ABSTRACT
In this study, we investigated the levels of polychlorinated dibenzo-p-dioxin and dibenzofuran (PCDD/PCDF) in soils from Jatropha plantations in three areas that were devastated during the Vietnam War, Ba Vi, Quang Tri, and Trang Bang, and in the Jatropha seeds from Trang Bang. The total toxic equivalent quantity (TEQ) in the soil was 2.1, 4.7, and 4.1 pg-TEQ/g-dry at Ba Vi, Quang Tri, and Trang Bang, respectively. These compounds were mainly of natural origin and were not attributed to “Agent Orange” contamination. The total TEQ in Jatropha seeds from Trang Bang was 1.2 TEQ/g-dry. The negative correlation between the ratios of PCDD/PCDFs in the seeds to those in the soil and the log Kow (octanol–water partition coefficient) were found to have correlation coefficients (r) of −0.96 and −0.82 (P<0.05), respectively. These results suggest that the main pathway for the transfer of dioxin was root uptake and translocation to the seeds. The Jatropha seeds, however, can be safely used to produce biodiesel fuel.
INTRODUCTION
During the Vietnam War (1961–1971), the United States military forces conducted operation “Ranch Hand,” which sprayed over 19.5 million gallons of herbicide onto Vietnam, Laos, and Cambodia to defoliate large forest and crop areas. Two-thirds of the chemical herbicides used were “Agent Orange,” which is a 50:50 mixtures of 2,4,5-trichlorophenoxyacetic acid (2,4,5-T) and 2,4-dichlophenoxyacetic acid (2,4-D). The 2,4,5-T defoliant that was used was contaminated with an extremely toxic substance, 2,3,7,8-tetra-chlorodibenzo-p-dioxin (TeCDD). Owing to their high hydrophobic and stable properties, dioxins are not degraded and are concentrated in human bodies, and can enter the food chain through breast milk (Nhu et al., 2009).
Surveys of areas heavily contaminated with dioxins in Vietnam, the hot spots [i.e., former US air bases, and storage sites such as Bien Hoa (Van Thuong et al., 2015), Da Nang (Hue et al., 2014), A So in A Luoi Valley (Dwernychuk et al., 2002), and Phu Cat] have been conducted, and soil remediation in these areas has begun. However, there have been few reports on the more desolate areas (Kishida et al., 2010) where “Agent Orange” was sprayed that have since been redeveloped for land use such as crop plantations.
Jatropha curcas, an oil-bearing shrub, can grow at high elevations in dry regions, on wastelands, and in hostile environments to preserve land from erosion and desertification (Hayashi, 2012) and it is widely distributed across Asia, America, and Africa. The harvested Jatropha fruits are composed of seeds (63 wt.%) covered with a pericarp (38 wt.%) (Brittaine and Lutaladio, 2010), and seed kernels that contain up to 60% oil (triglycerides). However, the seeds and seed oil cannot be used as nutrients as they are toxic and carcinogenic to humans and animals (Ahmed and Salimon, 2009; Li et al., 2010). To increase biodiesel fuel (BDF) production, the plantation area of Jatropha curcas has been expanded globally to tens of thousands of hectares (ha) in developing countries, including those in West Africa and India. Siang reported that approximately 33 million ha worldwide is used for Jatropha cultivation, which will result in the production of approximately 160 million tons of seed (Siang, 2009).
There has been great interest in the transfer of dioxins from the environment (ambient air and soil) to human beings via plants in the food chain. McLachlan has proposed air–plant pathways by gaseous and particle deposition via leaves and soil–plant pathways by volatilization (adsorption on foliage), adhesion of soil particles to the root, and root uptake translocation to the aerial part (McLachlan, 1997). Müller et al. reported that the major contamination pathway of polychlorinated dibenzo-p-dioxin and dibenzofuran (PCDD/PCDF) for the fruits of apple and pear trees is not by soil, but by air (Müller et al., 1993, 1994). Similarly, Hülster et al. have reported that cucumber plants (Cucumis sativus L.) are mainly contaminated by the deposition of airborne PCDD/PCDFs. In contrast, for zucchini and pumpkin (Cucurbita), root uptake and translocation to fruit were the main contamination pathways (Hülster and Marschner, 1993; Huelster et al., 1994).
This study aims to investigate dioxin contamination in the soil from Jatropha plantation farms 45 years after the end of the Vietnam War and the contamination in the Jatropha seeds, to examine the food cycle chain from the soil to seeds to ensure that Jatropha can be safely used as a BDF. Surveys on the soil were conducted in Ba Vi (Northern Vietnam), Quang Tri (Central Vietnam), and Trang Bang (Southern Vietnam). Jatropha fruits were collected at the plantation farm in Trang Bang, and the results were compared to evaluate the food cycle chain.
MATERIALS AND METHODS
SAMPLING
Soil samples were collected from Ba Vi and Quang Tri Jatropha cultivation areas, and both soil and seed samples were simultaneously collected at Trang Bang Seed Production Center in Vietnam; the sampling locations including coordinates and sampling conditions are summarized in Table 1 and the sampling sites in each plantation area are shown in Fig. 1.
