzel, Nat. Chem., 6, 242 (2014).
- 18) A. Yella, C. L. Mai, S. M. Zakeeruddin, S. N. Chang, C. H. Hsieh, C. Y. Yeh, and M. Grätzel, Angew. Chem., Int. Ed., 53, 2973 (2014).
- 19) Y. Kurumisawa, T. Higashino, S. Nimura, Y. Tsuji, H. Iiyama, and H. Imahori, J. Am. Chem. Soc., 141, 9910 (2019).
- 20) J. T. Dy, K. Tamaki, Y. Sanehira, J. Nakazaki, S. Uchida, T. Kubo, and H. Segawa, Electrochemistry, 77, 206 (2009).
- 21) J. K. Park, J. Chen, H. R. Lee, S. W. Park, H. Shinokubo, A. Osuka, and D. Kim, J. Phys. Chem. C, 113, 21956 (2009).
- 22) Y. Liu, H. Lin, J. T. Dy, K. Tamaki, J. Nakazaki, D. Nakayama, S. Uchida, T. Kubo, and H. Segawa, Chem. Commun., 47, 4010 (2011).
- 23) Y. Liu, H. Lin, J. Li, J. T. Dy, K. Tamaki, J. Nakazaki, D. Nakayama, C. Nishiyama, S. Uchida, T. Kubo, and H. Segawa, Phys. Chem. Chem. Phys., 14, 16703 (2012).
- 24) C. L. Mai, W. K. Huang, H. P. Lu, C. W. Lee, C. L. Chiu, Y. R. Liang, E. W. G. Diau, and C. Y. Yeh, Chem. Commun., 46, 809 (2010).
- 25) T. Zhang, X. Qian, P. F. Zhang, Y. Z. Zhu, and J. Y. Zheng, Chem. Commun., 51, 3782 (2015).
- 26) T. Higashino, Y. Kurumisawa, H. Iiyama, and H. Imahori, Chem. Eur. J., 25, 538 (2019).
- 27) T. Hamamura, J. T. Dy, K. Tamaki, J. Nakazaki, S. Uchida, T. Kubo, and H. Segawa, Phys. Chem. Chem. Phys., 16, 4551 (2014).
- 28) T. Hamamura, J. Nakazaki, S. Uchida, T. Kubo, and H. Segawa, Chem. Lett., 43, 655 (2014).
- 29) T. Hamamura, J. Nakazaki, S. Uchida, T. Kubo, and H. Segawa, Chem. Lett., 43, 796 (2014).
- 30) J. M. Ji, H. Zhou, Y. K. Eom, C. H. Kim, and H. K. Kim, Adv. Energy Mater., 10, 2000124 (2020).
- 31) K. Zeng, Y. Chen, W. H. Zhu, H. Tian, and Y. Xie, J. Am. Chem. Soc., 142, 5154 (2020).
- 32) J. Zou, Y. Wang, G. Baryshnikov, J. Luo, X. Wang, H. Ågren, C. Li, and Y. Xie, ACS Appl. Mater. Interfaces, 14, 33274 (2022).
- 33) M. Urbani, M. Grätzel, M. K. Nazeeruddin, and T. Torres, Chem. Rev., 114, 12330 (2014).
- 34) T. Higashino and H. Imahori, Dalton Trans., 44, 448 (2015).
- 35) K. Zeng, Z. Tong, L. Ma, W. H. Zhu, W. Wu, Y. Xie, and Y. Xie, Energy Environ. Sci., 13, 1617 (2020).
- 36) S. Nakade, T. Kanzaki, Y. Wada, and S. Yanagida, Langmuir, 21, 10803 (2005).
- 37) L. M. Peter, N. W. Duffy, R. L. Wang, and K. G. U. Wijayantha, J. Electroanal. Chem., 524–525, 127 (2002).
- 38) H. Imahori, S. Hayashi, T. Umeyama, S. Eu, A. Oguro, S. Kang, Y. Matano, T. Shishido, S. Ngamsinlapasathian, and S. Yoshikawa, Langmuir, 22, 11405 (2006).
- 39) J. S. Lindsey and R. W. Wagner, J. Org. Chem., 54, 828 (1989).
- 40) I. Scalise and E. N. Durantini, J. Photochem. Photobiol., A, 162, 105 (2004).
- 41) R. L. Ake and M. Gouterman, Theor. Chim. Acta, 15, 20 (1969).
- 42) M. Gouterman, R. A. Mathies, B. E. Smith, and W. S. Caughey, J. Chem. Phys., 52, 3795 (1970).
- 43) A. Harriman, J. Chem. Soc., Faraday Trans. 2, 77, 1281 (1981).
- 44) K. Kalyanasundaram and M. Neumann-Spallart, J. Phys. Chem., 86, 5163 (1982).
- 45) J. R. Jennings and Q. Wang, J. Phys. Chem. C, 114, 1715 (2010).
- 46) M. Asano, Y. Kaizu, and H. Kobayashi, J. Chem. Phys., 89, 6567 (1988).
- 47) J. S. Manka, D. B. Chugh, and D. S. Lawrence, Tetrahedron Lett., 31, 5873 (1990).
- 48) Y. Tachibana, J. E. Moser, M. Grätzel, D. R. Klug, and J. R. Durrant, J. Phys. Chem., 100, 20056 (1996).
- 49) P. Piatkowski, C. Martin, M. R. Di Nunzio, B. Cohen, S. Pandey, S. Hayse, and A. Douhal, J. Phys. Chem. C, 118, 29674 (2014).
- 50) F. Liu, K. L. Cunningham, W. Uphues, G. W. Fink, J. Schmolt, and D. R. Mcmillin, Inorg. Chem., 34, 2015 (1995).
- 51) A. Hagfeldt, G. Boschloo, L. Sun, L. Kloo, and H. Pettersson, Chem. Rev., 110, 6595 (2010).
 https://orcid.org/0000-0001-5091-4693
https://orcid.org/0000-0001-5091-4693
 https://orcid.org/0000-0002-2528-616X
https://orcid.org/0000-0002-2528-616X
 https://orcid.org/0000-0002-4971-574X
https://orcid.org/0000-0002-4971-574X
 https://orcid.org/0000-0001-8076-9722
https://orcid.org/0000-0001-8076-9722