2024 Volume 92 Issue 4 Pages 043026
2024 Volume 92 Issue 4 Pages 043026
The recent exponential growth in the Li ion batteries (LIB) market, is largely driven by the demand for electric vehicles and the general transition to a green and digital economy. It is therefore imperative to develop more effective and economic processes for recovering battery raw materials such as Li, Co, Cu and Ni. Moreover, these materials are all classified as critical or strategic (Cu, Ni) raw materials by the European Commission, and for Europe, it is of great importance to build a sustainable European supply chain for Li and other battery raw materials to decrease its dependency on import. In this work, we have studied the possibility to recover Li, Ni, Cu and Co from secondary raw materials like black mass (cathode and anode fraction from shredded end- of-life Li ion batteries), as well as Li from spodumene concentrate, spodumene being an important and available Li mineral. The approach has been to convert the metals in the raw materials to metal chlorides, by chlorination in LiCl-KCl (58 : 42) melts at 470 °C and CaCl2-NaCl-KCl (35 : 30 : 30) at 727 °C. With this method, the metals could potentially be reduced from the chloride matrix by subsequent sequential electrodeposition, utilizing their difference in nobility. Regarding black mass, the highest chlorination yields were obtained from uncalcined material (Li 64 %, Co and Ni 22–24 %, Cu 83 %, and Mn 49 %) in LiCl-KCl at 470 °C, the carbon in the black mass probably enhancing the chlorination rate. For spodumene concentrate, a high yield for Li (100 %) was obtained with Cl2 in CaCl2-NaCl-KCl at 727 °C, this melt composition being more oxoacidic and the higher temperature helping the chlorination kinetics.
The recent exponential growth in the Li ion batteries (LIB) market, is largely driven by the demand for electric vehicles and the general transition to a green and digital economy. For example, 82.4 % of all new cars sold in 2023 in Norway were fully electric. It is therefore imperative to develop more effective and economic processes for recovering battery raw materials such as Li, Co, Cu and Ni. Moreover, these materials are classified as critical or strategic (Cu, Ni) raw materials by the European Commission, and for Europe, it is of great importance to build a sustainable European supply chain for Li and other battery raw materials to decrease its dependency on import. In this work, we have studied the possibility to recover Li, Ni, Cu and Co from secondary raw materials like black mass (cathode and anode fraction from shredded end- of-life Li-ion batteries), as well as Li from spodumene concentrate, spodumene being an important and available Li mineral. The approach has been to convert the metals in the raw materials to metal chlorides, by chlorination in chloride melts at 470 °C and 727 °C according to the following equations, either by use of chlorine or HCl gas:
| \begin{equation} y\text{Cl}_{2}(\text{g}) + \text{M$_{x}$O$_{y}$}(\text{s}) = x\text{MCl}_{2y/x}\ (\text{l}) + y/2\text{O}_{2}\ (\text{g}) \end{equation} | (1) |
| \begin{equation} 2y\text{HCl}\ (\text{g}) + \text{M$_{x}$O$_{y}$}\ (\text{s}) = x\text{MCl}_{2y/x}\ (\text{l}) + y\text{H$_{2}$O}\ (\text{g}) \end{equation} | (2) |
The concept is illustrated in Fig. 1. With this method, the metals could potentially be reduced from the chloride matrix by subsequent sequential electrodeposition, utilizing their difference in nobility as seen from Fig. 2.1 In such a process, the Cl2 gas evolved at the anode during electrolysis could be looped back to the chlorination step.
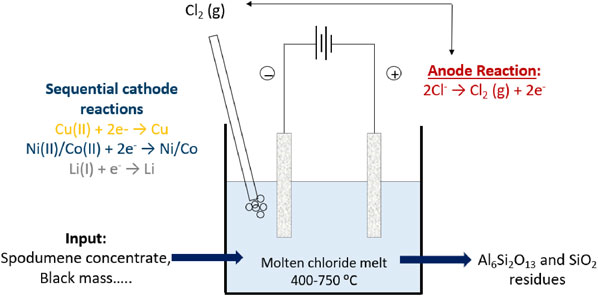
Concept of molten salt chlorination and subsequent electrolysis with chlorine looping.
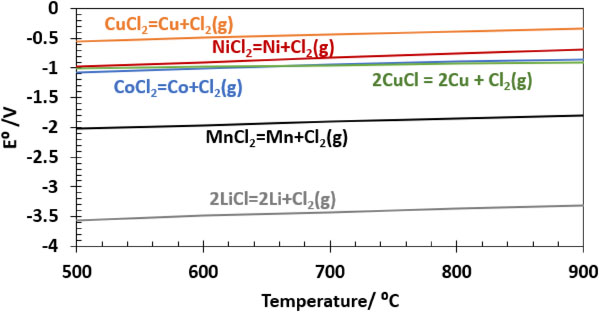
Standard potential for reduction of metal chlorides, calculated from ΔG° extracted from HSE.1
When it comes to Li ion battery recycling, the main approaches can broadly be divided into the categories pyrometallurgical, hydrometallurgical and direct recycling.2 The latter avoid the destruction of the battery materials and attempts to refresh or reactivate the cathode materials to recover properties lost during battery cycling.3
The traditional pyrometallurgy processes occur typically at high temperatures (∼1500 °C), the battery is smelted, and all carbon-based compounds are burned.2 The valuable metals Co, Ni and Mn become reduced and form an alloy, which can be further treated by hydrometallurgical processes to recover the individual elements. The advantages with this process are that it requires little mechanical pre-treatment and sorting, it is flexible and can treat many battery chemistries, and can to a certain degree be co-processed in primary Co, Ni and Cu smelters, however batteries are rendered a difficult feed due to the high fluorine, Al, Li, and organic content.4 The Li ends up in the slag, and in these processes this slag can have similar composition as spodumene concentrates, which are a major source for Li (besides Li-containing brines) and can be leached accordingly.4 A patent from 2018 claims 90 % Li yield by using a method that enhances the Li yield during slag leaching by depleting the leachate in aluminium.5 According to another recent patent describing a pyrometallurgical processes they achieved >95 % yields for Co, Cu, and Ni.6
Hydrometallurgical recycling methods utilize the high solubilities of Co, Ni, Mn and Li in acid. The batteries must be crushed, often preceded by thermal treatment, and the iron, copper, aluminium fractions must be mechanically sorted and removed. What remains to go to leaching and recovery of the more valuable metals (and graphite) is the black mass (cathode and anode fraction).