Table 1 Geographical locations, coordinates, and conditions for the sampling sites at Ba Vi, Quang Tri, and Trang Bang, Vietnam
| Jatropha Cultivation area | Ba Vi | Quang Tri (K1 area) | Trang Bang (Seed Production Center) |
|---|
| Location | 48 km northwest from Hanoi City in Northern Vietnam | 86 km northwest from Hue City in Central Vietnam | 100 km west of Saigon City in Southern Vietnam |
| Land area | 2.75 ha | 40 ha | 8 ha |
| Crop acreage | 21,600 m2 (90×240 m) | 20 ha (0.8–3 ha/sampling site) | 59,400 m2 (220×270 m) |
| Sampling date | 2013/11/8 | 2014/11/15 | 2014/11/13 |
| Climate | mist rain | fine | fine |
| Graphical coordination of 10 sampling sites | N21°8’43.1”, E105°21’7.3” | N21°8’42.4”, E105°21’8.3” | N16°43’9.9”, E106°43’39.0” | N16°43’9.4”, E106°43’39.6” | N11°9’9.7”, E106°22’16.4” | N11°9’8.0”, E106°22’14.1” |
| N21°8’44.4”, E105°21’9.1” | N21°8’44.7”, E105°21’7.5” | N16°43’9.8”, E106°43’41.9” | N16°43’9.7”, E106°43’40.7” | N11°9’5.5”, E106°22’11.7” | N11°9’5.5”, E106°22’10.1” |
| N21°8’46.2”, E105°21’7.9” | N21°8’46.1”, E105°21’9.2” | N16°43’10.9”, E106°43’41.5” | N16°43’9.8”, E106°43’41.9” | N11°9’4.9”, E106°22’9.2” | N11°9’1.1”, E106°22’13.8” |
| N21°8’47.4”, E105°21’9.6” | N21°8’48.9”, E105°21’9.7” | N16°43’9.2”, E106°43’43.4” | N16°43’9.2”, E106°43’43.4” | N11°9’2.4”, E106°22’15.6” | N11°9’3.8”, E106°22’17.5” |
| N21°8’49.0”, E105°21’8.5” | N21°8’47.8”, E105°21’8.0” | N16°43’11.0”, E106°43’44.4” | N16°43’10.0”, E106°43’45.3” | N11°9’4.9”, E106°22’18.9” | N11°9’5.9”, E106°22’5.1” |

According to the manual of soil sampling provided by the Ministry of the Environment [MOE], Government of Japan (MOE, Government of Japan, 2009), a soil sample was collected from each of the 10 identified blocks as follows; five portions of soils (>100 g each) were collected, one of which was at the center of the block and the other four at the crossing points at right angles with a 2 m circle radius. Then, the five portions of soil were mixed in a stainless-steel pan. After removing dead grasses, stones, and other contaminants, around 500 g of soil was stored in an aluminum-coated plastic bag. For fruit samples, approximately 1 kg of fruits were collected from the Jatropha trees planted within a 2 m circle radius from the center of the corresponding block and packed in a plastic bag. Sampling was conducted in November of 2014 when Jatropha trees bloomed with small white flowers, and green and/or yellow fruits are harvested throughout the year in the tropical area. These samples were stored in a cooler box and brought to the laboratory at the Center for Environment Monitoring, in Hanoi City.
SAMPLE ANALYSIS
Quantitative analysis of seventeen 2,3,7,8-substituted PCDD/PCDFs was performed using high-resolution gas chromatography coupled with high-resolution mass spectrometry (HRGC/HRMS), according to the 1613 method (US EPA, 1994).
SAMPLE PREPARATION FROM SOIL AND SEEDS
The soil samples were prepared using the following method (MOE, Government of Japan, 2009): soil samples were dried under moderate conditions in the laboratory and then crushed and sieved to a less than 2 mm size. A 50 g sub-sample was then put in a cylindrical filter paper. After the addition of surrogate compounds (40–80 pg) of the seventeen 2,3,7,8-substituted 13C12-labeled PCDD/PCDF isomers, the filter was set in the Soxhlet apparatus, and the extraction was conducted for 16 h using 300 mL of toluene. In each sample preparation step, PCDD/PCDF standard mixtures from Cambridge Isotope Laboratories (CIL Co., USA), which are in accordance with the 1613 method (US EPA, 1994), were used for the quantification of PCDD/PCDFs.
The Jatropha seed samples were prepared using the guidelines provided by the Ministry of Health, Labor, and Welfare [MOHLW], Government of Japan (MOHLW, Government of Japan, 2008) as follows: seed samples were dried under reduced pressure using a freeze-drying apparatus, after removing the pericarp from the fruit, the seeds were homogenized using an electronic mill (Wonder Blender WB-1). A 50 g sub-sample of the seed and surrogate compounds (40–80 pg) of the seventeen 2,3,7,8-substituted 13C12-labeled PCDD/PCDF isomers were put into a 300 mL of Erlenmeyer flask and mixed well. After adding 200 mL of 2 M aqueous sodium hydroxide solution, the mixed solution was left at room temperature overnight. Then, the mixture was moved to a separatory funnel and extracted three times with 100 mL of hexane. The extracts were then combined and concentrated. The obtained extract was cleaned up using two kinds of chromatography after removing the sulfur, if necessary, i.e., an alumina column and then an activated carbon/silica gel column. The final concentrated eluent was injected into the HRGC/HRMS apparatus after the addition of 13C12-labeled 1,2,3,4-TCDD as a syringe spike prior to the analysis.
