2022 年 2 巻 p. 60-66
2022 年 2 巻 p. 60-66
In Japan, the amount of freshwater and short-neck clams imported from countries, such as China, South Korea, and North Korea, has increased with the decrease in their domestic production. This calls attention to the contamination of marketed clams with toxic substances, such as heavy metals in aquatic environments, from the viewpoint of food safety and security, because their origin has been falsely indicated. Herein, first, we measured the concentrations of Cd, Cu, Pb, and Zn in marketed freshwater and short-neck clam samples to clarify the extent of their contamination. Consequently, we found that some of the freshwater clam samples were contaminated with both Cd and Pb at concentrations comparable to their maximum concentrations in bivalve mollusks imposed by the Commission of the European Communities. Further, we evaluated the sources of Pb in the contaminated clams on the basis of Pb isotope ratios. The result supported the hypothesis that the Pb in the contaminated freshwater clam samples originates predominantly from effluent and exhaust from mining or smelting, which is associated with old Pb ores formed in some countries, such as China and North Korea. On the contrary, it appears that the contamination of freshwater clam samples with Cd is attributable to the Cd contained as an impurity in effluent and exhaust from the mining or smelting.

All aquatic invertebrates accumulate trace metals in their tissues, regardless of whether or not these metals are essential to metabolism (Rainbow, 2002). In particular, as bivalve species such as oysters and mussels are sessile organisms, their tissue metal concentrations are expected to reflect the metal concentrations at their sites of collection. Hence, bivalves have been used to monitor the spatial and temporal trends of metal concentrations in coastal waters, as represented by the Mussel Watch Program, proposed by Goldberg as a worldwide monitoring program for the marine environment (Goldberg, 1975). However, metal concentration levels in organisms are not only the result of their bioavailability in the environment (Cossa, 1989). Environmental factors (e.g., temperature, salinity) and biotic factors (e.g., age, physiological conditions) are acting. Moreover, using only elemental concentrations for monitoring metal levels (Shiel et al., 2012) has certain limitations, such as a difficulty in determining whether bivalves are contaminated with metals in the case of relatively low metal concentrations. Additionally, it is impossible to distinguish between anthropogenic and natural sources of metals in bivalves on the basis of tissue metal concentrations alone. Conversely, a method using isotopes as tracers of metal sources in bivalves may be effective. Shiel et al. (2012, 2013) and Araújo et al. (2021) demonstrated that Cd, Zn, and Pb isotopes are successfully used to trace their sources in bivalves collected from the coasts of western Canada, the USA, and France. Among these isotopes, Pb isotopes have been widely used as a well-known “fingerprinting” tool to evaluate the sources of Pb in various environmental samples, including aerosols, water, sediment, and biota, because they are not fractionated in nature (Shiel et al., 2012, 2013).
In recent years, there have been increased concerns about human health with respect to the intake of toxic trace metals via food consumption. Acute and chronic toxic effects of trace metals on human health include gastrointestinal and kidney dysfunctions, nervous system disorders, skin lesions, vascular damage, immune system dysfunction, birth defects, and cancer (Balali-Mood et al., 2021). To ensure the safety of edible bivalves, the Codex Alimentarius Commission (CODEX STAN 193–1995) and the Commission of the European Communities (EC No. 466/2001) have imposed the maximum concentrations of some toxic trace metals such as Cd and Pb. For example, all oysters from sites within the Gironde estuary on the Atlantic coast of France were revealed to have Cd concentrations significantly above the 1.0 μg/g wet weight, the maximum Cd concentration imposed by the Commission of the European Communities, which led to the prohibition of the production, selling, and consumption of oysters from the Gironde estuary (Audry et al., 2004).
Among bivalves marketed in Japan, freshwater and short-neck clams have been consumed in large quantities. In recent years, the amount of these clams imported from countries such as China, South Korea, and North Korea has increased with the decrease in their domestic production (Komaru et al., 2010). This calls attention to the contamination of marketed clams with toxic trace substances such as heavy metals in aquatic environments from the viewpoint of food safety and security, because their origin has been falsely indicated (Komaru et al., 2010). Herein, first, we measured the concentrations of Cd, Cu, Pb, and Zn in marketed freshwater and short-neck clams to clarify the extent of their contamination. The results showed that some of the freshwater clam samples are contaminated with both Cd and Pb at moderately high concentrations. Furthermore, we evaluated the sources of these metals in the contaminated clams on the basis of Pb isotope ratios.