A clear distinction between pyro and hydro is not always easy to make, as the pyrometallurgical approach often must be followed by hydrometallurgical steps. A very efficient method seems to be reduction roasting followed by leaching. A recent work involved microwave carbothermic-assisted reduction followed by organic acid leaching to recover valuable metals from spent LiNi1/3Co1/3Mn1/3O2 cathode material.7 The cathode material was first reduced to Ni, Co, Li2CO3, and MnO under microwave carbothermic reduction, and then lithium was recovered from the reduced product by water leaching process with 99.6 % yield. Characterization showed that microwave irradiation leads to disorderly crystal structure and lattice cracks, which is beneficial to facilitate the reactivity of metals. Finally, Ni, Co, Mn enriched residues were dissolved in fumaric acid with leaching efficiencies of more than 97.6 %. Another publication describes the direct thermal treatment of industrial black mass with a temperature–controlled microwave process. Li2CO3 in the reduction products was recovered by carbonated water leaching, and 96.12 % of Lithium was extracted by water leaching and alkaline leaching. Finally, Co could be selectively leached by dilute sulfuric acid.8
Barrios et al. studied the recovery of the cathode material metals from spent lithium-ion batteries by gas-solid chlorination. LiCl could be recovered selectively at 400 °C, whereas all metals (Mn, Co, Ni, Li) were recovered with 100 % extraction yield at 900 °C (yield for each metal increasing with temperature).9 Moreover, González et al. carried out carbochlorination using CaCl2 as chlorinating agent and carbon black as reducing agent to extract, selectively, lithium chloride from black mass samples from spent Lithium-ion batteries.10 Quantitative lithium extraction was achieved at 700 °C for 60 min in the presence of carbon black and Co and Ni were recovered in their metallic state, and as metallic oxide. They could be separated magnetically and LiCl was recovered by water leaching.
As mentioned, spodumene is one of the main primary Li sources. Li extraction from spodumene concentrate can be broadly categorised in five approaches: (1) acid methods; (2) alkali methods; (3) sulphate roasting/autoclaving method; (4) carbonate roasting/autoclaving method; and (5) chlorinating method.11 The desired output material (used as precursors for Li ion batteries) are Li2CO3 and LiOH (emerging), however in chlorination the output material is LiCl, and this can be used as direct feed to molten chloride Li electrolysis process. A challenge with spodumene is that the very stable α-phase that dominates natural spodumene is resistant to the attack of common industrial chemicals. Therefore, it usually must undergo an energy demanding calcination at 1000–1100 °C for up to 2 h to transfer it into the more reactive β-phase. Li et al. concludes that H2SO4 roasting is still the dominant method for Li extraction from spodumene with reported extraction yield up to 96.9 %. However, drawbacks like acid gas emissions and challenging temperature control are mentioned.11
Li has effectively been extracted from spodumene as LiCl by chlorination; either by chlorine gas (gas-solid reaction) or chloride salts.11 Barbosa studied the recovery of Li from β-spodumene by chlorination at temperatures 1000–1100 °C in a fixed bed reactor and obtained a Li recovery yield above 95 %.12,13 The formed LiCl (l) had sufficiently high vapor pressure to be entrained by the gaseous stream whereas the reaction products remaining in the residue were Al6Si2O13 (mullite) and SiO2 (cristobalite).
In another work, Barbosa et al. used CaCl2 to extract Li from spodumene.14 They found that the best yield (90.2 %) was obtained at 900 °C with CaAl2Si2O8 as reaction product in addition to amorphous silica and LiCl. The LiCl was recovered as condensate with 98.6 % purity.
To our knowledge, no reports have been made of molten salts in combination with chlorinating gases to treat the mentioned raw materials, and this concept of molten salt chlorination might represent a feasible alternative to hydrometallurgical processes as well as gas-solid chlorination. To recover Li as molten LiCl directly from the ore allows recovery of Li in metallic form, LiCl being the feedstock for Li commercial scale electrolysis from LiCl-KCl melts at around 450 °C.15 Molten salt chlorination of the raw material/ore directly in the electrolyte used for Li electrowinning could give the possibility for immediate subsequent metal reduction. Li metal batteries possess higher energy density and are seen as the next-generation Li-batteries in “all-solid-state” technologies. This type of batteries uses an electrolyte in solid state, aiming to avoid the uncontrollable lithium dendrite growth during battery cycling, that otherwise exists in Li batteries with liquid electrolytes.