HRGC/HRMS ANALYSIS
The HRGC/HRMS apparatus used was a Micromass AutoSpec Ultima mass spectrometer (Waters Co. Ltd.) equipped with a 7890A gas chromatograph (Agilent Co. Ltd.). Two types of capillary columns were used as follows; a DB-5MS (60×0.32 mm i.d., 0.25 μm film thickness) capillary column (J & W Scientific, USA) was for determination of 2,3,7,8-TeCDD, 1,2,3,7,8-penta-chlorodibenzo-p-dioxin (PeCDD), 1,2,3,7,8-penta-chlorodibenzofuran (PeCDF), 1,2,3,4,7,8-hexa-chlorodibenzofuran (HxCDF), and 1,2,3,6,7,8-HxCDF from the seventeen 2,3,7,8-substituted PCDD/PCDF isomers. A DB-17 (60×0.32 mm i.d., 0.25 μm film thickness) capillary column (J & W Scientific, USA) was used for the determination of the twelve 2,3,7,8-substituted PCDD/PCDF isomers except for the five isomers previously described.
The analytical conditions of the GC were as follows; for the DB-5MS column, the column temperature was held at 130 °C for 2 min, increased to 200 °C at 30 °C/min, and then to 220 °C at 5 °C/min, held at 220 °C for 16 min, finally increased to 300 °C at 6 °C/min, and then held at 300 °C for 8 min. The sample injection mode was splitless, where the injection temperature was 260 °C and the injection volume 2 μL. For the DB-17 column, the column temperature was held at 130 °C for 2 min, increased to 200 °C at 30 °C/min, and then to 280 °C at 3 °C/min, and finally held at 280 °C for 30 min. Sample injection mode was the same as described for the DB-5MS column.
The analytical conditions for the MS were as follows; operation mode high-resolution EI-SIM (resolution>10,000), ionization voltage 36 eV, ionization current 600 μA, ion source temperature 280 °C.
QUANTIFICATION
Quantification was based on the isotope dilution mass spectrometry according to the 1613 method (US EPA, 1994). The method detection limits (MDLs) of the soil were 0.1–0.5 pg/g-dry for Te–hepta-chlorodibenzo-p-dioxin (HpCDD) and Te–hepta-chlorodibenzofurans (HpCDF) and 1.0 pg/g-dry for octa-chlorodibenzo-p-dioxin (OCDD) and octa-chlorodibenzofurans (OCDF). The MDLs of the fruits were 0.07–0.33 pg/g-dry for Te–HpCDD and Te–HpCDF and 0.67 pg/g-dry for OCDD and OCDF.
The recoveries from the soil were 59%–90% and those from seeds were 58%–76% for the seventeen 13C12-labeled PCDD/PCDF congeners. The values of the recovery satisfied the criteria (50%–120%) of the analytical method for soil (MOE, Government of Japan, 2009) and foods (MOHLW, Government of Japan, 2008).
The WHO Toxic Equivalent Quantity (TEQ) value for each sample was obtained from the concentrations of 2,3,7,8-substituted PCDD/PCDF using the toxic equivalency factors proposed by WHO in 2006 (Van den Berg et al., 2006).
STATISTICAL ANALYSIS
The data were presented as mean±SD, based on the analysis of samples at the location, and tested using one-way analysis of variance and Microsoft Office 2010 to determine the significance of the differences between the groups (Assaad et al., 2015). A difference was considered statistically significant if P<0.05.
RESULTS AND DISCUSSION
CONCENTRATIONS OF DIOXINS IN THE SOIL
The average concentrations of PCDD/PCDF and total TEQ in the soil samples from the Ba Vi, Quang Tri, and Trang Bang plantation areas are shown in Table 2. The profiles (TEQ base) of PCDD/PCDF in the soil from the three areas are shown in Fig. 2. The total TEQs in the soil from Ba Vi, Quang Tri, and Trang Bang were 2.1 pg-TEQ/g-dry, 4.7 pg-TEQ/g-dry, and 4.1 pg-TEQ/g-dry, respectively. The dominant homologues were OCDD, HpCDDs, and HxCDDs in the order of OCDD>HpCDDs>HxCDDs. The abundance of OCDD was in the range of 2,300–4,400 pg/g-dry and that of HpCDD was 81–110 pg/g-dry. The TEQ profiles of these three sampling sites were similar, although the PCDD/PCDFs for Ba Vi were low or undetectable. The obtained results were similar to those in the sediment from Can Gio and Hue, from which the total TEQs were 2.7 pg-TEQ/g-dry and 2.9 pg-TEQ/g-dry and the mean of OCDD was 200 pg/g-dry and 680 pg/g-dry, respectively. The total TEQ in the sediment from the urban area of Hanoi was 9.6 pg-TEQ/g-dry, and the mean for the OCDD was 390 pg/g-dry (Kishida et al., 2010). The total TEQ level from Hanoi was higher in comparison to those from the three plantation areas.