Samples of freshwater (19 samples) and short-neck clams (7 samples) that were marketed in Japan were collected, purchasing them at supermarkets and fish stores in Shizuoka City (Shizuoka Prefecture), Sagamihara City (Kanagawa Prefecture), and Tokyo in 2017. Fig. 1 shows the places of their production in Japan, which were written on the labels of their packages. The numbers in parentheses after the places of production represent the numbers of samples from the same places of production, which were collected separately at different supermarkets and fish stores. The typical species of freshwater clams are Corbicula japonica, Corbicula leana, and Corbicula sandai, whereas a typical species of short-neck clam is Ruditapes philippinarum. However, we did not conduct species identification using DNA base sequence analysis (Komaru et al., 2010).

Places of production of freshwater and short-neck clam samples in Japan. The numbers in parentheses after the places of production represent the numbers of samples from the same places of production, which were collected separately at different supermarkets and fish stores
The clam samples were shucked using a stainless steel spatula. The total soft tissues separated from more than 20 individuals were freeze-dried at once and ground for chemical analyses. The moisture content of each sample was measured by weighing the fresh homogenate on an analytical balance before and after freeze-drying.
CHEMICAL ANALYSESFor the determination of Cd, Cu, Pb, and Zn concentrations in the soft tissues of freshwater and short-neck clams, a 1.5-g dry sample was digested using HNO3–HClO4, and the digest was evaporated to approximate dryness. This process was repeated twice. Finally, the residue was dissolved in 1-M HNO3, and the original sample solution was diluted properly to eliminate the interferences caused by major elements in the solution. The metal concentrations in this solution were measured by inductively coupled plasma emission spectrometry (ICP-AES; Varian 730-ES). On the basis of the average concentrations of major elements (Ca, 17 mg/g; Na, 13 mg/g; K, 5.9 mg/g; P, 8.6 mg/g; normalized to dry weight) in freshwater clam (https://www.mext.go.jp/a_menu/syokuhinseibun/1365297.htm), their concentrations in the original sample solution are calculated as 890–2,600 mg/l. This suggests that spectral interferences by the major elements in the metal analysis by ICP-AES are almost eliminated, if the original sample solution is diluted by a factor of 10 (Nishikawa et al., 1993).
Herein, we did not confirm the validity of the metal analysis of clam samples using appropriate certified reference materials. However, we judged that the metal analysis of clam samples is valid on the basis of the following: The clam samples were satisfactorily digested using HNO3–HClO4, as shown by the resulting clear sample solution obtained, which is supported by the fact that the relative standard deviations of the three replicate measurements were generally <10%. Such good reproducibility is obtained when the sample was satisfactorily digested (Barbosa et al., 2019). Moreover, analyzing a certified reference material (River Water, JSAC 0302-3) from the Japan Society for Analytical Chemistry, the validity of metal analysis by ICP-AES was confirmed. The recovery efficiencies of the metals were generally in the range of 90 to 110%.
Using the abovementioned sample solution, the Pb isotope ratios (207Pb/206Pb and 208Pb/206Pb) in the soft tissues were measured via inductively coupled plasma quadrupole mass spectrometry (ICP-QMS; Varian 820-MS) (Kusunoki et al., 2012). The Pb concentration in the analytical solution was adjusted to 40 μg/l to eliminate the effect of different Pb concentrations on isotope analysis; however, when the Pb concentration in the original sample solution was less than 40 μg/l, the Pb concentration in the analytical solution was adjusted to 10 μg/l. The Pb isotope ratios in the solution NIST SRM 981 that were adjusted to a Pb concentration of 40 or 10 μg/l were measured before and after the isotope ratio measurement of the samples. These mean isotope ratios were used as the reference (207Pb/206Pb=0.9146±0.0003; 208Pb/206Pb=2.1681±0.0008) to determine the correction factors for Pb isotope ratios in the samples. The mean reproducibilities (1σ) for the samples with the Pb concentration of 40 μg/l were 0.28% for 207Pb/206Pb and 0.19% for 208Pb/206Pb, whereas the mean reproducibilities (1σ) for the samples with the Pb concentration of 10 μg/l were 0.44% for 207Pb/206Pb and 0.28% for 208Pb/206Pb.