The level of oxoacidity in a molten chloride is given by the ability of the O2− ion (electron pair donor), to exist in the “free” state.16 During molten salt chlorination, an insoluble oxide is transferred into a soluble chloride in the molten salt. To obtain this conversion in a successful way, the pO2− value in the melt, defined as −log aO2−, (analogue to pH in aqueous solutions) must be sufficiently high for the element in question to undergo solubilization. High pO2− is obtained by imposing a chlorinating agent such as for instance HCl or Cl2, exemplified below by the reaction between O2− and HCl:
| \begin{equation} \text{O}^{2 - }(\text{diss}) + 2\text{HCl}\ (\text{g}) = 2\text{Cl}^{ - }(\text{diss}) + \text{H$_{2}$O}\ (\text{g}) \end{equation} | (3) |
However, for different melts or “family of melts” (and temperatures), the same pO2− value does not correspond to the same oxoacidity level as explained by Combes and Picard.17,18 The co logarithm of the equilibrium constant (−log K = pK) for the above equation for different alkali and alkali earth chloride melt systems is compared and is a measure of the oxoacidity of the systems.17 The values indicate the acidity tendency of each cation in the following sequence:
| \begin{equation*} \text{K} = \text{Na} < \text{Li} \leq \text{Ba} < \text{Ca} < \text{Mg}. \end{equation*} |
Cl2 and HCl are the simplest chlorinating agents, Cl2 also being an oxidant. However, neither are capable of solubilizing the most stable metallic oxides such as Al2O3 and SiO2. Stronger chlorinating reagents are achieved by combining the oxidizing character of chlorine to an auxiliary reductant, which is usually carbon:
| \begin{equation} y\text{Cl}_{2}(\text{g}) + \text{M$_{x}$O$_{y}$}(\text{s}) + y/2\text{C}(\text{s}) = x\text{MCl}_{2y/x}\ (\text{l}) + y/2\text{CO}_{2}\ (\text{g}) \end{equation} | (4) |
In LiCl-KCl at 470 °C, carbon and chlorine may settle pO2− values above 20, whereas the Cl2/O2 system and HCl/H2O system obtains pO2− values in the ranges 11 and 13 respectively.16
By utilizing the difference in chlorinating power, melt acidity as well as the difference in vapor pressure for the various metal chlorides, a selective separation of elements from both single and complex oxide materials can be possible. Volatile chlorides such as AlCl3 and FeCl3 will leave the reactor in the gas phase and can be recovered using condenser columns, whereas other metal chlorides (e.g. LiCl) will remain in the molten phase.
Chlorination in molten chloride media sets apart from classical gas-solid phase chlorination in the way that it can take place at lower temperatures (ca. 500–900 °C instead of 900–1300 °C), the solvation of the oxide ion (O2−) and the produced metal cation (Mn+) in the melt favoring the dissolution reaction and gives stable complexes with the anions (Cl−) of the solvent.16 The fast heat transfer in the molten media makes the temperature control easier, giving opportunities for better selectivity.16 One also circumvents the problem of clogging up the fluidized bed that can occur in the classical gas-solid chlorination, and this direct extraction of the wanted metal chloride from the matrix, gives the opportunity for immediate subsequent electrodeposition.
The scope of this work is listed below:
| Reaction number | Reaction | ΔG°, kJ | Temperature, °C |
|---|---|---|---|
| 1 | 2β -LiAlSi2O6 + Cl2 (g) = 1/2 O2 (g) + 2LiCl + 1/3 Al6Si2O13 + 10/3 SiO2 | −90.65 | 470 |
| 2 | 2β -LiAlSi2O6 + Cl2 (g) = 1/2 O2 (g) + 2LiCl + 1/3 Al6Si2O13 + 10/3 SiO2 | −35.85 | 727 |
| 3 | CoO (s) + Cl2 (g) = CoCl2 (l) + 1/2 O2 (g) | −17.5 | 470 |
| 4 | CoO (s) + Cl2 (g) + 1/2 C = CoCl2 (l) + 1/2 CO2 (g) | −214.7 | |
| 5 | Co (s) + Cl2 (g) = CoCl2 (l) | −198.4 | |
| 6 | NiO (s) + Cl2 (g) = NiCl2 (l) + 1/2 O2 (g) | 10.0 | 470 |
| 7 | NiO (s) + Cl2 (g) + 1/2 C (s) = NiCl2 (l) + 1/2 CO2 (g) | −187.7 | |
| 8 | Ni (s) + Cl2 (g) = NiCl2 (l) | −161.0 | |
| 9 | MnO (s) + Cl2 (g) = MnCl2 (l) + 1/2 O2 (g) | −45.1 | 470 |
| 10 | MnO (s) + Cl2 (g) + 1/2 C = MnCl2 + 1/2 CO2 (g) | −242.7 | |
| 11 | Mn (s) + Cl2 (g) = MnCl2 (l) | −375.2 | |
| 12 | CuO (s) + Cl2 (g) = CuCl2 (l) + 1/2 O2 (g) | −23.1 | 470 |
| 13 | CuO (s) + Cl2 (g) + 1/2 C (s) = CuCl2 (l) + 1/2 CO2 (g) | −220.8 | |
| 14 | Cu (s) + Cl2 (g) = 2CuCl (l) | −110.9 | |
| 15 | 2CuO (s) + Cl2 (g) = 2CuCl (l) + O2 (g) | −20.0 | 470 |
| 16 | 2CuO (s) + Cl2 (g) + C (g) = 2CuCl (l) + CO2 (g) | −414.2 |
| Reaction number | Reaction | ΔG°, kJ | Temperature, °C |
|---|---|---|---|
| 1 | 2-β LiAlSi2O6 (s) + 2HCl (g) = 2LiCl (l) + 4SiO2 (s) + Al2O3 (s) + H2O (g) | −19.1 | 470 |
| 2 | Li2O (s) + 2HCl(g) = 2LiCl (l) + H2O (g) | −195.6 | 470 |
| 3 | CoO (s) + 2HCl (g) = CoCl2(l) + H2O (g) | −25.4 | 470 |
| 4 | Co (s) + 2HCl (g) = CoCl2 + H2 (g) | −0.32 | 470 |
| 5 | NiO (s) + 2HCl (g) = NiCl2 (l) + H2O (g) | 1.6 | 470 |
| 6 | Ni (s) + 2HCl (g) = NiCl2 (l) + H2O (g) | 36.9 | 470 |