Table 2 Average concentrations of PCDD/PCDF, total TEQ, and percentages of 2,3,7,8-TCDD/total TEQ in the soil and seeds from Ba Vi, Quang Tri, and Trang Bang, Vietnam
| Compounds | Ba Vi Soil (n=10) | Quang Tri Soil (n=10) | Trang Bang |
|---|
| Soil (n=10) | Seeds (n=10) |
|---|
| 2,3,7,8-TCDD | 0.13±0.08 | 0.49±0.37 | 0.25±0.16 | 0.17±0.09 |
| 1,2,3,7,8-PeCDD | tr | 1.1±0.48 | 0.64±0.40 | 0.26±0.22 |
| 1,2,3,4,7,8-HxCDD | 0.53±0.25 | 2.4±0.89 | 2.3±1.4 | 0.43±0.30 |
| 1,2,3,6,7,8-HxCDD | 0.72±0.48 | 3.9±1.4 | 3.7±2.3 | 0.85±0.72 |
| 1,2,3,7,8,9-HxCDD | 0.59±0.35 | 3.3±1.2 | 4.8±2.9 | 0.24±0.17 |
| 1,2,3,4,6,7,8-HpCDD | − | 81±170 | 110±190 | 3.6±2.5 |
| OCDD | 4,500±1,600 | 3,300±1,200 | 2,300±1,400 | 3.6±3.1 |
| 2,3,7,8-TCDF | 0.12±0.11 | 0.12±0.06 | 0.13±0.08 | 0.40±0.28 |
| 1,2,3,7,8-PeCDF | tr | tr | 0.39±0.24 | 0.76±0.50 |
| 2,3,4,7,8-PeCDF | tr | 0.28±0.09 | 0.40±0.26 | 0.88±0.90 |
| 1,2,3,4,7,8-HxCDF | 0.28±0.09 | 1.7±0.63 | 0.64±0.40 | 0.77±0.73 |
| 1,2,3,6,7,8-HxCDF | tr | 1.1±0.41 | 0.33±0.18 | 0.71±0.66 |
| 1,2,3,7,8,9-HxCDF | 0.35±0.31 | 0.25±0.00 | 0.33±0.17 | 0.64±0.63 |
| 2,3,4,6,7,8-HxCDF | tr | 0.25±0.00 | 0.25±0.00 | 0.23±0.13 |
| 1,2,3,4,6,7,8-HpCDF | 0.28±0.09 | 2.1±0.77 | 1.4±0.89 | 3.8±2.8 |
| 1,2,3,4,7,8,9-HpCDF | 0.33±0.24 | 0.29±0.12 | tr | 0.43±0.33 |
| OCDF | 0.68±0.64 | 0.50±0.00 | 0.59±0.28 | 1.5±1.4 |
| Total TEQ*1 | 2.1±0.45 | 4.7±2.8 | 4.1±3.4 | 1.2±0.77 |
| TCDD (%)*2 | 6.7±4.5 | 9.1±6.0 | 6.6±2.0 | 15±5.9 |
Values are expressed as mean±standard deviation (SD) (pg/g-dry). For statistical evaluation, values that are less than the MDL were estimated as half of the MDL.
tr; <MDL. −; missing value; the values were too low to be evaluated.
*1 Toxic equivalent quantity (TEQ): pg-TEQ/g-dry, WHO-2006TEF.
*2 Percentages of 2,3,7,8-TCDD to total TEQ.
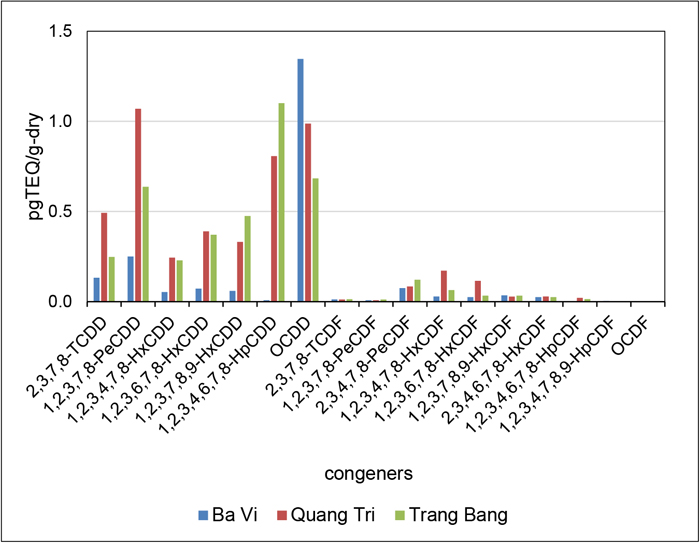
To consider Agent Orange, the average percentages of 2,3,7,8-TCDD in the total TEQ are presented in Table 2. The values in the soil from these three areas were in the range of 6.6–9.1%, which is very low in comparison to those from A So, Ta Bat, and A Luoi with which were 99%, 86%, and 92%, respectively, and were located near the hot spots of the former US air bases (Dwernychuk et al., 2002), and also to that from Can Gio with the value of 30%, and was located 50 km from the former US marine base (Kishida et al., 2010). These results indicated that the PCDD/PCDFs at the sites in this investigation were not a result of Agent Orange contamination.