The analytical results obtained in this study are summarized in Table 1. The concentrations of Cd, Cu, Pb, and Zn in freshwater and short-neck clam samples are shown in Fig. 2. The samples of freshwater (19 samples) and short-neck clams (7 samples) were denoted by serial numbers (F-1–F-19 and S-1–S-7, respectively) without specifying the places of their production. Moreover, the metal concentrations in the clam samples were normalized to wet-sample weight on the basis of the moisture contents of the samples. Some amounts of metals in the soft tissues of clam samples may have originated from the bottom mud and be sand-mixed into soft tissues (i.e., a crustal origin). However, we assumed that the amounts of metals originating from mixed bottom mud and sand are very small on the basis of the enrichment factors (EFs) of metals in the soft tissue samples, as mentioned below. EF is defined as EF=(X/Al)sample/(X/Al)crust, where X and Al are the concentrations of elements X and Al, respectively, in a soft tissue sample and an average crustal material (Krauskopf and Bird, 1995). The calculated results showed markedly large EFs of metals (340–95,000 for Cd; 21–6,100 for Cu; 2–360 for Pb; and 130–55,000 for Zn; Table 1), suggesting that these metals are enriched primarily in soft tissues themselves.
| Moisture content (%) | Concentration (μg/g) | Enrichment Factor | Pb isotope ratios (mean±1σ) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cd | Cu | Pb | Zn | Cd | Cu | Pb | Zn | 207Pb/206Pb | 208Pb/206Pb | ||
| Freshwater clam | |||||||||||
| F-1 | 87 | 0.051 | 1.2 | 0.004 | 18 | 4,300 | 380 | 5 | 4,500 | 0.841±0.005 | 2.085±0.009 |
| F-2 | 89 | 0.16 | 2.3 | 0.010 | 19 | 52,000 | 2,700 | 50 | 18,000 | 0.863±0.003 | 2.111±0.006 |
| F-3 | 82 | 0.16 | 2.5 | 0.012 | 28 | 4,500 | 260 | 5 | 2,300 | 0.854±0.005 | 2.100±0.004 |
| F-4 | 90 | 0.11 | 1.9 | 0.011 | 22 | 95,000 | 6,100 | 140 | 55,000 | 0.847±0.007 | 2.100±0.015 |
| F-5 | 84 | 0.15 | 2.5 | 0.004 | 22 | 4,600 | 280 | 2 | 2,000 | 0.855±0.002 | 2.103±0.003 |
| F-6 | 81 | 0.15 | 3.0 | 0.032 | 31 | 4,600 | 340 | 15 | 2,700 | 0.863±0.005 | 2.108±0.004 |
| F-7 | 84 | 0.33 | 2.1 | 0.062 | 15 | 4,000 | 93 | 11 | 510 | 0.847±0.003 | 2.101±0.006 |
| F-8 | 84 | 0.050 | 2.3 | 0.10 | 25 | 530 | 91 | 16 | 760 | 0.863±0.002 | 2.117±0.003 |
| F-9 | 84 | 0.18 | 3.3 | 0.10 | 23 | 660 | 44 | 6 | 240 | 0.852±0.002 | 2.099±0.003 |
| F-10 | 74 | 0.20 | 4.4 | 0.19 | 42 | 1,700 | 130 | 25 | 1,000 | 0.856±0.003 | 2.108±0.008 |
| F-11 | 86 | 0.14 | 2.5 | 0.26 | 19 | 340 | 21 | 9 | 130 | 0.851±0.001 | 2.101±0.001 |
| F-12 | 80 | 0.17 | 4.5 | 0.15 | 25 | 1,100 | 110 | 15 | 470 | 0.855±0.001 | 2.106±0.003 |
| F-13 | 90 | 0.29 | 2.7 | 0.10 | 12 | 4,100 | 140 | 21 | 490 | 0.850±0.002 | 2.103±0.004 |
| F-14 | 88 | 1.08 | 4.0 | 1.54 | 15 | 17,000 | 220 | 360 | 670 | 0.902±0.003 | 2.201±0.006 |