| 7 | MnO (s) + 2HCl (g) = MnCl2 (l) + H2O (g) | −53.5 | 470 |
| 8 | Mn (s) + 2 HCl (g) = MnCl2 (l) + H2O (g) | −175.6 | 470 |
| 9 | CuO (s) + 2HCl (g) = CuCl2 (l) + H2O (g) | −31.5 | 470 |
| 10 | Cu (s) + HCl (g) = CuCl2 (l) + H2 (g) | 83.6 | 470 |
| Reaction number | Reaction | ΔG°, kJ | Temperature, °C |
|---|---|---|---|
| 1 | 2-β LiAlSi2O6 (s) + CaCl2 (l) = 2LiCl (l) + 2SiO2 (s) + CaAl2Si2O8 (s) | 15.4 | 727 |
| 2 | CoO (s) + LiCl (l) = CoCl2 (l) + Li2O (s) | 170.3 | 470 |
| 3 | CoO (s) + KCl (l) = CoCl2 (l) + K2O (s) | 442.3 | |
| 4 | NiO (s) + LiCl (l) = NiCl2 (l) + Li2O (s) | 197.4 | 470 |
| 5 | NiO (s) + KCl (l) = NiCl2 (l) + K2O (s) | 469 | |
| 6 | MnO (s) + LiCl (l) = MnCl2 (l) + Li2O (s) | 142.4 | 470 |
| 7 | MnO (s) + KCl (l) = MnCl2 (l) + K2O (s) | 414.8 | |
| 8 | CuO (s) + LiCl (l) = CuCl2 (l) + Li2O (s) | 164.2 | 470 |
| 9 | CuO (s) + KCl (l) = CuCl2 (l) + K2O (s) | 436.6 |
The general idea was to use eutectic LiCl-KCl (58 : 42) at 470 °C for all the raw materials, since this is the melt used for Li electrolysis, thus introducing the feed material directly in the event of subsequent metal electrolysis. This system was used for the black mass trials. However, for reasons discussed more in the results section, CaCl2-NaCl-KCl (30 : 35 : 35) at 727 °C was in addition chosen as reaction media for the chlorination of the spodumene concentrate.
Three different raw materials have been treated in two different molten salt systems; spodumene concentrate, and two types of black mass noted BM-1 and BM-2.
2.1 Characterization and pre-treatment of the input materialsThe spodumene concentrate consists mainly of lithium aluminium silicate (α-spodumene), feldspar and quartz with typically 2 % Li. The monoclinic, high density α-spodumene is extremely stable, so the material underwent calcination to convert it to the tetragonal allotrope β-spodumene. Before calcination, to decide on a feasible calcination temperature and make sure the material would not melt, the thermal expansion and melting behaviour was studied by optical dilatometry.
After receiving the two black mass materials, they underwent different approaches with respect to pre-treatment in the laboratory prior to chlorination. BM-1, of unknown battery chemistry and pre-treatment-history from the supplier’s side, was calcined at 550 °C to remove the carbon before chlorination. BM-2 was a Lithium nickel manganese cobalt (NMC) material, and this had undergone pyrolysis and Li removal at the suppliers. To study if carbon could have a positive effect of the chlorination yield, it was desired to chlorinate BM-2 with some carbon present, so this material was not calcined, and had only some of the carbon removed by flotation. Both BM-1 and BM-2 were analysed for carbon content by an IR combustion technique (before and after their respective pre-treatments) and BM-2 was in addition characterized by Electron probe micro-analysis (EPMA). All input materials were analysed by ICP-MS and X-Ray Diffraction method.
2.2 Chlorination set up and procedure.Figure 3 shows the chlorination reactor. The apparatus was a gas tight quartz cell placed in a furnace. The materials involved had to withstand the corrosive atmosphere of HCl and chlorine gas, so neoprene and viton were used as hose and O-ring materials. A type S thermocouple (Pt/Pt-10 % Rh) was used to monitor the temperature continuously, and the gas- in tube was a quartz tube. The gas flow was set by mass flow controllers calibrated for each gas, using a gas flow rate in the range 30–50 mL/min. In case of clogging of the gas system a pressure relief valve was mounted on the inlet to the furnace and the pressure was monitored using a pressure transmitter. The whole experimental set-up was placed in a walk-in fume hood. When not introducing HCl or Cl2, the system was kept under argon purge. Scrubber flasks of 6 M NaOH were placed in the outlet-line, efficiently removing any excess and un-reacted Cl2 or HCl, avoiding emissions to the atmosphere. 90 g salt and 10 g input material were used in all the trials. After inserting the raw material and salt in the furnace, the system was left at 200 °C under argon atmosphere overnight to dry, and then heated to the wanted experimental temperature the next morning. To follow the chlorination yield with time, bath samples for ICP-MS analysis of the target elements were taken before starting the chlorination (using a quartz tube with a frit at the end to avoid contamination from particles of the raw material), and every hour through the experiment. For safety reasons, before opening the cell for sampling, the melt was bubbled with argon for 1 hour to strip off the chlorine/HCl gas. After cooling, the residue of the raw material was collected, washed, and weighed, and the chemical composition analysed with ICP-MS. Table 4 shows an overview of the different experiments carried out.