The strong predominance of OCDD in the PCDD/PCDF profiles is considered to have a natural origin (Gaus et al., 2001; Gadomski et al., 2004) or to originate from contaminants, i.e., combustion products of pentachlorophenol (PCP) (Baker and Hites, 2000; Masunaga et al., 2001). PCP was once one of the most widely used biocides in the United States, but it has not been used for rice cultivation in Vietnam. Products from the de novo synthesis of PCP have a strong predominance of OCDD and HpCDDs (OCDD>HpCDDs) and a low content of other PCDD/PCDF homologues. However, the PCDD/PCDF patterns in Ba Vi, Quang Tri, and Trang Bang disagreed with those attributed to PCP synthesis. Accordingly, the high occurrence of OCDD in the Jatropha plantation area is not attributed to PCP.
In terms of naturally occurring OCDD, Gaus et al. have reported that PCDD/PCDF profiles that are characterized as the predominance of OCDD, low or undetectable PCDFs, and specific 2,3,7,8-substituted HxCDD distributions (Gaus et al., 2001). Table 2 shows the PCDD/PCDF concentrations in Ba Vi, Quang Tri, and Trang Bang. The levels of OCDD were the highest, and those of PCDF were low or undetectable. In terms of 2,3,7,8-substituted HxCDD distributions, the two ratios of 1,2,3,7,8,9-HxCDD and 1,2,3,6,7,8-HxCDD to the total 2,3,7,8-substituted HxCDDs from natural origin were 53% and 30%, but the two ratios in the sediment sample corresponding to the year of 1995 were 38% and 35% (Gaus et al., 2001). The two ratios obtained from the three plantation areas were in the range of 32%–44% and 35%–40%, respectively. These values were similar to those in the sediment of the 1995 year described previously. The results suggested that contamination and/or alternation of PCDDs from a natural origin may have occurred. Therefore, PCDD/PCDFs in the soil samples from Ba Vi, Quang Tri, and Trang Bang were mainly attributed to a natural origin.
DIOXINS IN JATROPHA SEEDS
The results of the average concentrations of PCDD/PCDF and total TEQ in the Jatropha seeds from Tang Bang are shown in Table 2. The total TEQ in the seeds was 1.2 pg-TEQ/g-dry, and the value was lower by approximately 1/4-fold than that in the soil. HpCDD and OCDD had very low levels compared to those of the soil, in which the concentrations were 3.6 pg/g-dry and 3.6 pg/g-dry, respectively.
According to the survey results on dioxins regarding the agriculture field and crops in Japan reported by the MOE, Government of Japan and Ministry of Agriculture, Forestry, and Fisheries [MOAFF], Government of Japan (MOE, Government of Japan and MOAFF, Government of Japan, 2002), the average TEQ values (PCDD/PCDF and co-planar polychlorinated biphenyl (Co-PCB)) of the edible parts of 30 types of crops (rice, fruits, and vegetables) were 0.010 pg-TEQ/g-wet in the range of 0–0.19 pg-TEQ/g-wet, whereas the values in the cultivated soil were 21 pg-TEQ/g-dry in the range of 0.0017–200 pg-TEQ/g-dry. The PCDD/PCDF levels in the crops were lower by 1/10–1/100-fold than that in Jatropha seeds.
The average TCDD/TCDFs in pine needles collected from eleven urban areas in Japan were 7.1 pg/g-wet in the range of 0.6–17 pg/g-wet (Aozasa et al., 1996), which are contaminated mainly by air–plant transfer, i.e., deposition, absorption, and adhesion (Welsch-Pausch et al., 1995; Uegaki et al., 2004). In whole fruits of apples and pears the average TCDD/TCDFs were in the range of 1–4 ng/kg FW, and the higher range in the peel in comparison to the pulp indicated that airborne PCDD/PCDFs are the major pathway for fruit contamination (Müller et al., 1993). These TCDD/TCDF levels were comparable to those of the Jatropha seeds examined.