| F-15 | 88 | 0.24 | 2.3 | 0.14 | 21 | 1,900 | 66 | 18 | 480 | 0.898±0.002 | 2.201±0.004 |
| F-16 | 87 | 0.84 | 3.3 | 1.19 | 21 | 3,500 | 50 | 76 | 250 | 0.907±0.003 | 2.228±0.009 |
| F-17 | 81 | 0.11 | 3.4 | 0.03 | 21 | 2,700 | 300 | 11 | 1,500 | 0.859±0.004 | 2.113±0.005 |
| F-18 | 85 | 0.17 | 2.8 | 0.14 | 18 | 1,800 | 110 | 22 | 530 | 0.890±0.001 | 2.191±0.002 |
| F-19 | 84 | 0.14 | 3.7 | 0.059 | 32 | 35,000 | 3,500 | 240 | 24,000 | 0.861±0.002 | 2.110±0.004 |
| Short-neck clam | |||||||||||
| S-1 | 85 | 0.016 | 0.72 | 0.020 | 14 | 7,800 | 1,300 | 150 | 20,000 | 0.857±0.005 | 2.115±0.007 |
| S-2 | 87 | 0.016 | 0.55 | 0.026 | 12 | 3,000 | 370 | 74 | 6,300 | 0.857±0.002 | 2.113±0.005 |
| S-3 | 88 | 0.14 | 0.50 | 0.013 | 8.8 | 24,000 | 310 | 34 | 4,200 | 0.857±0.004 | 2.109±0.007 |
| S-4 | 85 | 0.056 | 0.79 | 0.022 | 12 | 4,800 | 250 | 29 | 2,900 | 0.852±0.002 | 2.101±0.006 |
| S-5 | 89 | 0.047 | 0.79 | 0.005 | 12 | 4,600 | 280 | 7.8 | 3,300 | 0.855±0.003 | 2.098±0.003 |
| S-6 | 86 | 0.080 | 0.88 | 0.033 | 12 | 9,100 | 360 | 58 | 3,900 | 0.855±0.003 | 2.116±0.003 |
| S-7 | 87 | 0.066 | 0.62 | 0.032 | 9.3 | 17,000 | 570 | 120 | 6,600 | 0.856±0.004 | 2.123±0.005 |

Concentrations of Cd, Cu, Pb, and Zn (normalized to wet-sample weight) in freshwater (F-1–F-19) and short-neck (S-1–S-7) clam samples. The maximum concentrations of Cd and Pb in bivalve mollusks imposed by the Commission of the European Communities (EC No. 466/2001) are shown in the figures
Fig. 2 shows that the Cd and Pb concentrations in the freshwater clam samples F-14 and F-16 are fairly high. The Cd concentrations in these samples (F-14, 1.08 μg/g; F-16, 0.84 μg/g) were lower than the 2-μg/g wet weight maximum Cd concentration in marine bivalve mollusks imposed by the Codex Alimentarius Commission (CODEX STAN 193–1995), but were comparable to the 1.0 μg/g wet weight maximum Cd concentration in bivalve mollusks imposed by the Commission of the European Communities (EC No. 466/2001). On the contrary, the Pb concentrations in these samples (F-14, 1.54 μg/g; F-16, 1.19 μg/g) were also comparable to the 1.5 μg/g wet weight maximum Pb concentration imposed by the EC (there is no regulation of Pb concentration in marine bivalve mollusks imposed by the CODEX). Therefore, we estimate that some of the freshwater clams marketed in Japan are fairly contaminated with both Cd and Pb.
Conversely, the Cu and Zn concentrations in the freshwater clam samples were higher than those in the short-neck clam samples, showing that their tissue concentrations vary depending on the species. Moreover, for both clam samples, the concentrations of Cu and Zn were much higher than those of Cd and Pb, but there were relatively small differences in the Cu and Zn concentrations among the places of production. This may be because Cu and Zn are essential to various biological functions (Cossa, 1989).