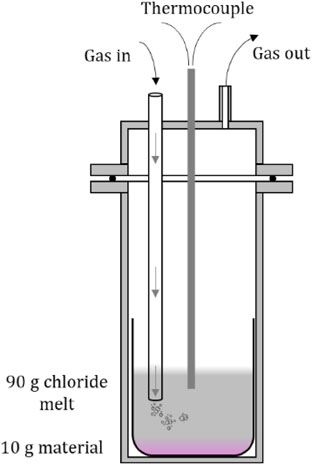
Close up of the chlorination reactor.
| Input material | Pre-treatment | Characterization | Chloride melt | Temperature | Chlorinating gas (Cl2 99.999 %, HCl 99.9995 %) |
|---|---|---|---|---|---|
| Spodumene concentrate | Calcined, 1000 °C | XRD, ICP-MS | LiCl-KCl | 470 °C | HCl |
| Spodumene concentrate | Calcined, 1000 °C | LiCl-KCl | 470 °C | Cl2 | |
| Spodumene concentrate | Calcined, 1000 °C | CaCl2-NaCl-KCl | 727 °C | Cl2 | |
| BM-1 | Calcined, 550 °C | XRD, ICP-MS, Carbon (Leco) | LiCl-KCl | 470 °C | Cl2 |
| BM-1 | Calcined, 550 °C | LiCl-KCl | 470 °C | HCl | |
| BM-2 | Not calcined, Carbon only partly removed by flotation |
XRD, ICP-MS, Carbon (Leco), Electron micro probe analysis | LiCl-KCl | 470 °C | Cl2 |
Figure 4 shows the thermal expansion and melting behaviour spodumene concentrate before calcining, carried out with optical dilatometer. The sample seems to start to soften and expand around 960 °C, and at 1065 °C a maximum in volume is observed. Shrinking starts at 1080 °C and at 1359 °C the sample is completely molten. According to Dessemond et al., decrepitation leads to a lattice expansion of 27 % when heating α-spodumene to a temperature above 870 °C.18 The spodumene mineral density decreases to 2.45 g cm−3 following the decrepitation process, which suggests a more open atomic structure, and thus more reactive. The temperature reported for an efficient decrepitation process typically ranges from 870 °C to 1100 °C. To keep safely away from the shrinking phase, the calcination temperature was chosen to 1000 °C.
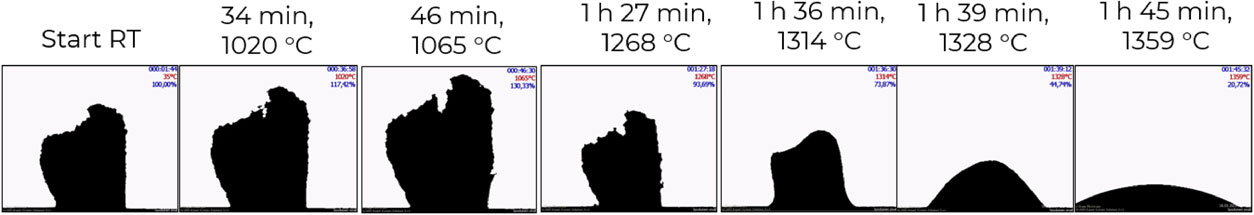
Melting behaviour of spodumene from optical dilatometry.
Figure 5 shows XRD of calcined and uncalcined spodumene. It shows an almost 100 % transition from α to β spodumene after calcination at 1000 °C. However, the reaction pathway of α to β transition may take place via γ spodumene (depending on heating rate and mechanical pre-treatment), and the XRD shows still some unconverted γ phase present, that might have a negative impact on the Li extraction if present in larger amounts.19,20

X-Ray Diffractograms of Spodumene concentrate: Uncalcined compared to calcined for 1 hour and 5.5 hours at 1000 °C.
Black mass is a complex material, its composition depending on battery chemistry and pre-treatment. Figure 6 shows a micrograph as well as elemental mapping of BM-2, and Fig. 7 and Fig. 8 show XRD of BM-1 and BM-2, all as received.