DIOXIN COMPOSITION IN JATROPHA SEEDS
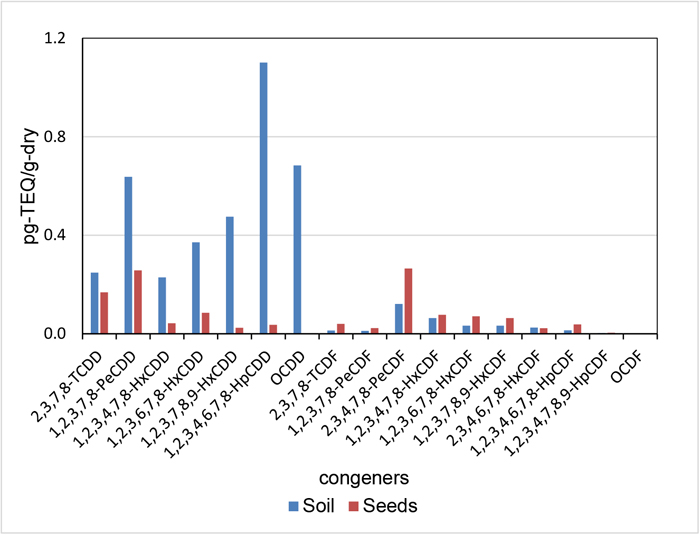
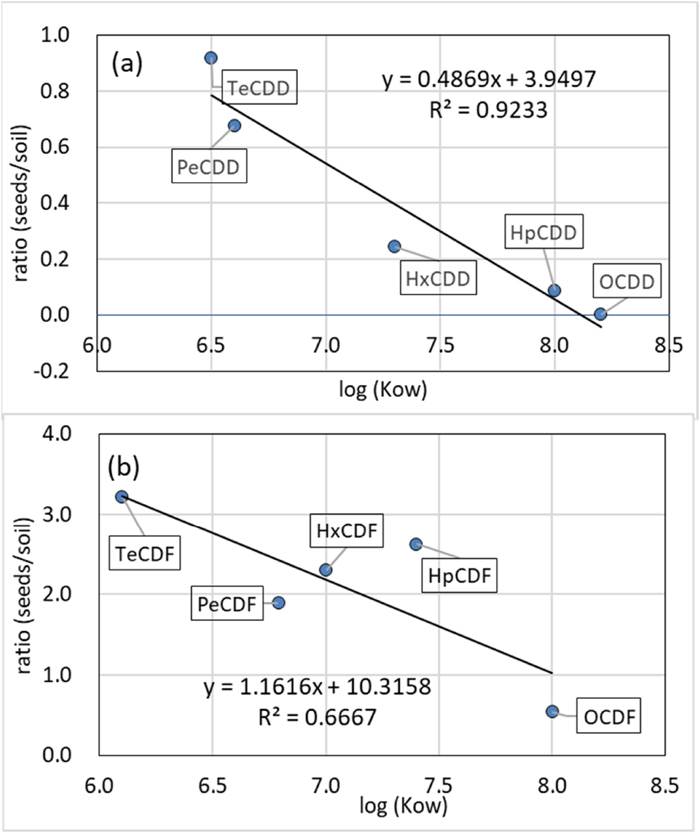
The profiles (TEQ base) of PCDD/PCDFs in the Jatropha seeds and soil are shown in Fig. 3. For the PCDD congeners, the highest ratio of seeds/soil was 0.92 for 2,3,7,8-TCDD and this decreased to 2×10−3 for OCDD. The negative correlation between the ratios of PCDD and Te−OCDD congeners with a correlation coefficient (r) of –0.97 (P<0.05) was observed as shown in Fig 4-(a). For the PCDF congeners, the highest ratio for seeds/soil was 3.2 for 2,3,7,8-TCDF, and it was 0.54 for OCDF. The negative relationship between the ratios of PCDF and Te−OCDF congeners with an r of –0.73 was observed as shown in Fig. 4-(b). These results indicate that the concentration of PCDD/PCDF congeners in seeds increased with a decrease in the number of substituted chlorine of congeners despite their lipophilic properties.
TRANSFER OF DIOXINS FROM THE SOIL TO JATROPHA SEEDS
Huelster et al. (1994) reported that for zucchini and pumpkin (Cucurbita), the root uptake of PCDD/PCDFs and subsequent translocation to the shoots and into the fruits were the main contamination pathways. However, cucumber plants (C. sativus L.) were mainly contaminated by the deposition of airborne PCDD/PCDFs.
Zhang et al. (2017) have reviewed the studies on the uptake of organic pollutants (e.g., PCDD/PCDFs, PCB, and herbicides) by plants from the air and roots. The important factors for root uptake and translocation to plants are the octanol–water partition coefficient (Kow) (Dioxin Control Office MOE, 2004) and root extractable lipid content.
The correlations between the ratios of the PCDD/PCDF concentrations in seeds to those in soil and the log Kow for Te–OCDD/OCDF congeners are shown in Fig. 5. The correlation between the ratios of the seeds/soil and log Kow of the congeners were observed for PCDD with an r of –0.96 (P<0.05) and that for PCDF with an r of –0.82 (P<0.05). These results indicate that the concentrations of PCDD/PCDF in seeds were correlated with those in the aqueous phase in soil. These phenomena can be explained as follows; the PCDD/PCDFs participated in water phase i.e. interstitial water were translocated from the root by the transpiration stream (Higashio et al., 2011), and accumulated in fruits and seeds that contained more than 60% lipids (triglycerides). The behavior of PCDD/PCDFs was very similar to that in zucchini (Cucurbita pepo) (Inui et al., 2008) and in agricultural crops (Zhang et al., 2009). Accordingly, it was supposed that the main pathway of PCDD/PCDFs to Jatropha seeds was not air uptake, but root uptake, and they were translocated and accumulated in seeds.
CONCLUSION
The PCDD/PCDF concentrations in soil were investigated at three Jatropha plantation areas, Ba Vi, Quang Tri, and Trang Bang and simultaneously those in Jatropha seeds from Trang Bang. The total TEQs were in the range of 2.1–4.7 pg-TEQ/g-dry. The values were low compared to the urban area of Hanoi, and may be of natural origin. At Trang Bang, the average total TEQs observed in the Jatropha seeds was 1.2 pg-TEQ/g-dry. From the physicochemical study, it was suggested that the main pathway of PCDD/PCDFs to Jatropha seeds was the root uptake, followed by translocation and accumulation in seeds. The level of PCDD/PCDFs in the seeds might be safe to produce BDF which is available on the market.