Pb ISOTOPE RATIOS IN MARKETED FRESHWATER AND SHORT-NECK CLAM SAMPLESThe Pb isotope ratios, which are expressed as 207Pb/206Pb and 208Pb/206Pb in this study, in the freshwater and short-neck clam samples are shown in Fig. 3. The line shown in the figure is based on the Pb growth curve presented by Cumming and Richards (1975). The Pb isotope ratios change with time, approximately from the top right to bottom left of this line, which is attributed to radiogenic production from isotopes of U and Th (238U → 206Pb; 235U → 207Pb; 232Th → 238Pb), which means that the end point of the line corresponds to the present time. However, if the Pb was separated from U and Th in the past, the Pb isotope ratios did not change after that. Thus, the Pb ore deposits (primarily galena, PbS) formed in the ancient times of Earth have relatively high Pb isotope ratios. The Pb isotope ratios in the clam samples were compared with those in the surface (polluted by Pb) and background (unpolluted by Pb) layers of sediment cores in Tokyo Bay (Hirao et al., 1986; Sakata et al., 2018) and Lake Shinji (Kusunoki et al., 2012). Anthropogenic Pb in surface sediments were observed to be enriched in Tokyo Bay and Lake Shinji probably owing to the discharge of river bottom sediments polluted by Pb (Sakata et al., 2018) and the long-range transport of Pb from the Asian continent (Kusunoki et al., 2012; Kusunoki and Sakata, 2018), respectively. It is noteworthy that the Pb isotope ratios in the background sediments in Tokyo Bay and Lake Shinji are almost identical to those in Japanese background soils (207Pb/206Pb=0.846±0.004; 208Pb/206Pb=2.093±0.011; Sakata et al., 2021). Hence, it is reasonable to assume that the Pb isotope ratios in the background sediments in Tokyo Bay and Lake Shinji are the background values in Japanese aquatic regions.

Pb isotope ratios (207Pb/206Pb and 208Pb/206Pb) in freshwater (F-1–F-19) and short-neck (S-1–S-7) clam samples. These values are compared with those in the surface (polluted by Pb) and background (unpolluted by Pb) sediments in Tokyo Bay (Hirao et al., 1986; Sakata et al., 2018) and Lake Shinji (Kusunoki et al., 2012)
Fig. 3 shows that the Pb isotope ratios in the freshwater clam samples F-14, F-15, F-16, and F-18 are considerably higher than those in other freshwater clam samples and the short-neck clam samples. As mentioned in the preceding section, the freshwater clam samples F-14 and F-16 have fairly high Pb concentrations. This suggests that these samples are contaminated with Pb having high Pb isotope ratios. Conversely, the Pb isotope ratios in the other freshwater clam samples and the short-neck clam samples varied almost within the range of the values in the surface sediments to the background sediments in Tokyo Bay and Lake Shinji. Thus, it is likely that these clam samples were collected from unpolluted or slightly polluted aquatic regions, which reflects the recent pollution status of Japanese aquatic environments. Fig. 3 also shows that the Pb isotope ratios in the freshwater clam samples F-14, F-15, F-16, and F-18 are located above the Pb growth curve, suggesting that radiogenic Pb was produced in a Th–rich environment, because 208Pb comes from the decay of Th.
As shown in Fig. 3, the freshwater clam samples F-14, F-15, F-16, and F-18 had much higher Pb isotope ratios than the other samples. Such high Pb isotope ratios have never been observed in Japanese aquatic regions such as Tokyo Bay and Lake Shinji. Additionally, they cannot be explained by the discharge of domestic waste waters, including treated water from sewage treatment plants (Sakata et al., 2018) and mining or smelting effluent associated with Pb ores in Japan. The Japanese Pb ores have relatively low Pb isotope ratios because the formation age of Pb ores is considerably young. Mabuchi and Hirao (1982) reported that the mean Pb isotope ratios (±1σ) of 21 typical ores are 0.843±0.027 for 207Pb/206Pb and 2.091±0.053 for 208Pb/206Pb. Therefore, the high Pb isotope ratios in the freshwater clam samples F-14, F-15, F-16, and F-18 are probably attributable to the Pb originating from Pb ores formed in the ancient times of Earth.
As described in the Introduction, the amount of freshwater and short-neck clams in Japan, imported from countries such as China, South Korea, and North Korea, has increased with the decrease in their domestic production. Hence, the Pb isotope ratios in the freshwater clam samples F-14, F-15, F-16, and F-18 were compared with those in typical Pb ores in China (Sangster et al., 2000) and North Korea (Mabuchi and Hirao, 1982) whose data are available, as shown in Fig. 4. Except for two Pb ores in China, the Pb ores in China and North Korea have high Pb isotope ratios. According to the report by Li et al. (2020), the Pb isotope ratios (207Pb/206Pb=0.843–0.848; 208Pb/206Pb=2.094–2.101) of background soils in Southeast China are close to those of Japanese background soils (Sakata et al., 2021; Fig. 4). If it is assumed that the Pb isotope ratios in Japanese background soils are identical to those of background Pb in China and North Korea, the Pb isotope ratios in the freshwater clam samples are explainable by a mixture of the Pb from old Pb ores with high Pb isotope ratios in China and North Korea and the background Pb (Fig. 4). This supports the hypothesis that the Pb in those freshwater clam samples F-14, F-15, F-16, and F-18 originates predominantly from effluent and exhaust from mining or smelting associated with old Pb ores formed in countries such as China and North Korea. Thus, the places of their production in Japan, which were written on the labels of the packages, may be disguised (Komaru et al., 2010). In particular, the freshwater clam samples F-14 and F-16 have fairly high Pb concentrations (Fig. 2), suggesting that they are strongly affected by effluent and exhaust from mining or smelting associated with Pb ores.