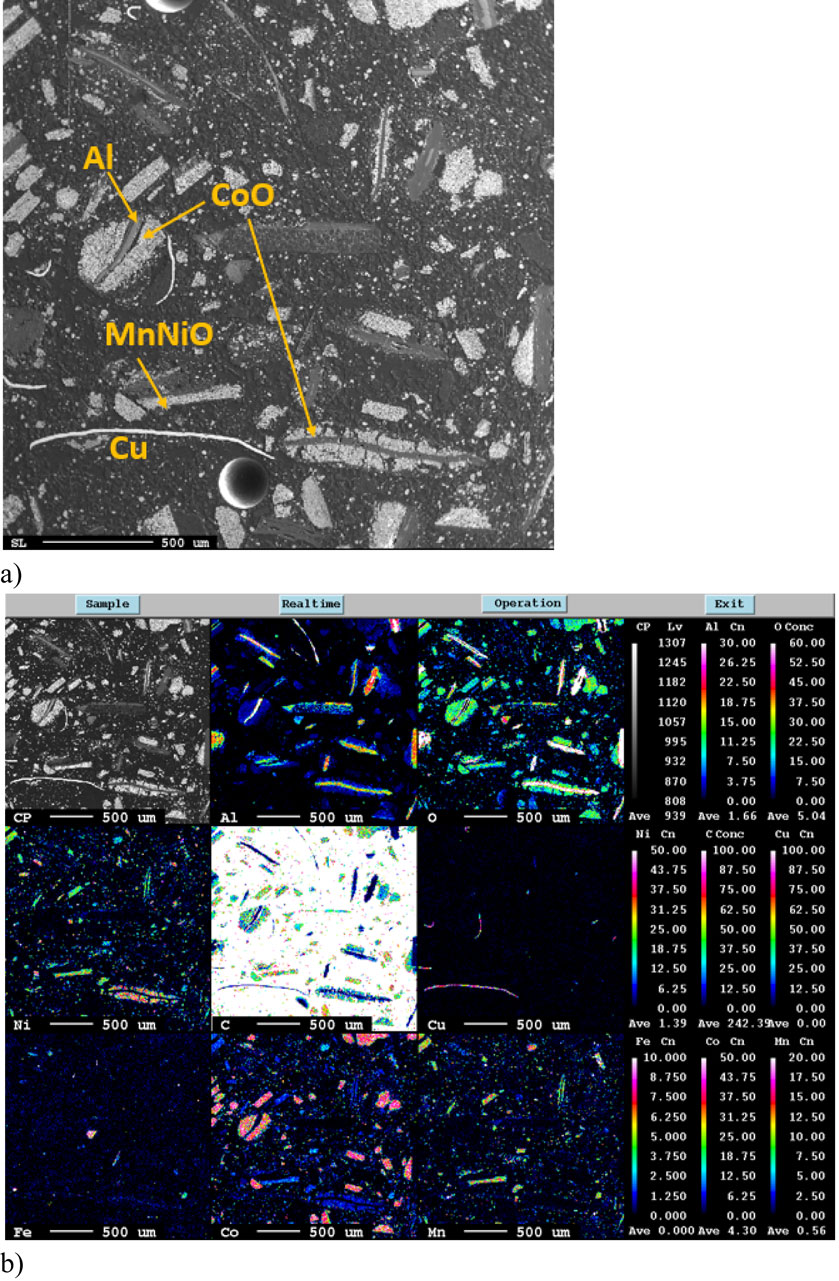
Electron Micro Probe of BM-2 as received, a) SEM image, b) elemental mapping.

XRD of BM-1, as received.
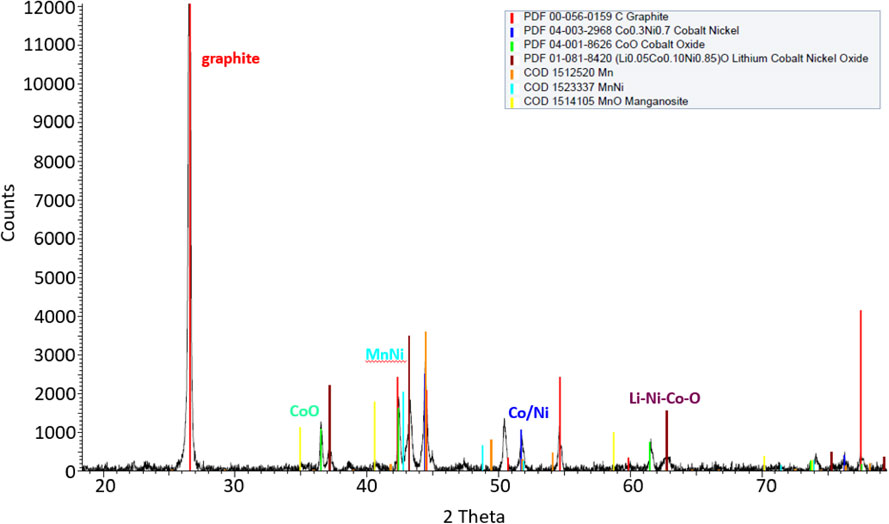
XRD of BM-2, as received.
The original cathode structure of the active materials is retained after shredding and can be recognised in Fig. 6 as characteristic structure of layered Co, Ni and Mn oxides. There is also some cross-contamination from the aluminium and copper fractions (Al and Cu being back contacts for the cathode and anode respectively), seen as characteristic Cu needles.
A lot of graphite is present in the received material, represented by the strong diffraction peaks in the XRD shown in Fig. 7 and Fig. 8, and seen from the IR combustion analysis shown in Table 5 not surprisingly as this is the main constituent of the anode in Li ion batteries. As also highlighted in Table 5, the carbon content in BM-1 when used as input material was reduced to 0.34 % (due to calcination), whereas the C content in the BM-2 was 12.2 % (some C removed by flotation). Another interesting feature for both types of black mass and seen from the XRD (both Fig. 7 and Fig. 8) is that they contain metallic Co and Ni, possibly reduced by the graphite present during some heating during pre-treatment.
| BM-1 | BM-2 | |
|---|---|---|
| C content in raw material as received (wt%) | 19.3 | 44.2 |
| Mass loss during calcination (wt%) | 15.9 | N.A |
| C content after calcination (wt%) | 0.34 | N.A |
| C content after flotation (wt%) | N.A | 12.2 |
Table 6 shows the metal content in the spodumene and black mass used as input materials, analysed by ICP-MS and Table 7 sums up the chlorination yield achieved for the targeted elements for the various input materials and parameters.