ACKNOWLEDGMENTS
The authors acknowledge financial support from the Science and Technology Research Partnership for Sustainable Development (SATREPS, Project: Multi-beneficial Measure for the Mitigation of Climate Change by the Integrated Utilization of Biomass Energy in Vietnam and Indochina countries), JST-JICA.
CONFLICT OF INTEREST
The authors have none of any financial interests or personal relationships that could have influenced the work in this paper.
REFERENCES
- Ahmed, W.A., Salimon, J., 2009. Phorbol ester as toxic constituents of tropical Jatropha curcas seed oil. Eur. J. Sci. Res. 31(3), 429–436.
- Aozasa, O., Ikeda, M., Nakao, T., Ohta, S., Miyata, H., Huang, C., Tsai, H., 1996. Air pollution by PCDDs, PCDFs and non-ortho coplanar PCBs in Japan using pine needle as a biomonitoring indicator. Organohalogen Compd. 28, 3–8.
- Assaad, H.I., Hou, Y., Zhou, L., Carroll, R.J., Wu, G., 2015. Rapid publication-ready MS-Word tables for two-way ANOVA. Springerplus 4, 33. doi: 10.1186/s40064-015-0795-z.
- Baker, J.I., Hites, R.A., 2000. Siskiwit lake revisited: Time trends of polychlorinated dibenzo-p-dioxin and dibenzofuran deposition at Isle Royale. Michigan Environ. Sci. Technol. 34(14), 2887–2891. doi: 10.1021/es991280p.
- Brittaine, R., Lutaladio, N., 2010. Jatropha: A Smallholder Bioenergy Crop the Potential for Pro-Poor Development. Food and Agriculture Organization of The United Nations, Rome.
- Dioxin Control Office Ministry of the Environment, 2004. Handbook of Model for Dioxin Behavior. https://warp.da.ndl.go.jp/info:ndljp/pid/11546002/www.env.go.jp/chemi/dioxin/hand/handbook.pdf.
- Dwernychuk, L.W., Cau, H.D., Hatfield, C.T., Boivin, T.G., Hung, T.M., Dung, P.T., Thai, N.D., 2002. Dioxin reservoirs in southern Viet Nam-A legacy of agent orange. Chemosphere 47(2), 117–137. doi: 10.1016/S0045-6535(01)00300-9.
- Gadomski, D., Tysklind, M., Irvine, R.L., Burns, P.C., Andersson, R., 2004. Investigations into the vertical distribution of PCDDs and mineralogy in three ball clay cores from the United States exhibiting the natural formation pattern. Environ. Sci. Technol. 38(19), 4956–4963. doi: 10.1021/es049579h.
- Gaus, C., Brunskill, G.J., Weber, R., Päpke, O., Müller, J.F., 2001. Historical PCDD inputs and their source implications from dated sediment cores in Queensland (Australia). Environ. Sci. Technol. 35(23), 4597–4603. doi: 10.1021/es011086e.
- Hayashi, K., 2012. Global trend of Jatropha Research and its use: potential of Jatropha plant for the development in sub Saharan Africa, Chapter 1, pp. 13–18, Japan International Research Center for Agricultural Sciences, Tsukuba.
- Higashio, H., Ippoushi, K., Ito, H., Azuma, K., 2011. Absorption and transfer of dioxins from soil in several vegetables. Hort. Res. 10(4), 467–473.
- Hue, N., Nam, V., Thuong, N., Huyen, N., Phuong, N., Hung, N., Tuan, N., Son, L., Minh, N., 2014. Determination of PCDD/Fs in breast milk of women living in the vicinities of Da Nang Agent Orange hot spot (Vietnam) and estimation of the infant’s daily intake. Sci. Total Environ. 491–492, 212–218. doi: 10.1016/j.scitotenv.2014.02.054.
- Huelster, A., Mueller, J.F., Marschner, H., 1994. Soil-plant transfer of polychlorinated dibenzo-p-dioxins and dibenzofurans to vegetables of the cucumber family (Cucurbitaceae). Environ. Sci. Technol. 28(6), 1110–1115. doi: 10.1021/es00055a021.
- Hülster, A., Marschner, H., 1993. Transfer of PCDD/PCDF from contaminated soils to food and fodder crop plants. Chemosphere 27(1–3), 439–446. doi: 10.1016/0045-6535(93)90324-X.
- Inui, H., Wakai, T., Gion, K., Kim, Y.S., Eun, H., 2008. Differential uptake for dioxin-like compounds by zucchini subspecies. Chemosphere 73(10), 1602–1607. doi: 10.1016/j.chemosphere.2008.08.013.
- Kishida, M., Imamura, K., Takenaka, N., Maeda, Y., Viet, P.H., Kondo, A., Bandow, H., 2010. Characteristics of the abundance of polychlorinated dibenzo-p-dioxin and dibenzofurans, and dioxin-like polychlorinated biphenyls in sediment samples from selected Asian regions in Can Gio, Southern Vietnam and Osaka, Japan. Chemosphere 78(2), 127–133.