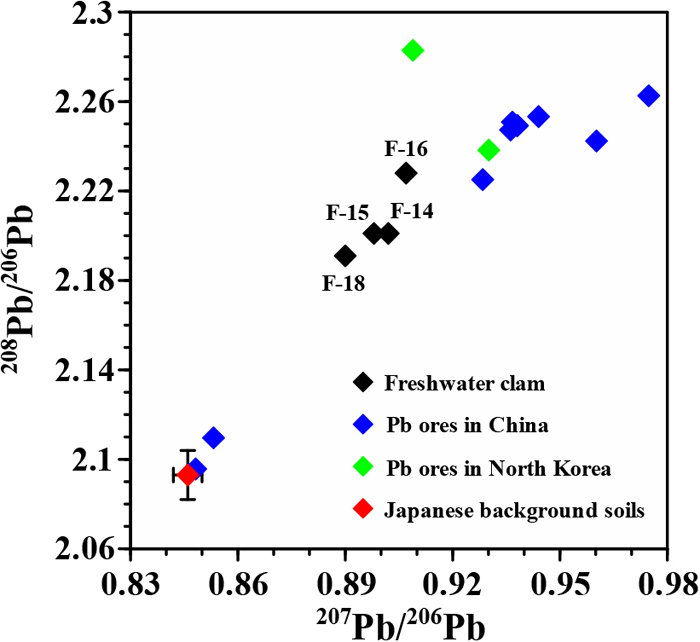
Pb isotope ratios (207Pb/206Pb and 208Pb/206Pb) in freshwater clam samples F-14, F-15, F-16, and F-18. These values are compared with those in typical Pb ores in China (Sangster et al., 2000) and North Korea (Mabuchi and Hirao, 1982) and Japanese background soils (mean±1σ; Sakata et al., 2021). It is assumed that the Pb isotope ratios in Japanese background soils are identical to those of background Pb in China and North Korea
Conversely, Pb-contaminated freshwater clams initially grown in foreign countries may be farmed for a certain period in Japanese aquatic regions. In this case, the concentration and isotope ratios of Pb in these freshwater clams are low, owing to the mixing of Pb from the foreign countries and Pb in Japan, depending on the period of farming in Japan and the biological half-time of Pb in clams. Eguchi et al. (2010) observed that in Maizuru Bay, Japan, the Pb concentration in mussels grown in uncontaminated areas increased significantly during 13 days after they were transferred to Pb-contaminated areas.
Generally, Cd is present as an impurity (primarily CdS) in Pb ores and other sulfide ores. It appears that the contamination of freshwater clam samples with Cd is attributable to the Cd accompanying Pb in effluent and exhaust from the mining or smelting. However, Fig. 2 shows that there is no significant difference between the Cd and Pb concentrations in the freshwater clam samples F-14 and F-16. This may be explained by the finding that Cd is more highly enriched than Pb in oyster and mussel samples collected from the coasts of western Canada and the USA (Shiel et al., 2012).
The results presented here revealed that some of the freshwater clams marketed in Japan are fairly contaminated with both Cd and Pb, which were comparable to their maximum concentrations in bivalve mollusks imposed by the Commission of the European Communities. Moreover, the results tended to support the hypothesis that the Pb and Cd in the contaminated freshwater clam samples originates predominantly from effluent and exhaust from mining or smelting associated with old Pb ores formed in countries such as China and North Korea. Thus, there is no doubt that these freshwater clams were imported from foreign countries with false indications of their origin. Hence, more attention should be paid to the contamination of marketed clams with toxic substances such as heavy metals from the viewpoint of food safety and security.
We thank Dr. K. Kusunoki (Shizuoka University) for providing useful information for this study.