| Input material | Al | Co | Cu | Fe | Li | Mn | Ni | P | Si | Ca | K |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spodumene concentrate | 11.7 | 0.67 | 1.99 | 0.07 | 27.6 | 1.4 | 0.35 | ||||
| BM-1 calcined | 5.2 | 19.5 | 15.5 | 32.45 | 2.4 | 4.5 | 8.7 | 1.75 | |||
| BM-2 C partly removed by flotation | 3.4 | 29 | 5.3 | 0.41 | 2.1 | 6.4 | 17.7 | 0.6 | 0.26 |
| Input material | Chloride melt | Temperature | Chlorinating gas | % yield based on ICP-MS analysis | ||||
|---|---|---|---|---|---|---|---|---|
| Li | Co | Cu | Ni | Mn | ||||
| Spodumene concentrate | LiCl-KCl | 470 °C | HCl | 0* | — | — | — | — |
| Spodumene concentrate | LiCl-KCl | 470 °C | Cl2 | 11.39* | — | — | — | — |
| Spodumene concentrate | CaCl2-NaCl-KCl | 727 °C | Cl2 | 100 | — | — | — | — |
| BM-1 calcined (0.4 wt% C) | LiCl-KCl | 470 °C | Cl2 | 17.96* | 2.9 | 36 | 2.5 | 6 |
| *BM-1 calcined (0.4 wt% C) | LiCl-KCl | 470 °C | HCl | 10.0* | 1.7 | 16.1 | 0.2 | 14.6 |
| BM-2, uncalcined, Carbon only partly removed by flotation (12 wt% C) |
LiCl-KCl | 470 °C | Cl2 | 64.1* | 24.1 | 83.5 | 22.4 | 49.4 |
*Based on ICP analysis of the residue and not on bath samples.
The chlorination yield was mainly calculated from the analysed content in melt samples, but for Li in LiCl-KCl melts, the yield is based on the content in the residue material after chlorination. The reason for this was that the base line concentration of Li in these melts were far too high compared to any increase in due to conversion to LiCl from the oxidic Li in the raw material, and thus the change was difficult to detect. However, for spodumene, practical challenges with the quantitative “harvesting” of the residue gave uncertain numbers when calculating the yield this way, so it was decided to chlorinate spodumene concentrate in CaCl2-NaCl-KCl (30 : 35 : 35) instead, a melt with no initial LiCl present. The experiment had to be carried out at 727 °C due to the higher melting point of the mixture. However, this CaCl2-NaCl-KCl melt at 727 °C is more oxo acidic than the LiCl-KCl mixture at 470 °C,18 and they are therefore not directly comparable. For future work, is a better idea to use the BaCl2-CaCl2-NaCl (23.5 : 24.5 : 52) as “verification melt” for LiCl-KCl, since according to Picard, at 600 °C, their oxoacidic properties are more alike (LiCl-KCl being a little more acidic).18 As long as the “vision” is to reduce Li directly from the melt, neither the mentioned CaCl2 nor the BaCl2 systems will be suitable in a potential process since Ba, Ca and Na will likely be reduced before Li. But as mentioned, if Li can be extracted successfully in the BaCl2 system at 600 °C, it is likely that it will take place also in LiCl-KCl.
Figure 9a and Fig. 9b show how the Li concentration and corresponding yield in CaCl2-NaCl-KCl changes with increasing chlorination duration, stabilizing after approximately 3 hours, however it can also be observed that quite a lot of the Li from the spodumene is spontaneously dissolved by the CaCl2-NaCl-KCl melt before the chlorination started. According to the thermodynamic evaluations, (Table 3, Reaction 1) this is not a spontaneous reaction, however it is in line with what was observed by Barbosa et al.14 They observed a yield of 75.5 % after 75 minutes at 700 °C (solid salt), comparable to our results; 75.7 before adding chlorine gas (the chlorination with Cl2 started typically 30–60 minutes after the salt was melted). It might be possible that the Li yield also would have increased with time without the use of chlorine gas, at least to a certain extent.
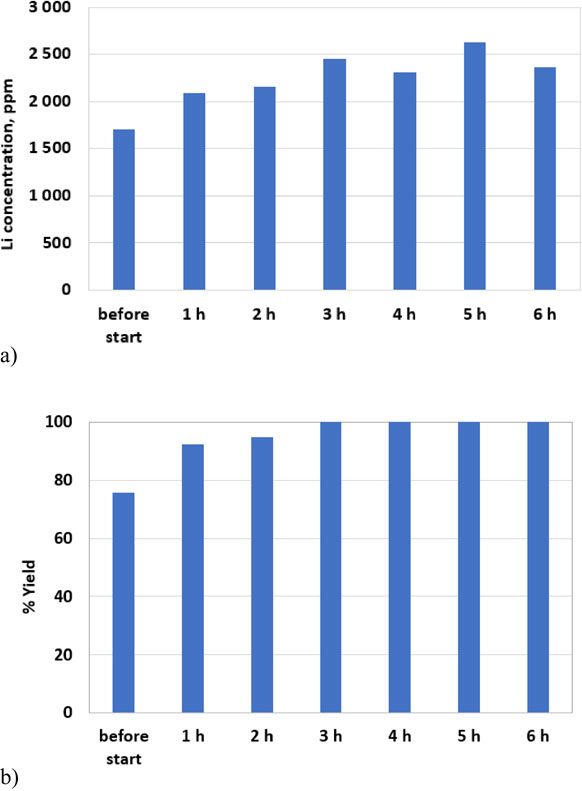
Li concentration (a) and corresponding yield (b) (from ICP-MS analysis of bath samples) in CaCl2-NaCl-KCl as function of chlorination time (Spodumene concentrate, Cl2 gas, 727 °C).
A very high yield for Lithium from spodumene concentrate is obtained with Cl2 (g) in CaCl2-NaCl-KCl at 727 °C (100 %) after 3 hours of chlorination, something that is caused by the higher operating temperature, enhancing the kinetics as well as the stronger oxo acidic nature of this melt (see also discussion above) compared to the LiCl-KCl system. The very high yield for Li from spodumene concentrates in the CaCl2-NaCl-KCl system is very promising, however as discussed above Li metal cannot be directly reduced from this melt. Similar to González et al., LiCl could probably be water leached from the melt after cooling,10 but that would be a whole different concept.