- Li, C.Y., Devappa, R.K., Liu, J.X., Lv, J.M., Makkar, H.P.S., Becker, K., 2010. Toxicity of Jatropha curcas phorbol esters in mice. Food Chem. Toxicol. 48(2), 620–625. doi: 10.1016/j.fct.2009.11.042.
- Masunaga, S., Yao, Y., Ogura, I., Nakai, S., Kanai, Y., Yamamuro, M., Nakanishi, J., 2001. Identifying sources and mass balance of dioxin pollution in Lake Shinji Basin, Japan. Environ. Sci. Technol. 35(10), 1967–1973. doi: 10.1021/es001729a.
- McLachlan, M.S., 1997. A simple model to predict accumulation of PCDD/Fs in an agricultural food chain. Chemosphere 34(5–7), 1263–1276. doi: 10.1016/S0045-6535(97)00424-4.
- Ministry of the Environment, Government of Japan and Ministry of Agriculture Foresty and Fishery, Government of Japan, 2002. Survey results of dioxins related to agricultural soil and crops in 2002. http://www.env.go.jp/water/dojo/no-diox/14no-dio.pdf. (in Japanese)
- Ministry of Health, Laber, and Walfare, Government of Japan, 2008. Method for Measuring Dioxins in Foods—Interim Guidelines—. http://www.nihs.go.jp/mhlw/shokuhin/dioxin-gl.pdf. (in Japanese)
- Ministry of the Environment, Government of Japan, 2009. Soil survey measurement manual for dioxins. http://www.env.go.jp/air/air/tech/dojomanualh2103.pdf. (in Japanese)
- Müller, J.F., Hülster, A., Päpke, O., Ball, M., Marschner, H., 1993. Transfer pathways of PCDD/PCDF to fruits. Chemosphere 27(1–3), 195–201. doi: 10.1016/0045-6535(93)90293-E.
- Müller, J.F., Hülster, A., Päpke, O., Ball, M., Marschner, H., 1994. Transfer of PCDD/PCDF from contaminated soils into carrots, lettuce and peas. Chemosphere 29(9–11), 2175–2181. doi: 10.1016/0045-6535(94)90384-0.
- Nhu, D.D., Kido, T., Naganuma, R., Sawano, N., Tawara, K., Nishijo, M., Nakagawa, H., Hung, N.N., Thom, L.T.H., 2009. A GIS study of dioxin contamination in a Vietnamese region sprayed with herbicide. Environ. Health Prev. Med. 14(6), 353–360. doi: 10.1007/s12199-009-0107-8.
- Siang, C.C., 2009. Jatropha curcas L.: Development of a new oil crop for biofuel. Tokyo, Ieej.
- Uegaki, R., Seike, N., Eun, H., Ueji, M., 2004. Polychlorinated dibenzo-p-dioxins, dibenzofurans, and coplanar polychlorinated biphenyls (dioxins) in silage corn: the influence of contaminated soil and air. Grassl. Sci. 50, 132–138.
- US EPA, 1994. Method 1613: tetra-through octa-chlorinated dioxins and furans by isotope dilution HRGC/HRMS. United States Environmental Protection Agency, Washington.
- Van den Berg, M., Birnbaum, L.S., Denison, M., De Vito, M., Farland, W., Feeley, M., Fiedler, H., Hakansson, H., Hanberg, A., Haws, L., Rose, M., Safe, S., Schrenk, D., Tohyama, C., Tritscher, A., Tuomisto, J., Tysklind, M., Walker, N., Peterson, R.E., 2006. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 93(2), 223–241. doi: 10.1093/toxsci/kfl055.
- Van Thuong, N., Hung, N.X., Mo, N.T., Thang, N.M., Huy, P.Q., Van Binh, H., Nam, V.D., Van Thuy, N., Son, L.K., Minh, N.H., 2015. Transport and bioaccumulation of polychlorinated dibenzo-p-dioxins and dibenzofurans at the Bien Hoa Agent Orange hotspot in Vietnam. Environ. Sci. Pollut. Res. 22(19), 14431–14441. doi: 10.1007/s11356-014-3946-9.
- Welsch-Pausch, K., McLachlan, M.S., Umlauf, G., 1995. Determination of the principal pathways of polychlorinated dibenzo-p-dioxins and dibenzofurans to lolium multiflorum (Welsh Ray Grass). Environ. Sci. Technol. 29(4), 1090–1098. doi: 10.1021/es00004a031.
- Zhang, C., Feng, Y., Liu, Y., Chang, H., Li, Z., Xue, J., 2017. Uptake and translocation of organic pollutants in plants: A review. J. Integr. Agric. 16(8), 1659–1668. doi: 10.1016/S2095-3119(16)61590-3.
- Zhang, H., Chen, J., Ni, Y., Zhang, Q., Zhao, L., 2009. Uptake by roots and translocation to shoots of polychlorinated dibenzo-p-dioxins and dibenzofurans in typical crop plants. Chemosphere 76(6), 740–746. doi: 10.1016/j.chemosphere.2009.05.030.
 https://orcid.org/0000-0003-4163-116X
https://orcid.org/0000-0003-4163-116X