Figure 10a show the concentration and Fig. 10b the respective yields for some of the target elements with time (0–3 hours) during chlorination of BM-2 in LiCl-KCl. Contrary to thermodynamic predictions (Table 3) some Cu and Mn dissolves spontaneously before the chlorination starts. This was also the case for BM-1. In addition to the elements shown small amounts of Al (∼50 ppm), S and Si (∼100 ppm) was found in the molten chloride.

Concentration (a) and corresponding yield (b) of target elements (from ICP-MS analysis of bath samples) in LiCl-KCl with chlorination time (0–3 hrs, BM-2, Cl2 gas, 450 °C).
As seen in Table 7, compared to BM-1, better yields were obtained for BM-2 for all targeted elements. The highest were for Cu (83.5 %) and Li (64.1 %), the metals “ranked” Cu > Li > Mn > Co > Ni for both types of black mass. The higher yields for BM-2 are likely due to the presence of carbon, helping to establish a higher pO2− as discussed in the introduction. Possibly the lack of calcining, avoiding oxidation of the transition oxides and metallic phases might play a role. A draw back with using carbon is of course that CO2 can become a reaction product, but introducing carbon in the system might also lead to the off gas containing more toxic gases like COCl2 and CO.
Except for manganese, HCl gave the lowest chlorination yield for all systems studied.
For the black mass, comparisons with Barrios (gas-solid chlorination of spent Li ion cathode materials) and González (carbochlorination of black mass in CaCl2) are very relevant. The former recovered 40 % Li and none of the other metals at 400 °C.9 With increasing temperature the other elements also became chlorinated in the following sequence: Li > Mn > Ni > Co, Ni being easier than Co as opposed to our work. Their work achieved lower recovery rates for all metals except for Mn at 500 °C, and Co was not obtained at all before surpassing 500 °C, so the molten chloride chlorination and carbon might have some advantages in this lower temperature region. However, the temperature influenced the yield greatly, especially for Co where the yield increased from 12.37 % to 61.3 % to 100 % when going from 600 to 800 to 900 °C. During carbochlorination of black mass in CaCl2, Gonzales recovered Li as LiCl and Co and Ni as metals.10 There was no sign of carbothermic reduction of the transition metal oxides in our case, however in their study carbon seems to have been in larger excess as carbon black was added in addition to the carbon in the black mass, whereas in the current study carbon had partly been removed before the chlorination.
Both for spodumene concentrate and black mass, the obtained concentrations of the target elements (Fig. 9a, Fig. 10a) could perhaps be increased by increasing the weight ratio between the solid input material and molten chloride melt, something that would be an advantage in the case of subsequent electrowinning. Other suggestions for further optimisation would be to study the effect of the chlorine gas flow rate and temperature, and analogous to Barrios selective and better recovery of the various metals from black mass could be tried out by increasing the temperature stepwise. To characterize the black mass and spodumene residues by XRD would also give valuable insight to the reaction mechanisms.
The possibility to recover Li, Ni, Cu and Co from secondary raw material black mass (cathode and anode fraction from shredded end- of-life Li ion batteries), as well as Li from spodumene concentrate by molten salts chlorination has been investigated. The chlorinating agents HCl (g), Cl2 (g) and Cl2 + C (g) have been applied in LiCl-KCl and CaCl2-NaCl-KCl melts. Regarding black mass, the highest chlorination yields were obtained from uncalcined material (Li 64 %, Co and Ni 22–24 %, Cu 83 %, and Mn 49 %) in LiCl-KCl at 470 °C, the carbon in the black mass probably enhancing the chlorination rate. Further optimisation with respect to temperature, gas flow rate, raw material pre-treatment and particle size distribution could improve the chlorination yields.
For spodumene concentrate, a very high yield for Li (100 %) was obtained with Cl2 in CaCl2-NaCl-KCl at 727 °C, the more oxo acidic melt and higher temperature probably helping the chlorination kinetics.
The work concerning the spodumene concentrate has been funded by the EU Framework Horizon Europe, LiCORNE project (GA 101069644). The black mass studies were funded by SINTEF AS.
Karen Osen: Conceptualization (Equal), Data curation (Equal), Funding acquisition (Equal), Investigation (Equal), Methodology (Equal), Writing – original draft (Lead)
Ana Maria Martinez: Conceptualization (Lead), Funding acquisition (Lead), Methodology (Equal)
Anne Støre: Data curation (Lead), Investigation (Equal), Methodology (Equal)
Cathrine K. W. Solem: Data curation (Supporting), Investigation (Equal)
Zhaohui Wang: Data curation (Equal), Investigation (Equal)
Kent-Robert Molvik: Investigation (Equal)
Aksel Roll-Matthiesen: Investigation (Supporting)
Stian Sundby: Investigation (Supporting)
Samuel Senanu: Data curation (Equal)
The authors declare no conflict of interest in the manuscript.
Horizon Europe: GA 101069644
A part of this paper has been presented in 2023 Joint Symposium on Molten Salts (Presentation 3B05).
K. Osen, A. M. Martinez, A. Støre, C. K. W. Solem, Z. Wang, K.-R. Molvik, A. Roll-Matthiesen, S. Sundby, and S. Senanu: Equal Contribution
A. Roll-Matthiesen and S. Sundby: Present address: Norwegian Institute of Technology, Sem Saelands vei 12, 7034-Trondheim, Norway